Back to Journals » ImmunoTargets and Therapy » Volume 11
The Meat of the Matter: Understanding and Managing Alpha-Gal Syndrome
Authors Macdougall JD, Thomas KO, Iweala OI
Received 12 April 2022
Accepted for publication 2 September 2022
Published 15 September 2022 Volume 2022:11 Pages 37—54
DOI https://doi.org/10.2147/ITT.S276872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Michael Shurin
Jessica D Macdougall,1,2,* Kevin O Thomas,1,2,* Onyinye I Iweala1,2
1Department of Medicine, Thurston Arthritis Research Center, Division of Rheumatology, Allergy, and Immunology, Chapel Hill, NC, 27599, USA; 2Department of Pediatrics, University of North Carolina Food Allergy Initiative, Division of Allergy and Immunology, University of North Carolina School of Medicine, Chapel Hill, NC, 27599, USA
*These authors contributed equally to this work
Correspondence: Onyinye I Iweala, Department of Medicine, University of North Carolina at Chapel Hill, 3300 Thurston Building, CB#7280, Chapel Hill, NC, 27599-7280, USA, Tel +1-984-974-2645, Fax +1-984-974-2660, Email [email protected]
Abstract: Alpha-gal syndrome is an unconventional food allergy, characterized by IgE-mediated hypersensitivity responses to the glycan galactose-alpha-1,3-galactose (alpha-gal) and not to a food-protein. In this review, we discuss how alpha-gal syndrome reframes our current conception of the mechanisms of pathogenesis of food allergy. The development of alpha-gal IgE is associated with tick bites though the possibility of other parasites promoting sensitization to alpha-gal remains. We review the immune cell populations involved in the sensitization and effector phases of alpha-gal syndrome and describe the current understanding of why allergic responses to ingested alpha-gal can be delayed by several hours. We review the foundation of management in alpha-gal syndrome, namely avoidance, but also discuss the use of antihistamines, mast cell stabilizers, and the emerging role of complementary and alternative therapies, biological products, and oral immunotherapy in the management of this condition. Alpha-gal syndrome influences the safety and tolerability of medications and medical devices containing or derived from mammalian products and impacts quality of life well beyond food choices.
Keywords: mammalian meat, red meat allergy, galactose-alpha-1, 3-galactose, tick
Introduction
Alpha-gal syndrome (AGS), also known as alpha-gal allergy, red meat allergy, or mammalian meat allergy (MMA), is characterized by the generation of immune-mediated hypersensitivity responses to the carbohydrate galactose-alpha-1,3-galactose (alpha-gal). Alpha-gal moieties are common in nature and present in non-primate mammals such as cows, pigs, and sheep. They are also present in various food products derived from those animals including dairy products, meat, and their innards. Consumption of those food products may result in symptoms ranging from urticaria to potentially lethal anaphylaxis.1 Individuals suffering from AGS may also be exposed to alpha-gal through various pharmaceutical products that contain alpha-gal such as the cancer drug cetuximab.2 Alpha-gal syndrome stands apart from conventional, IgE-mediated food allergies because the IgE driving the allergy forms against a sugar (alpha-gal) rather than a protein. Notably, sensitization to alpha-gal, ie, the development of alpha-gal IgE, is associated with a tick bite in most cases, rather than epithelial surface exposure to dietary alpha-gal itself.3 Since the description of AGS in 2008, there is growing recognition of other food allergies driven by IgE to carbohydrate, in which the IgE develops after exposure to an external sensitizer, rather than the food itself.4 These include IgE-mediated anaphylaxis to short-chain galacto-oligosaccharides (scGOS) in supplemented cow’s milk formula and allergy to GOS-supplemented beverages.5,6 In these carbohydrate-driven food allergies, sensitization to GOS moieties is driven by exposure to and allergy to the dust mite B. tropicales in the case of scGOS in cow’s milk and exposure to sea squirts for GOS-supplemented beverages.6 While the hypersensitivity reactions following the ingestion of scGOS occur within minutes,5 similar to the timeframe for allergic responses to food protein, allergic symptoms following the ingestion of alpha-gal typically happen hours after eating mammalian meat. This time course is highly unusual for IgE-mediated food allergy symptoms, which classically appear within minutes of food consumption. This paper reviews the current understanding of the pathogenesis of alpha-gal syndrome that might explain this delayed symptom onset. In addition, the epidemiology, diagnosis, management, clinical implications, and quality of life impacts of alpha-gal syndrome are discussed.
