Back to Journals » OncoTargets and Therapy » Volume 13
The miR-1224-5p/ELF3 Axis Regulates Malignant Behaviors of Pancreatic Cancer via PI3K/AKT/Notch Signaling Pathways
Authors Kong L, Liu P , Zheng M, Wang Z, Gao Y, Liang K, Wang H, Tan X
Received 10 February 2020
Accepted for publication 31 March 2020
Published 23 April 2020 Volume 2020:13 Pages 3449—3466
DOI https://doi.org/10.2147/OTT.S248507
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Lingming Kong,1 Peng Liu,1 Mingjun Zheng,2 Zhongpeng Wang,3 Yang Gao,1 Keke Liang,1 Huaitao Wang,1 Xiaodong Tan1
1Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang 110004, People’s Republic of China; 2Department of Obstetrics and Gynecology, University Hospital, LMU Munich, Marchioninistr. 15, Munich 81377, Germany; 3Department of Cardiology, The First Hospital of China Medical University, Shenyang 110001, People’s Republic of China
Correspondence: Xiaodong Tan
Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang 110004, People’s Republic of China
Email [email protected]
Purpose: Aberrant expression of microRNAs contributes to the progression of pancreatic cancer by targeting downstream genes. A novel regulatory axis, miR-1224-5p/ELF3, was identified by bioinformatic analysis and experimental verification. Studies of the underlying molecular mechanisms behind this axis lead to a better understanding of the development of pancreatic cancer.
Materials and Methods: The differential expression of miR-1224-5p and ELF3 was verified based on Gene Expression Omnibus (GEO) datasets and clinical samples. The relationship between miR-1224-5p and ELF3 was demonstrated by luciferase assay and Western blot. The related signaling pathways of the miR-1224-5p/ELF3 axis in pancreatic cancer were investigated by gene set enrichment analysis (GSEA) and verified by Western blot. An analysis between ELF3 expression and immune infiltration was performed. Cellular and animal experiments were utilized to explore the effects of miR-1224-5p and ELF3 in pancreatic cancer.
Results: Suppressed expression of miR-1224-5p in pancreatic tumor tissues and cancer cells was identified first. Furthermore, miR-1224-5p is correlated with clinicopathological features, and decreased expression of miR-1224-5p indicates poor prognosis. miR-1224-5p serves as a tumor suppressor and inhibits malignant behaviors of pancreatic cancer based on in vivo and in vitro assays. The putative target gene ELF3 was predicted by bioinformatic analysis and confirmed by dual-luciferase reporter assay. Overexpression of ELF3 can improve the malignant behaviors of pancreatic cancer and demonstrates poor prognosis and advanced clinical stage. The inhibitory role of miR-1224-5p in pancreatic cancer is manifested by its direct targeting of ELF3. A negative correlation between ELF3 expression and immune cell infiltration was identified, suggesting an immunosuppressive state resulting from ELF3 overexpression. The PI3K/AKT/Notch signaling pathways and epithelial-to-mesenchymal transition (EMT) are important underlying mechanisms.
Conclusion: The miR-1224-5p/ELF3 axis may serve as a new diagnostic, therapeutic, and prognostic biomarker in pancreatic cancer. The related PI3K/AKT/Notch/EMT signaling pathways greatly promote the elucidation of the progression of pancreatic cancer.
Keywords: miR-1224-5p, ELF3, immune infiltration, proliferation, migration, invasion
Introduction
Pancreatic cancer is characterized by advanced clinical presentation, rapid progression, and early metastasis. Pancreatic cancer has a poor prognosis, and the five-year survival rate is below 5%.1,2 Most patients who present at the hospital are at an advanced stage with less opportunity for radical treatment. Although tremendous efforts have been made to improve the diagnosis and treatment of pancreatic cancer, pancreatic cancer still ranks fourth in cancer-related death.3 Better understanding the mechanisms behind the occurrence and development of pancreatic cancer requires further analysis to increase the overall survival rate and identify novel targets for early diagnosis and specific therapy.4
microRNAs, which are 18–22 nucleotide long, serve as important epigenetic regulators and control gene expression at the post-transcriptional level. microRNAs are significantly associated with malignant behaviors of tumors, including tumorigenesis, proliferation, invasion, migration, cell cycle regulation, and apoptosis, by regulating the expression of specific genes.5–7 Previous studies have shown that microRNA-21, microRNA-221, and microRNA-155 play oncogenic roles in pancreatic cancer, while microRNA-34, microRNA-200, microRNA-let-7, microRNA-15a, microRNA-506, microRNA-96, microRNA-17-92, and microRNA-145 might function as tumor suppressors.8 Because each microRNA has hundreds of targets, alterations of microRNAs could significantly change the expression of various genes involved in multiple key signaling pathways in pancreatic cancer. Several studies have suggested that serum and plasma miRNAs (miR-16 and miR-196a) could serve as novel diagnostic and prognostic biomarkers with high sensitivity and specificity when combined with CA-199.9,10 Therefore, the development of targeting specific miRNAs could offer novel methods for the diagnosis, prognosis, and therapy of pancreatic cancer.