Mechanisms of Pathogenesis
Antibody Responses to Alpha-Gal
Naturally occurring anti-gal antibodies have been found in each of the major immunoglobulin isotypes (IgA, IgG, and IgM) and are thought to exist because of constant exposure to gastrointestinal bacteria that express alpha-gal on their surfaces.7 The presence of anti-alpha gal IgM and IgG antibodies has also been associated with a reduced risk of infection with Plasmodium, the causative agent of malaria.8 Anti-gal antibodies target Plasmodium sporozoites, blocking their trafficking from skin to liver and promoting skin-restricted sporozoite killing.8 If the parasite reaches erythrocytes in the bloodstream after mosquito bite, however, the presence of these antibodies does not lessen disease severity.9 Alpha-gal has also been detected on the surface of certain Mycobacterium species,10 leading to the speculation that anti-gal antibodies could protect against or control mycobacterial infection.9 Alpha-gal antibodies have been hypothesized to modulate coronavirus disease 2019 (COVID-19) infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2, reviewed in more detail below),11,12 although this is controversial.13 IgM and IgG2 anti-gal isotypes are present in high titers within the human body, raising concerns over the impact of these antibodies on xenotransplantation.14
Allergic Sensitization to Alpha-Gal
Alpha-gal specific IgE has been associated with hypersensitivity reactions,7 but similar to conventional IgE-mediated food allergies, the presence of circulating alpha-gal specific IgE does not necessarily equate to clinical symptoms of alpha-gal syndrome.15 This is illustrated in a recent study by Wilson et al that found cohorts of children from Ecuador and Kenya who possessed detectable levels of alpha-gal IgE but expressed no clinical signs of the allergy.15 Patients with AGS also tend to have higher levels of alpha-gal specific IgG1 and IgG3 compared to non-allergic patients and lower levels of IgG4.15–17 Food-specific IgG4 is considered a marker of tolerance to food antigens,18 but there is no such association with IgG1 or IgG3. We speculate that the presence of circulating alpha-gal specific IgG1 and IgG3 subclasses may identify individuals with increased risk of developing clinical allergy to alpha-gal, whereas alpha-gal IgG4 may promote a tolerized response to the antigen and may signify those less likely to develop mammalian meat allergy. However, currently, there is no published evidence to suggest that the presence of alpha-gal specific IgG1 and IgG3 subclasses incurs an increased risk of developing clinical allergy.
Sensitization to alpha-gal typically occurs following a tick bite. However, it remains unclear why insect bite is the primary route of induction of clinical alpha-gal syndrome, particularly when individuals are continuously exposed to alpha-gal through the host microbiome. In the southeastern United States, the main tick associated with AGS is Amblyomma americanum, known colloquially as the lone star tick.3 Various other tick species have also been linked to the induction of the allergy (see Table 1 and reviewed in Carson et al).19 One mechanism by which clinically relevant ticks may sensitize their host to alpha-gal involves host exposure to tick saliva as the tick feeds.20–22 The saliva, salivary glands, and secretory glands of A. americanum contain or are in close contact with alpha-gal residues.20 In addition, continued blood feeding by a tick can result in upregulation of alpha-gal epitopes in the saliva.22
 |
Table 1 Tick Species Associated with Alpha-Gal Sensitization |
Tick saliva from Ixodes ricinus, which has been associated with the development of AGS, can interfere with the maturation and migration of specialized antigen-presenting dendritic cells.23 Dendritic cells are central initiators of allergic responses to conventional protein allergens, presenting the allergens to naive T cells and creating a microenvironment that pushes T cells toward a pro-allergic T-helper (Th) 2 response.24 In the case of dendritic cells exposed to Ixodes ricinus saliva, this exposure prevents these dendritic cells from inducing pro-inflammatory Th1 or Th17 responses and favors the development of type 2 or Th2 pro-allergic responses.23 In addition, the presence of alpha-gal on glycoprotein may increase the ease with which dendritic cells take up antigen, even though they seem to process the alpha-gal glycoproteins at a slower rate than those without alpha-gal.25 Additional research is required to determine whether the presence of alpha-gal influences how dendritic cells interact with T cells and whether this may push them toward an allergic phenotype.
It has also been proposed that skin resident mast cells and circulating basophils are recruited to the site of the tick bite and stimulated to produce IL-4, a type 2, pro-allergic cytokine.19 The presence of tick saliva-associated factors that act as adjuvants of immune responses, coupled with host alarmins, cytokines and other chemical defenses released by the breached skin epithelial barrier, may create a cytokine environment that pushes antibody-producing, alpha-gal specific B cells to make IgE against alpha-gal.19,26 Transcriptional profiling of circulating peripheral blood mononuclear cells,27 and animal models of AGS,28 suggest that CD4+ Th2 cells, important in conventional allergies, may also play a role in allergic sensitization in AGS. The exact role of these cells, which recognize peptide allergen rather than glycans, in AGS is not entirely clear. Single-cell multi-omics sequencing of B-cells from alpha-gal sensitized individuals with atherosclerotic disease has identified a population of chemokine receptor (CCR)6+, CXCR4hi, class-switched memory B cells as potential alpha-gal IgE producers in patients with coronary artery disease.29 However, the T-cell dependence of this B-cell population is not established. This has opened the door for the possibility that unconventional or semi-variant T cell populations that recognize alpha-gal glycolipids, as well as unconventional, T-cell independent, alpha-gal specific B cells may be key players in the development of alpha-gal IgE.19 Short-lived, circulating plasmablasts, instead of long-lived bone marrow resident plasma cells, have also been proposed as the alpha-gal IgE producing B cell subset. This hypothesis is supported by the observation that alpha-gal IgE has been shown to wane relatively quickly over time (over 6 to 12 months) in a subset of individuals with AGS, especially if they avoid getting additional tick bites.30,31
Alpha-Gal Syndrome and the Effector Phase
In contrast to conventional allergy to food protein where allergic symptoms develop minutes after ingestion, allergic symptoms to alpha-gal typically develop hours after alpha-gal ingestion.32,33 Consistent with this delay, basophils (allergic effector cells also associated with immune responses to ticks) taken from alpha-gal allergic individuals undergoing open mammalian meat challenge expressed increased amounts of CD63, a marker of basophil activation, with peak expression 4 hours after meat ingestion.33 The delay in the development of allergic symptoms does not seem to be secondary to delays in the ability of basophils to react once stimulated with alpha-gal glycan. Using both direct and indirect basophil activation tests, we and others have shown that alpha-gal glycoprotein and glycolipid can activate basophils sensitized with alpha-gal specific IgE, even if sensitized basophils are only stimulated with alpha-gal glycan for 45 minutes.34,35 In addition, patients with AGS who are skin tested with the alpha-gal-rich cancer chemotherapeutic monoclonal antibody cetuximab develop erythematous, pruritic whelps to percutaneous and intradermal testing within 15 to 20 minutes.36 Thus, the delay in allergic symptom onset may be due to the time it takes for alpha-gal to traffic to the bloodstream following oral ingestion.19 This may be due to differences in how people with alpha-gal syndrome metabolize alpha-gal glycolipids and glycoproteins. For example, both before and after the oral pork challenge, individuals with circulating alpha-gal specific IgE were found to have significant differences in metabolic pathways of carbohydrates, lipids, and proteins compared to unsensitized, non-allergic individuals.37 After consuming pork, participants with alpha-gal syndrome had lipid blood levels that remained at baseline for several hours, compared to 2 hours in control participants.37 Additional studies have shown that alpha-gal glycoproteins do not move easily across intestinal epithelial cells,38 or may not be able to cross at all.35 Moreover, alpha-gal glycosylation can increase the resistance of mammalian proteins to digestion in vitro.35 In sum, delayed allergic reactions in patients with AGS after consuming alpha-gal may be due to delays in the processing, packaging, and transportation of alpha-gal, whether in glycolipid or glycoprotein form, across the intestinal epithelium and into the bloodstream.19 While a cell-mediated delayed type hypersensitivity response is an intriguing alternative explanation for the delayed symptoms in alpha-gal syndrome, the nature of the clinical signs and symptoms of AGS are more consistent with an IgE-driven hypersensitivity response than cytotoxic T-cell or macrophage-driven, cell-mediated responses which are associated more frequently with mucocutaneous lesions and tissue damage.39 To date, there are no experimental models linking cytotoxic T cells and macrophages to symptom onset or progression in AGS.