In our research, we identified a novel tumor suppressor, miR-1224-5p, in pancreatic cancer based on three GEO datasets. In addition, the diagnostic and prognostic values of miR-1224-5p were investigated based on The Cancer Genome Atlas (TCGA) database. Then, the E74-like ETS transcription factor 3 (ELF3) gene was recognized as the target of miR-1224-5p based on integrated bioinformatic analysis and experimental verification. ELF3 plays an important oncogenic role in several tumors, such as lung cancer,11 liver cancer,12 and breast cancer,13 via the PI3K/AKT, ERK and EMT signaling pathways. To further investigate the specific functions of ELF3 in pancreatic cancer, Kyoto encyclopedia of genes and genomes (KEGG) pathway, gene ontology (GO), and gene set enrichment analysis (GSEA) studies were performed based on the TCGA and STRING databases. Our results showed that ELF3 could promote the progression of pancreatic cancer via the PI3K/AKT/Notch/EMT signaling pathways based on in vivo and in vitro experiments and bioinformatic analysis.
Materials and Methods
Cell Lines
Four human pancreatic cancer cell lines (AsPC-1, Capan-2, PANC-1, and SW1990) and the human normal pancreatic cell line hTERT-HPNE were purchased from American Type Culture Collection. AsPC-1 cells were grown in RPMI-1640 medium containing 10% FBS. Capan-2 cells were maintained in McCoy’s 5a modified medium supplemented with 10% FBS. PANC-1 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. SW1990 cells were cultured in Leibovitz’s L-15 medium with 10% FBS. hTERT-HPNE cells were grown in 75% DMEM without glucose and 25% M3 Base medium supplemented with 5% FBS, 5.5 mM D-glucose, 10 ng/mL human recombinant EGF and 750 ng/mL puromycin.
Tissue Collection
Fresh pancreatic tumors and matched adjacent normal tissue were obtained at the time of surgery from 2018 to 2019. The tissues were properly stored in liquid nitrogen.
Bioinformatic Analysis and Survival Analysis
Three miRNA microarray datasets (GSE119794,14 GSE32678,15 and GSE2427916), which consisted of tumor tissues and normal tissues of the pancreas, were downloaded from the GEO database to identify differentially expressed miRNAs. The raw data of these datasets were extracted and normalized according to the methods provided by the data contributors. The relative expression level of miR-1224-5p between the normal and tumor groups was calculated based on Student’s t-test. P < 0.05 was defined as significantly different.
The GEPIA database provided differential gene expression analysis of 33 kinds of cancers based on integrated analysis of the TCGA and GTEx databases. The differentially expressed genes with |log2foldchange|≥1 and P < 0.05 were selected based on 179 pancreatic cancer samples and 171 normal pancreas samples.17
The miRNA-seq, RNA-seq, and clinical data of 179 pancreatic cancer patients were downloaded from the TCGA website on June 15, 2019. The relationship between clinicopathological characteristics and ELF3 or miR-1224-5p expression levels was visualized by GraphPad Prism 7. The survival plots for ELF3 and miR-1224-5p were created by the R package “survival” based on TCGA data, and survival times less than 30 days were excluded to avoid potential bias. The detailed data on ELF3 and miR-1224-5p expression and corresponding clinicopathological information are provided in Tables S1–S2.
GO, KEGG Pathway, GSEA, and Immune Infiltration Analyses
To elucidate the mechanisms behind the miR-1224-5p/ELF3 axis in pancreatic cancer, we performed KEGG pathway and GO analyses of molecular function (MF), biological process (BP), and cellular component (CC) based on the STRING database.18 GSEA was utilized to find relative signaling pathways of ELF3 based on the mRNA data of 179 pancreatic cancer samples from the TCGA database.19,20 The immune infiltration levels of six immune cell types associated with ELF3 expression were analyzed based on the TIMER database.21,22
Western Blot
The detailed procedures for Western blotting were performed as previously described.23 Equal amounts of protein (30 µg) were loaded per lane. The protein was separated by different concentrations of SDS-PAGE gel and detected by specific antibodies. Antibody information is provided in Table S3.
RNA Extraction and Quantitative Real-Time PCR
RNA extraction and qRT-PCR procedures were performed according to the methods described in a previous study.24 The detailed RT-PCR primer sequences are provided in Table S4. GAPDH and U6 were utilized as internal controls to normalize ELF3 and miR-1224-5p expression, respectively. Fold changes in ELF3 and miR-1224-5p were measured by the 2−ΔΔCT method.