The clinical presentation of AGS, including the development of pruritic, red wheals and plaques that do not leave marks on the skin, and wheal and flare responses to percutaneous and intradermal testing with alpha-gal support a role for mast cells in the allergic responses to alpha-gal.32 In an AGS mouse model, serum levels of mouse mast cell protease 1, a readout for mast cell degranulation in mice, rose in animals with circulating alpha-gal IgE that were challenged with a homogenate of pork kidney.21 Yet, serum tryptase elevations after an allergic reaction, the gold standard for mast cell degranulation in humans, occurred in only 30% of patients with AGS with allergic symptoms after oral mammalian meat challenges and peaked 4 hours after meat consumption.33 AGS is similar to conventional food allergy in this respect since tryptase is also not consistently elevated during anaphylaxis to food protein allergens.40 Future studies of both AGS and conventional food allergy may identify alternative readouts for mast cell activation aside from tryptase that are more consistently linked to food-allergen induced mast cell activation.
Epidemiology and Diagnosis
Epidemiology
Alpha-gal syndrome cases arising after hard-bodied tick bites have been reported on every continent except Antarctica.41 One study analyzed Google searches for the term “Alpha-gal allergy” over a 15-year period from 2004 to 2019 as an “infodemiologic” surrogate for the extent of the global reach of this condition. The authors found the highest search volumes in Sweden, the United States, Canada, Australia, and South Africa.42 In Southern Africa, the mechanism of sensitization is still not entirely clear, but may be secondary to tick bite, as alpha-gal glycoproteins have been identified in regionally endemic ticks,43 or to other alpha-gal-bearing endoparasites.43,44
The prevalence of alpha-gal sensitization varies depending on the region of the world, population studied, and the cut-off value for a positive alpha-gal IgE level. As discussed previously, sensitization to alpha-gal does not guarantee the presence of symptoms,45 and the serum IgE level is not predictive of symptoms or severity of reactions.46 For example, a study of 300 German forest service employees and hunters in rural Germany found that 35% had alpha-gal IgE ≥0.10 kU/L, and 19.3% with alpha-gal IgE levels ≥0.35%.45 Alpha-gal IgE levels 0.35 kU/L or more did not guarantee the presence of symptoms since only 8.6% of those with alpha-gal IgE 0.35 kU/L and higher had symptomatic meat allergy.45 Larger studies from Denmark (>3400 participants)47 and Sweden (2201 individuals)48 report lower sensitization rates among these populations of 5.547 and 5.9%,48 respectively. Studies from Germany, Italy, South Africa, Kenya, Ecuador, and the United States have demonstrated higher rates of sensitization to alpha-gal in those living in rural versus urban areas.3,15,45,49–51 The alpha-gal sensitization rates among rural children in lower- and middle-income countries may be due to increased exposures to alpha-gal-containing endo- and ectoparasites.15,43
In the southeastern United States, initial sensitization rates ranged from 15% to 25% based on cohorts of approximately 50–250 patients.52,53 Recently, a study incorporating 122,068 serum samples from >100,000 unique patients in the United States demonstrated an alpha-gal IgE sensitization rate of 32.4%. The states with the highest numbers included Arkansas, Virginia, Kentucky, Oklahoma, and Missouri.54 The higher than expected alpha-gal sensitization rates in this cohort may be due to the fact that samples are sent from individuals for whom there is strong suspicion for mammalian meat allergy and do not fully represent the general population. However, this study also reported a 6-fold increase in the number of positive tests from 2011 to 2018,54 suggesting that sensitization rates to alpha-gal in the US may be rising.
Alpha-gal syndrome is most frequently reported in Australia and the southeastern United States.41 In fact, within the southeastern US, alpha-gal syndrome is a leading cause of anaphylaxis among adults and adolescents. For instance, Pattanaik et al noted that the number of idiopathic anaphylaxis cases declined in one Tennessee practice, while numbers of AGS-associated anaphylaxis increased.55 Alpha-gal syndrome accounted for one-third of anaphylaxis cases in this single-center retrospective review, while idiopathic anaphylaxis cases dropped from 59% in 2006 to 35% in 2018.55 A Spanish study demonstrated that the frequency of alpha-gal IgE in patients presenting with urticaria or anaphylaxis was 15%,56 suggesting that alpha-gal syndrome should be seriously considered in patients who present with unexplained hives, swelling, or spontaneous allergic reactions.
Risk Factors
Risk factors that are associated with development of mammalian meat allergy following tick bite include male sex;48 hypersensitivity responses to medications, including the cancer chemotherapeutic cetuximab36,57–59 and medications containing the mammalian product gelatin;41,60–62 A and O blood type;7,63,64 a history of idiopathic anaphylaxis and systemic mastocytosis;65 or history of bovine/porcine bioprosthetic heart valves.41 As detailed above, specific occupations like forest worker45,66 and rural home environment67 are also associated with high rates of alpha-gal IgE sensitization. Those with an outdoor occupation or living in rural areas are at higher risk of being sensitized to alpha-gal presumably due to increased risk of tick bites from clinically relevant ticks.67 Rates of polysensitization to stinging insect venom,68,69 inhalant aeroallergens,47,48,70 and food allergens (milk, egg, soy, peanut, fish48 and wheat48,68), are higher in individuals with AGS compared to the general population. However, a history of atopic disease is not necessary or sufficient to develop AGS,68,71 and it is not clear whether patients with AGS are at increased risk of developing other allergic diseases, including conventional food protein allergy.19
By contrast, the expression of B antigen on blood cells seems to confer some measure of resistance to the development of an alpha-gal allergy, presumably due to the B antigen and alpha-gal sharing a similar molecular structure.7,64,72 Compared to other blood types, alpha-gal sensitized individuals with the B blood type are below the expected frequency when compared to the general population.64 Those AGS-affected individuals with the B blood type also tend to have lower levels of alpha-gal specific IgE, if any at all.7
Diagnosis
Detection of serum alpha-gal specific IgE is typically used to support a diagnosis of red meat allergy caused by alpha-gal syndrome, whereas an oral food challenge, if performed and if positive, confirms it. There are variations in the source of alpha-gal sugar used in these serum-based specific IgE assays, but typically beef thyroglobulin or the cancer biological product cetuximab, both alpha-gal glycoproteins, are used.73,74 Sim et al found that they could distinguish patients with alpha-gal syndrome from those with beef or pork protein allergy by assessing the varying specific IgE levels to cetuximab and/or beef thyroglobulin versus specific IgE to bovine or porcine serum albumin which contain no galactose-alpha-1,3-galactose.73
An AGS diagnosis is made if individuals have a clinical history consistent with AGS and a positive serum IgE specific to alpha-gal. In addition, in many cases where AGS is suspected, the total IgE is obtained since tick bites frequently induce rises in total IgE levels, even in those individuals with no history of atopy and ordinarily low/normal IgE levels.68 In these cases, the ratio of alpha-gal IgE to total IgE becomes important.75 Specifically, if alpha-gal IgE antibodies are ≥2 IU/mL or >2% of the total IgE there is more likely to be a clinically relevant allergy.75 Some have reported that the alpha-gal specific IgE test may not be sufficient to catch all red/mammalian meat allergic patients in tick-endemic areas.76 One study evaluating twenty-six patients with mammalian meat allergy demonstrated that there are individuals with known tick exposure and clinical symptoms consistent with AGS that do not have elevated alpha-gal specific IgE.76 This suggests that in some cases, a clinical diagnosis may be necessary based on tick exposure, reaction history following mammalian meat ingestion, and response to dietary elimination of mammalian meat.