CCK-8 Assay
The CCK-8 kit provided by Beyotime Biotechnology was utilized to assess cell proliferative ability. AsPC-1 or PANC-1 cells were seeded into 96-well plates with 2000 cells in each well in triplicate wells. Ten microliters of CCK-8 solution was added to each well after the cells attached to the well surface, and the plates were incubated in the cell incubator for 1 hour. Then, the OD value was measured by a microplate spectrophotometer (Bio-Rad Laboratories, USA).
Colony Formation Assay
Cells that received different treatments were digested and plated in 6-well plates with 700 cells per well. The culture medium of each well was replaced every 3 days for ten to fourteen days. When visible colonies were formed, the colonies were fixed with methyl alcohol, and crystal violet was used to stain the colonies.
Wound-Healing Assay
For cellular migratory capacity in vitro, cells that received different treatments were seeded into 6-well plates (Guangzhou Jet Bio-Filtration Co., Ltd) in triplicate.25 A 200 μL sterile pipette tip was utilized to draw lines on the surface of each well. The previous medium was removed, and the cells were washed three times with PBS. Then, 2 mL of serum-free medium was added to each well, and culture was continued for 24 hours.
Transwell Invasion Assay
For cellular invasive ability in vitro, the transwell method (Corning Co., USA) was employed according to the methods previously described in our study.26 The upper transwell chamber was precoated with Matrigel Matrix (Becton, Dickinson and Company, USA). A total of 50,000 cells in 0.1 mL culture medium without serum were placed in the upper chamber, and the lower chamber was filled with 0.7 mL culture medium containing 10% FBS. The residual cells in the top surface of the chamber were dislodged softly using a swab after one day of coculture. Then, 4% paraformaldehyde and crystal violet were added to fix and stain the invading cells in the lower surface of the chamber sequentially. Five stochastic images obtained from each chamber were utilized to calculate the mean number of invading cells.
Cell Transfection
Three siRNAs specifically targeting ELF3 were provided by GenePharma Biotechnology (Shanghai, China). The three siRNA sequences were as follows: siELF3-1: CCATGAGGTACTACTACAA; siELF3-2: GCCATTGACTTCTCACGAT; and siELF3-3: CCTCATGAAGTGGGAGAAT. Since siELF3-1 was more effective than siELF3-2 and siELF3-3 after transfection of AsPC-1 and PANC-1 cells, siELF3-1 was selected to design shRNA against ELF3. Lentiviruses containing ELF3-shRNA (5ʹ-CCATGAGGTACTACTACAA-3ʹ) were generated using the pHBLV-U6-ZsGreen-puro lentiviral RNAi expression system that was provided by Hanbio Biotechnology Co. Ltd. (Shanghai, China). miR-1224-5p mimics, miR-1224-5p inhibitor, and miR-NC (negative control) were designed and synthesized by GenePharma Biotechnology (Shanghai, China). The overexpression plasmid (gV144-ELF3) and miR-1224-agomir were provided by GeneChem Biotechnology (Shanghai, China) for animal experiment. Lipofectamine 3000 (Thermo Fisher Scientific, Invitrogen, USA) was used for cell transfection depending on the official guidelines.
Luciferase Reporter Experiment
For the luciferase assay, the TargetScan, miRanda, and miRWalk websites were utilized to predict the potential binding sites between ELF3 mRNA and hsa-miR-1224-5p. The 3ʹ untranslated region (UTR) fragments of ELF3 with the wild-type binding site of hsa-miR-1224-5p or the mutated binding site of hsa-miR-1224-5p were designed and chemically constructed by GeneChem Biotechnology (Shanghai, China). HEK293T cells (100,000) were seeded in triplicate into 24-well plates one day before transfection, and then miR-mimics or miR-NC was cotransfected with the wild-type or mutant plasmid, respectively, using Lipofectamine 3000 (Invitrogen, USA). The relative luciferase activity was recorded 48 hours after transfection using the Dual-Luciferase Reporter Assay System (Promega, USA).
Immunohistochemical Assay
The detailed immunohistochemical assay procedures were performed according to the methods described in our previous study.27 The staining intensity score of each section was graded based on the following scores: 0 for negative; 1 for weak; 2 for moderate; and 3 for strong. In addition, the staining extent score depended on the rate of positive cells and was defined as follows: 0 for less than 5%; 1 for 5% to 25%; 2 for 25% to 50%; 3 for 50% to 75%; and 4 for greater than 75%. The final score was measured using the following equation: final score = score of staining intensity × score of staining extent. Final scores between zero and seven were defined as low expression, and final scores from eight to twelve were regarded as overexpression. Ten visual fields were randomly selected from every section to calculate the average score for each section.
Xenograft Tumor Assay
A subcutaneous tumor model was utilized to assess the function of ELF3 and miR-1224-5p in pancreatic cancer progression in vivo according to previously described methods.28 BALB/c nude mice (28 days old, female) were provided by Huafukang Biotechnology (Beijing, China). An SPF animal laboratory was used to house the nude mice. A total of 1 × 106 cells from different groups in 0.1 mL PBS solution were subcutaneously injected in the left axillary area. Volumes of subcutaneous tumors were measured every week using a Vernier caliper. Subcutaneous tumor volumes were obtained according to the equation V (volume) = (L × W × W)/2, where L represents the long axis of the tumor, and W represents the short axis of the tumor. After 21 days, the mice were sacrificed to surgically obtain tumoral tissues for subsequent immunohistochemical analysis.