Clinical Manifestations
AGS is unique, as its clinical manifestations can be delayed in onset. While symptoms typically occur immediately after food-protein ingestion in conventional IgE-mediated food-protein allergies, symptoms in AGS frequently appear more than 2 hours after alpha-gal ingestion77 and may not manifest until 7 or 8 hours post-ingestion.33 Diagnosis of AGS can be difficult since the affected individual may not associate the hypersensitivity reaction with a particular food given the delayed symptom onset. There are data to show that a portion of individuals with idiopathic anaphylaxis (IA) actually have alpha-gal sensitization, demonstrating the importance of considering alpha-gal syndrome in the differential diagnosis of IA.56,77,78 While reactions are classically delayed at least 3 hours, some patients do experience symptoms within minutes.79 Immediate reactions occurring within one hour of ingestion in those with AGS may be secondary to the type of food consumed (for example, internal organs rich in alpha-gal) or due to other cofactors including alcohol consumption and exercise80 and frequently involve the gastrointestinal tract.79
Reactions in a single individual can vary depending on the dose and type of food ingested.77 For example, some foods are considered high-risk, including foods derived from internal organs (eg, pork kidney), and symptoms may occur more rapidly following ingestion of these mammalian products than after eating mammalian muscle meat.81,82 Similar to other IgE-mediated food allergies, cofactors, including exercise, non-steroidal anti-inflammatory drugs, and alcohol, can also impact the threshold for reaction.1,77,78 Alpha-gal syndrome can resolve spontaneously, especially since alpha-gal IgE wanes over time in those who avoid repeat bites from clinically relevant tick species.30 However, an individual may be re-sensitized or have increased reaction severity with repeat tick exposure.75
Alpha-gal syndrome can be present with a spectrum of symptoms, including but not limited to, urticaria, angioedema, respiratory distress, emesis, diarrhea, reflux, and abdominal pain.31,77,78 In an observational study of 261 patients, clinical manifestations and timing of reactions were similar between children and adults.68 There are individuals whose symptoms solely involve the gastrointestinal system;79,83 this manifestation was present in up to 20% of individuals with AGS in a South African cohort.79 In a Swedish cohort with AGS, 90% had urticaria, 74% had gastrointestinal symptoms, and 50% had anaphylaxis.70 Greater than half of the study participants were atopic and anaphylaxis with pulmonary symptoms were more common in this subgroup.70 We found a similar distribution of symptoms in a small cohort of patients recruited for a proof-of-concept transcriptional profiling study. Of the 15 participants with AGS, 93% reported cutaneous symptoms, 73% gastrointestinal symptoms, and 33% or less described respiratory, oropharyngeal, or cardiovascular symptoms (Table 2).27
 |
Table 2 Common Symptoms and Symptom Distribution in Study Participants with AGS (n = 15)a |
Management
Avoidance of Mammalian Meat and Associated Products
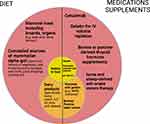
As with conventional IgE-mediated food-protein allergies, there is no cure for AGS. The mainstay of AGS management is to recommend avoidance of the allergen. In the case of AGS, first and foremost, patients are advised to avoid mammalian meat, including internal organs (Figure 1). Internal organs and fattier cuts of meats have been associated with increased risk for developing symptoms and progressing to more severe allergic reactions.31,77 Individuals with AGS should also be counseled to be wary of less obvious sources of mammalian alpha-gal, including beef broth, fatback or bacon in vegetables, pork encasings for poultry sausage, gravy drippings, and lard in biscuits1 (Figure 1). Dairy products do not need to be avoided routinely. In fact, patients with AGS can be encouraged to include moderate amounts of dairy products in the diet, particularly hard cheeses and skim or low-fat milk, since this has been associated with increased likelihood of spontaneous resolution of AGS.1,31 However, we do recommend dairy elimination from the diet if a patient continues to have symptoms despite strict avoidance of mammalian meat.1,75,77 From our clinical experience, approximately 80% of patients will experience symptom resolution with the elimination of mammalian meat products alone, while an additional 15% should have symptoms resolved with the removal of dairy in addition to mammalian meat.1,31 However, a small proportion of AGS patients (~5%) must also avoid gelatin-containing foods, including in some puddings and yogurts75 (Figure 1).
It is important to consider other types of exposures when a patient continues to have reactions despite removing all obvious products containing alpha-gal from their diet.31 In some cases, alpha-gal containing mammal products are introduced during the manufacturing process for some foods and other products, including pharmaceuticals, and cosmetics.31,84 In addition, it has been proposed that carrageenan, a high molecular weight polysaccharide derived from red seaweed and which contains the galactose-alpha-1,3-galactose (alpha-gal) epitope, may also cause IgE-mediated symptoms of hypersensitivity in a minority of patients with AGS.85 Carrageenan is used in several food products, including dairy products, frozen desserts, canned and cured meats, emulsified sauces, jelly candies, and some powdered products.85 While there are case reports of IgE-mediated allergic reactions to carrageenan,85,86 to date, there have been no reports demonstrating that such patients are also sensitized to alpha-gal. There are also no reported cases of patients with alpha-gal syndrome experiencing severe allergic responses or anaphylaxis to carrageenan. This may be due to the fact that the galactose-alpha-1,3-galactose moiety in carrageenan alternates with a beta-1,4-linked D-galactose sugar and may not always be exposed to the host immune system unless there is enzymatic breakdown of carrageenan by specific colonic microbes to expose the alpha-gal moiety.85 Thus, we counsel that the majority of individuals with AGS should be able to tolerate products with carrageenan and with trace amounts of alpha-gal, such as mammal-derived glycerin, and strict avoidance is not required.1 However, if patients remain symptomatic with these products in the diet, then as with gelatin, they should be cautious with their use or avoid these components altogether.
Management Using Pharmaceuticals
When patients report continued symptoms despite avoidance of alpha-gal containing products or if they are at high risk for exposure, pharmaceutical management of allergic symptoms may be warranted.1,31 Notably, there are no published observational studies or randomized clinical trials exploring the efficacy of any medicine for symptom control in alpha-gal syndrome. From our clinical experience, however, medications to consider include oral antihistamines,1,31 oral cromolyn solution (a mast cell stabilizer),1,31 oral corticosteroid (provided as a short burst and taper for acute reactions),31 omalizumab,31 and metformin.31 Interestingly, a few patients receiving omalizumab were able to reintroduce small amounts of mammal meat into the diet, while six patients with AGS receiving metformin prior to bariatric surgery added back both dairy and mammal meat to their diets.31
Complementary/Alternative Management
There have been recent case reports utilizing complementary medicine approaches in the management of AGS, specifically Soliman Auricular Allergy Treatment (SAAT), a form of auricular acupuncture.87 For example, a case series reported by Bernal et al described 137 patients who presented for SAAT at two different clinical centers. Treatment was not randomized, not placebo-controlled, and was unblinded. The majority of patients reported cutaneous and gastrointestinal symptoms and 93% of them had removed mammalian meat from the diet. Of the 126 out of 137 patients undergoing SAAT with post SAAT documented follow-up, 96% (121/126) reported remission of their AGS symptoms.87 This case series introduces a potential role for complementary and alternative therapies in the management of AGS and possibly other IgE-mediated food allergies. However, as these were case reports and the mechanism of action by which auricular acupuncture mitigates IgE-mediated alpha-gal syndrome remains unclear, further studies are clearly needed to assess the mechanism of action, efficacy, and safety of this treatment method.