Statistical Analysis
The statistical analysis was completed using SPSS (version 19.0, SPSS Co., America) or R software (R version 3.6.1). Two-tailed Student’s t-test or one-way ANOVA were utilized for statistical comparisons among different groups. Each experiment was repeated at least three times.
Results
miR-1224-5p Is Downregulated in Pancreatic Tumor Tissues, and Its Expression Is Correlated with Clinicopathological Features and Prognosis
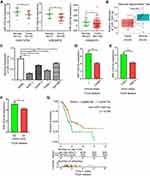
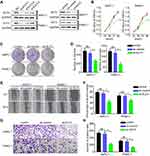
To identify differentially expressed miRNAs between pancreatic normal and tumor tissues, three GEO datasets, GSE119794 (10 tumor and 10 normal tissues), GSE32678 (25 tumor and 7 normal tissues) and GSE24279 (136 tumor and 22 normal tissues), were obtained from the GEO website. Analysis of the three datasets indicated that miR-1224-5p was commonly downregulated in pancreatic tumor tissues, as shown in Figure 1A. Then, RT-PCR was performed to examine miR-1224-5p expression in 20 pairs of pancreatic tumors and matched adjacent normal tissues. miR-1224-5p was confirmed to be downregulated in tumor tissues, which was consistent with the publicly available sequencing results (Figure 1B). In addition, the relative expression level of miR-1224-5p between a pancreatic normal cell line and cancer cell lines was detected. The cancer cell lines AsPC-1, Capan-2, PANC-1, and SW1990 showed lower miR-1224-5p expression levels than hTERT-HPNE cells (Figure 1C).
To understand how miR-1224-5p expression affects pancreatic cancer development, we evaluated correlations between miR-1224-5p and clinicopathological or survival information based on the TCGA database. As shown in Figure 1D–F, low expression of miR-1224-5p was significantly correlated with advanced clinical stage, advanced tumor stage, and lymphatic node metastasis. In addition, low expression of miR-1224-5p was correlated with a poor prognosis (Figure 1G).
miR-1224-5p Inhibits the Migration, Invasion, and Proliferation of Pancreatic Cancer Cells
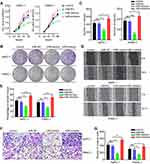
To study the possible effect of miR-1224-5p on pancreatic cancer, the AsPC-1 and PANC-1 cell lines were selected and transfected with miR-1224-5p mimics, inhibitor, or miR-NC. CCK-8 and colony formation experiments were conducted to detect the proliferative ability. The proliferation of AsPC-1 and PANC-1 cells was significantly inhibited after transfection of miR-1224-5p mimics in contrast to the miR-NC group, but cell proliferation was improved after transfecting miR-1224-5p inhibitor in compared to the miR-NC group (Figure 2A–C). To evaluate cell migratory and invasive ability, wound-healing and transwell experiments were performed, respectively. Overexpression of miR-1224-5p significantly reduced the migratory and invasive ability by transfecting miR-1224-5p mimics into AsPC-1 and PANC-1 cells compared with the negative control group. In addition, inhibition of miR-1224-5p expression through transfection of the inhibitor promoted the migratory and invasive ability in both cell lines (Figure 2D–G).
ELF3 Is a Target Gene of miR-1224-5p
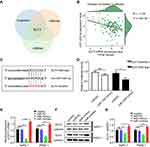
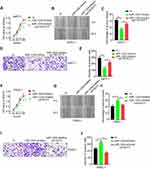
The TargetScan, miRanda, and miRWalk databases were employed to predict potential target genes of miR-1224-5p. ELF3 was chosen as a possible target gene of miR-1224-5p since it was predicted by all three databases (Figure 3A). We also found a significantly inverted relationship between ELF3 and miR-1224-5p expression based on 174 pancreatic cancer samples from the TCGA database (Figure 3B, Table S5). To evaluate the regulatory relationship between miR-1224-5p and ELF3, we synthesized two luciferase reporter vectors containing wild-type or mutant ELF3 sequences of the 3ʹ UTR (Figure 3C). Subsequently, miR-1224-5p mimics or miR-NC were transfected along with the different luciferase reporter vectors into HEK293T cells. Overexpression of miR-1224-5p significantly reduced the luciferase activity of the vector containing the wild-type ELF3 3′ UTR. However, luciferase activity did not change significantly in cells transfected with the vector containing the mutant ELF3 3′ UTR (Figure 3D). miR-1224-5p mimics or inhibitor were transfected into AsPC-1 and PANC-1 cells, and ELF3 expression was significantly decreased or increased, respectively, at both the mRNA (Figure 3E) and protein levels (Figure 3F–G). Collectively, the above results showed that miR-1224-5p could inhibit ELF3 expression by directly targeting ELF3 3′ UTR sequences.
ELF3 Is Upregulated in Pancreatic Tumor Tissues, and ELF3 Expression Is Associated with Clinicopathological Features and Prognosis
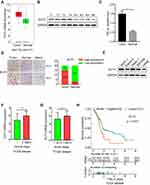
The GEPIA database provided differentially expressed genes based on TCGA and GTEx databases, which included 179 pancreatic tumor and 171 normal tissues. ELF3 was clearly upregulated in tumor tissues in comparison to normal tissues (Figure 4A). Then, we used Western blotting and immunohistochemistry to analyze ELF3 expression in paired pancreatic tumor and normal tissues based on our own collected samples. ELF3 was markedly overexpressed in pancreatic tumor tissues in contrast to normal tissues (Figure 4B–D). We also analyzed the expression level of ELF3 in pancreatic normal and cancerous cell lines by Western blotting, and ELF3 was significantly higher in AsPC-1, Capan-2, PANC-1 and SW1990 cells than in hTERT-HPNE cells (Figure 4E). ELF3 upregulation was significantly correlated with late clinical stage and advanced tumor stage based on TCGA clinicopathological information (Figure 4F–G). Moreover, ELF3 overexpression was markedly correlated with poor prognosis in pancreatic cancer patients (Figure 4H). Collectively, the above data indicated that ELF3 was overexpressed in pancreatic tumor tissues and might serve as a new diagnostic or prognostic biomarker.
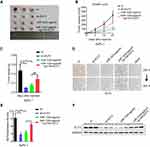
Knockdown of ELF3 Inhibits Malignant Behaviors of Pancreatic Cancer Cells
To explore the carcinogenic role of ELF3 in pancreatic cancer, three ELF3-targeting siRNAs were used to transfect AsPC-1 and PANC-1 cells. The transfection efficiency was detected by Western blot. Since siELF3-1 was more effective than siELF3-2 and siELF3-3 after transfection of AsPC-1 and PANC-1 cells (Figure 5A), the siELF3-1 sequence was utilized to generate a lentivirus to stably knock down ELF3 expression in the two cell lines. In contrast to the sh-control group, knockdown of ELF3 significantly reduced cell proliferation and colony formation rate in AsPC-1 and PANC-1 cell lines (Figure 5B–D). Regarding the migratory and invasive abilities, wound-healing and transwell experiments showed that knockdown of ELF3 could markedly reduce the migration and invasion of AsPC-1 and PANC-1 cells (Figure 5E–H).
Suppression of ELF3 Is Needed for the miR-1224-5p Inhibitory Effects on Proliferation, Migration, and Invasion in Pancreatic Cancer
To analyze whether the biological functions of miR-1224-5p were achieved by repressing ELF3, we restored ELF3 expression in miR-1224-5p-overexpressing AsPC-1 cells by cotransfection of miR-1224-5p mimics and the ELF3 overexpression plasmid. We found that proliferative, migratory and invasive abilities significantly increased after restoration of ELF3 expression compared with those in the miR-1224-5p overexpression group (Figure 6A–E). Furthermore, the expression of both miR-1224-5p and ELF3 was inhibited in PANC-1 cells by cotransfection with miR-1224-5p inhibitor and sh-ELF3. Inhibition of ELF3 partially eliminated the effect of miR-1224-5p knockdown (Figure 6F–J). Collectively, these results suggested that miR-1224-5p played an inhibitory role in pancreatic cancer progression by repressing ELF3 expression.
miR-1224-5p Inhibits Pancreatic Cancer Growth by Targeting ELF3 in vivo
A subcutaneous tumor model was established to assess the cell proliferation ability in nude mice. miR-1224-5p agomir was used to transfect AsPC-1 cells. The nude mice injected with AsPC-1 cells overexpressing miR-1224-5p showed a significantly smaller volume of xenograft tumors compared with the NC group. In addition, knockdown of ELF3 in AsPC-1 cells produced a similar result (Figure 7A–C). Cotransfection of the ELF3 overexpression plasmid and miR-1224-5p agomir partially relieved the inhibitory effect of miR-1224-5p overexpression (Figure 7A–C). Furthermore, Western blot and immunohistochemical analyses were used to examine the ELF3 expression level in the excised tumors from different groups. A similar result was obtained whereby miR-1224-5p overexpression could clearly inhibit ELF3 expression (Figure 7D–F). Overall, miR-1224-5p inhibited pancreatic cancer growth by suppressing ELF3 expression, which was further confirmed in vivo.
The miR-1224-5p/ELF3 Axis Regulates Pancreatic Cancer Progression via PI3K/AKT/Notch Signaling Pathways and Is Associated with Immunologic Derangement
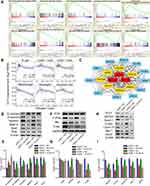
The new regulatory axis miR-1224-5p/ELF3 in pancreatic cancer based on the above results has been identified, but the underlying molecular mechanisms remain unclear. GSEA was used to find related signaling pathways based on the TCGA database according to the accepted guidelines. After analysis, we found several signaling pathways related to ELF3, including the notch signaling pathway, tight junction, cell adhesion molecules cams and base excision repair, which indicated that ELF3 might contribute extensively to pancreatic cancer progression (Figure 8A). In addition, ELF3 was closely associated with cytokine-cytokine receptor interaction, autoimmune thyroid disease, and graft versus host disease signaling pathways, suggesting that ELF3 might cause a potential immunologic derangement. Therefore, we performed immunological infiltration analysis of six immune cell types based on the TCGA database. High expression of ELF3 had an extensive negative correlation with infiltration by the six immune cell types, and significant correlations were identified between neutrophils, macrophages, and dendritic cells (Figure 8B). The above analysis indicated that high expression of ELF3 might lead to an immunosuppressive state in pancreatic cancer. The results of GO and KEGG analyses from the STRING database are provided in Table 1 and Figure S1. The protein-protein interaction (PPI) network was built and visualized based on the STRING database and Cytoscape software (version 3.7.2). This result indicated that ELF3 served as a hub gene in the network and had a close relationship with Notch1 (Figure 8C). We observed downregulation of Notch signaling pathway proteins, including Notch-1, C-myc, Cyclin D1, Hes-1, and VEGF, after knockdown of ELF3 in AsPC-1 and PANC-1 cells, suggesting that ELF3 might promote malignant behaviors of pancreatic cancer via a novel Notch signaling pathway (Figure 8H–I). It has been reported that high expression of ELF3 could promote epithelial-to-mesenchymal transition and is associated with malignant behaviors in hepatic carcinoma.12 A similar result was also found whereby silencing ELF3 increased E-cadherin and decreased N-cadherin, Vimentin, MMP-9, Snail, and Slug protein expression levels in pancreatic cancer cell lines (Figure 8D–E). Since PI3K and AKT have been recognized as important upstream pathways regulating EMT changes, we found that P-PI3K and P-Akt expression was markedly inhibited after knockdown of ELF3 (Figure 8F–G). Overall, ELF3 might play a novel oncogenic role in pancreatic cancer via various signaling pathways.
 |
Table 1 KEGG Pathway and Gene Ontology (GO) Analysis Containing Molecular Function (MF), Biological Process (BP), and Cellular Component (CC) Based on the STRING Database |
Discussion
Pancreatic cancer is a malignant solid tumor with the unique features of early metastasis, late diagnosis, and poor prognosis. Elucidation of the underlying mechanism in tumor progression and identification of new biomarkers for diagnosis, treatment, and prognosis are urgently needed to increase the overall survival rate of pancreatic cancer patients. Emerging studies have demonstrated that aberrant expression of microRNAs has an extensive impact on the occurrence and progression of different kinds of tumors and can act as novel diagnostic, prognostic, and specific therapeutic targets. Based on the development of whole-genome sequencing, the TCGA and GEO databases offer abundant high-throughput sequencing information and corresponding clinical data. In our study, the new regulatory axis miR-1224-5p/ELF3 was identified in pancreatic cancer via integrated bioinformatic analysis and experimental verification.
Previous research has suggested that miR-1224-5p is downregulated in lung cancer, glioma, and intestinal-type gastric cancer. Overexpression of miR-1224-5p predicts good prognosis in intestinal-type gastric cancer and glioma.29–31 miR-1224-5p inhibits the progression of gastric cancer and glioma by directly inhibiting FAK and CREB1.29,31 In addition to its role in cancer, overexpression of miR-1224-5p observably inhibits the migration, invasion, and proliferation of keloid fibroblasts and represses proliferation of hepatocytes after acute liver failure.32,33 In this study, we first determined that miR-1224-5p is downregulated in pancreatic cancer tissues based on three GEO datasets and clinical sample verification. Furthermore, overexpression of miR-1224-5p inhibited cell proliferation, migration, and invasion of pancreatic cancer in vivo and in vitro. miR-1224-5p has been identified as a novel prognosis-related biomarker for pancreatic cancer. A further large-scale study is needed to test the prognostic efficiency of miR-1224-5p in pancreatic cancer. To further analyze the underlying mechanism behind miR-1224-5p, three miRNA target prediction databases were integrated to find the direct target of miR-1224-5p. ELF3 was selected as the direct target gene of miR-1224-5p and verified by experiments.
ELF3 is an important member of the ETS transcription factor family, playing oncogenic or suppressive roles depending on the different tumor types. For instance, overexpression of ELF3 in lung cancer, HER2+ breast cancer, and liver cancer can facilitate the progression of tumors.11–13 However, a previous study showed that loss of ELF3 induces epithelial to mesenchymal transition in ovarian cancer, indicating that ELF3 acts as a tumor suppressor.34 It has also been demonstrated that overexpression of ELF3 in the cholangiocarcinoma cell line can significantly inhibit its proliferation.35 However, the specific function of ELF3 in pancreatic cancer has not been recognized previously. We first demonstrated that knockdown of ELF3 expression can significantly inhibit the proliferation, migration, and invasion of pancreatic cancer cells. Rescue experiments were also performed to verify that suppression of ELF3 is required for the inhibitory role of miR-1224-5p in pancreatic cancer. GSEA and immune infiltration analysis revealed that high expression of ELF3 might contribute to the immunosuppressive state of pancreatic cancer, but detailed mechanisms behind this phenomenon require further study. In previous studies, it has been shown that ELF3 can promote the proliferation, migration, and invasion of hepatic carcinoma cells by promoting EMT changes.12 A similar result was also obtained in our study, in which knockdown of ELF3 in pancreatic cancer cells increased E-cadherin and decreased N-cadherin, Vimentin, MMP-9, Snail, and Slug protein expression levels. In addition, another study has shown that high expression of ELF3 facilitates proliferation and invasion in lung cancer by regulating PI3K/AKT/ERK signaling pathways.11 The PI3K and AKT signaling pathways are recognized as novel upstream pathways regulating the EMT process.36 We observed that P-PI3K and P-Akt expression levels were markedly inhibited after knockdown of ELF3 in two pancreatic cancer cell lines, indicating that ELF3 may promote EMT by activating the PI3K/AKT pathways.
Mounting evidence has indicated that the Notch signaling pathway is important in the occurrence and progression of pancreatic cancer.37 Based on the STRING database, we identified a close interaction between ELF3 and Notch1. Subsequently, several key pathways were predicted by GSEA and the STRING database, including the Notch signaling pathway, microRNAs in cancer, pathways in cancer, and positive regulation of the Notch signaling pathway. The above evidence indicates that ELF3 might act as a key regulator involved in various cancer-related pathways. After knockdown of ELF3 in pancreatic cancer, we observed significant downregulation of Notch signaling pathway proteins, including Notch-1, C-myc, Cyclin D1, Hes-1, and VEGF. The Notch signaling pathway is identified as a new downstream pathway behind ELF3 in pancreatic cancer. Further studies on the direct molecular mechanisms between ELF3 and the Notch signaling pathway are needed in future research.
Conclusion
In conclusion, we establish the novel regulatory axis miR-1224-5p/ELF3 in pancreatic cancer. Several important signaling pathways behind this axis have been identified, including the PI3K, AKT, EMT and Notch pathways. Since miR-1224-5p and ELF3 are closely associated with diagnosis or prognosis in pancreatic cancer, a further large-scale experiment is required to verify whether they could be new biomarkers for pancreatic cancer.
Ethics and Consent Statement
The Ethics Committee of Shengjing Hospital of China Medical University approved this research. Written informed consent was obtained from every patient participated in this study. This research was conducted in accordance with the Declaration of Helsinki. The animal experiments were performed according to the guidelines for use and care of animals approved by Shengjing Hospital of China Medical University. The certificate number 2018PS01K and 2018PS25K were used for clinical samples collection and animal experiment respectively.
Acknowledgments
The results published here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. The authors would like to thank Busheng Xue for the suggestions on the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81902953, 30973501); Natural Science Foundation of Liaoning Province (grant number 180530068); the Outstanding Young Doctor Fund of China Medical University (grant number QGZD2018050); Liaoning BaiQianWan Talents Program (grant number 3200417003); 345 Talent Project of Shengjing Hospital of China Medical University. The sponsors had no involvement in any of the stages from study design to submission of the paper for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi:10.1056/NEJMra0901557
2. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861. doi:10.3748/wjg.v24.i43.4846
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
4. Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12(6):319–334. doi:10.1038/nrclinonc.2015.53
5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
6. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–234. doi:10.1038/ncb0309-228
7. Li Y, Sarkar FH. MicroRNA targeted therapeutic approach for pancreatic cancer. Int J Biol Sci. 2016;12(3):326–337. doi:10.7150/ijbs.15017
8. Guo S, Fesler A, Wang H, Ju J. microRNA based prognostic biomarkers in pancreatic cancer. Biomark Res. 2018;6:18. doi:10.1186/s40364-018-0131-1
9. Liu J, Gao J, Du Y, et al. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer. 2012;131(3):683–691. doi:10.1002/ijc.26422
10. Gao L, He SB, Li DC. Effects of miR-16 plus CA19-9 detections on pancreatic cancer diagnostic performance. Clin Lab. 2014;60(1):73–77. doi:10.7754/Clin.Lab.2013.121210
11. Wang H, Yu Z, Huo S, et al. Overexpression of ELF3 facilitates cell growth and metastasis through PI3K/Akt and ERK signaling pathways in non-small cell lung cancer. Int J Biochem Cell Biol. 2018;94:98–106. doi:10.1016/j.biocel.2017.12.002
12. Zheng L, Xu M, Xu J, et al. ELF3 promotes epithelial-mesenchymal transition by protecting ZEB1 from miR-141-3p-mediated silencing in hepatocellular carcinoma. Cell Death Dis. 2018;9(3):387. doi:10.1038/s41419-018-0399-y
13. Kar A, Gutierrez-Hartmann A. ESE-1/ELF3 mRNA expression associates with poor survival outcomes in HER2(+) breast cancer patients and is critical for tumorigenesis in HER2(+) breast cancer cells. Oncotarget. 2017;8(41):69622–69640. doi:10.18632/oncotarget.18710
14. Lin J, Wu YJ, Liang X, et al. Network-based integration of mRNA and miRNA profiles reveals new target genes involved in pancreatic cancer. Mol Carcinog. 2019;58(2):206–218. doi:10.1002/mc.22920
15. Donahue TR, Tran LM, Hill R, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18(5):1352–1363. doi:10.1158/1078-0432.CCR-11-1539
16. Bauer AS, Keller A, Costello E, et al. Diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis by measurement of microRNA abundance in blood and tissue. PLoS One. 2012;7(4):e34151. doi:10.1371/journal.pone.0034151
17. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
18. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
19. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
20. Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi:10.1038/ng1180
21. Li T, Fan J, Wang B, et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi:10.1158/0008-5472.CAN-17-0307
22. Li B, Severson E, Pignon JC, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi:10.1186/s13059-016-1028-7
23. Jin H, Liu P, Kong L, et al. Identification of RE1-silencing transcription factor as a promoter of metastasis in pancreatic cancer. Front Oncol. 2019;9:291. doi:10.3389/fonc.2019.00291
24. Zhang X, Gao F, Zhou L, Wang H, Shi G, Tan X. UCA1 regulates the growth and metastasis of pancreatic cancer by sponging miR-135a. Oncol Res. 2017;25(9):1529–1541. doi:10.3727/096504017X14888987683152
25. Liu P, Kong L, Jin H, Wu Y, Tan X, Song B. Differential secretome of pancreatic cancer cells in serum-containing conditioned medium reveals CCT8 as a new biomarker of pancreatic cancer invasion and metastasis. Cancer Cell Int. 2019;19:262. doi:10.1186/s12935-019-0980-1
26. Liu P, Weng Y, Sui Z, et al. Quantitative secretomic analysis of pancreatic cancer cells in serum-containing conditioned medium. Sci Rep. 2016;6:37606. doi:10.1038/srep37606
27. Wu Y, Tan X, Liu P, et al. ITGA6 and RPSA synergistically promote pancreatic cancer invasion and metastasis via PI3K and MAPK signaling pathways. Exp Cell Res. 2019;379(1):30–47. doi:10.1016/j.yexcr.2019.03.022
28. Jin H, Liu P, Wu Y, et al. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109(9):2946–2956. doi:10.1111/cas.13737
29. Qian J, Li R, Wang YY, et al. MiR-1224-5p acts as a tumor suppressor by targeting CREB1 in malignant gliomas. Mol Cell Biochem. 2015;403(1–2):33–41. doi:10.1007/s11010-015-2334-1
30. Nymark P, Guled M, Borze I, et al. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosom Cancer. 2011;50(8):585–597. doi:10.1002/gcc.20880
31. Wang J, Wen T, Li Z, et al. MicroRNA-1224 inhibits tumor metastasis in intestinal-type gastric cancer by directly targeting FAK. Front Oncol. 2019;9:222. doi:10.3389/fonc.2019.00222
32. Yao X, Cui X, Wu X, et al. Tumor suppressive role of miR-1224-5p in keloid proliferation, apoptosis and invasion via the TGF-beta1/Smad3 signaling pathway. Biochem Biophys Res Commun. 2018;495(1):713–720. doi:10.1016/j.bbrc.2017.10.070
33. Roy S, Bantel H, Wandrer F, et al. miR-1224 inhibits cell proliferation in acute liver failure by targeting the antiapoptotic gene Nfib. J Hepatol. 2017;67(5):966–978. doi:10.1016/j.jhep.2017.06.007
34. Yeung TL, Leung CS, Wong KK, et al. ELF3 is a negative regulator of epithelial-mesenchymal transition in ovarian cancer cells. Oncotarget. 2017;8(10):16951–16963. doi:10.18632/oncotarget.15208
35. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi:10.1038/ng.3375
36. Zhao J, Ou B, Han D, et al. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3beta/beta-catenin pathways. Mol Cancer. 2017;16(1):70. doi:10.1186/s12943-017-0629-4
37. Gao J, Long B, Wang Z. Role of notch signaling pathway in pancreatic cancer. Am J Cancer Res. 2017;7(2):173–186.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.