Immunotherapy and Desensitization
While there is one oral immunotherapy treatment approved by the US Food and Drug Administration (FDA) for the treatment of peanut allergy, otherwise known as peanut OIT,19 there are no FDA-approved OIT treatments for AGS. The purpose of OIT is to feed a food-allergic individual escalating doses of food allergen until the person reaches a therapeutic treatment dose, which is administered daily to desensitize the allergic individual to the food in question. OIT mitigates allergic responses to food by modulating cellular and immunoglobulin responses to food allergen.18 Unal et al reported two cases of successful beef desensitization in adult patients with AGS utilizing a 27-day desensitization protocol starting with 0.00005 mg of beef extract twice daily and terminating in 100g serving size of beef daily.88 In addition, there is a case report of successful beef desensitization in a pediatric patient in Turkey who underwent a 24-day buildup protocol and was able to tolerate 120 g of beef daily following completion of desensitization.89 In each of these cases, the patients were instructed to continue daily consumption to maintain desensitization.88,89
In our clinical experience, individuals with AGS who can tolerate small amounts of dairy are more likely to experience remission of their allergic disease.1,31 Notably, a small, unblinded pilot study, with 7 participants with AGS who underwent open food challenge, showed that OIT with 6 mg of alpha-gal daily in the form of cow’s milk was safely tolerated over a 3-year period in these participants (https://clinicaltrials.gov/ct2/show/results/NCT02350660). Significantly, this study had a small number of participants, was not randomized or placebo-controlled and did not report on the efficacy of the treatment. In addition, this pilot study has not been subject to peer review, limiting the conclusions that can be drawn, but it suggests that cow’s milk as OIT for the dietary management of AGS is likely to be well tolerated. However, additional, therapeutic dose-finding studies and randomized, placebo-controlled studies to assess the safety and efficacy of such treatment in larger populations of participants with AGS are critical and necessary prior to implementation in the clinical setting.
Clinical Implications of Alpha-Gal Syndrome
AGS not only affects a patient’s diet but also impacts other components of health and disease. Below, we discuss the different facets of an individual’s medical care that may be influenced by an AGS diagnosis.
Cardiovascular/Heart Disease
Products that may contain alpha-gal are commonplace throughout the medical field31,84 (Figure 1). As such, they pose a potential risk to individuals who suffer from AGS. These individuals may be unable to take certain pharmaceuticals or receive certain prosthetics. Prior to surgery and other procedures, the risk of a severe peri-procedural allergic reaction caused by exposure to alpha-gal should be considered and measures to mitigate this taken when possible. For example, the use of heparin and bioprosthetic heart valves are of particular interest during cardiac surgery. Heparin is used in high doses during cardiopulmonary bypass surgeries to prevent the formation of blood clots; it is derived from bovine lungs and porcine mucosa and has been found to have detectable levels of alpha-gal.90 In light of this finding, Hawkins et al postulated that patients with AGS would have higher rates of anaphylaxis compared to controls if they received heparin anticoagulation. In their review of over 8800 patients seen at a single institution for cardiac surgery evaluation, they found 17 patients sensitized to alpha-gal, four (24%) of whom underwent cardiac surgery and suffered anaphylactic shock. Among the eight patients with alpha-gal serum IgE levels checked within 90 days of surgery, the rate of anaphylaxis was 50% (four of eight patients).90
The presence of alpha-gal in prosthetic heart valves obtained from pigs is also a concern. A study by Kuravi et al found that AGS serum reacted strongly to various animal-derived implants including cardiac patches, vascular grafts, and heart valves.91 Since these implants typically reside permanently in the body, the immune system’s response to them is ongoing. This may cause chronic inflammation with potentially serious implications for the patient, such as early valve failure and coronary heart disease, though further studies are needed to clearly elucidate a link.91 Research is underway to design methods for de-cellularizing valves and removing the majority of xenoantigens, including alpha-gal, without compromising their structural integrity.92,93
In December 2020, the FDA approved a genetically altered line of pigs known as “Gal-Safe Pigs” to generate medical products and to use as a potential human food source (https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-intentional-genomic-alteration-line-domestic-pigs-both-human-food, accessed 11 April 2022). These galactosyltransferase knockout pigs (GTKO) have been genetically engineered to lack the enzyme galactosyltransferase, which creates alpha-1,3 linkages between galactoses. As a result, these animals lack alpha-gal antigen on their cells, providing a potentially safe source for various xenotransplantation products like tissues, organs, and valves.94 A study by McGregor et al found that the genetic alteration induced in these GTKO pigs has no substantial impact on the structural integrity of the pericardium,95,96 suggesting that the tissue could be a direct substitute for the pericardium of standard pigs which are used in biomedical devices.96 Meat from these animals is also believed to be safe for individuals with AGS to consume (https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-intentional-genomic-alteration-line-domestic-pigs-both-human-food, accessed 11 April 2022). However, pre-clinical trials examining the safety of this meat using currently available animal models of alpha-gal syndrome21,28,97 and clinical trials of this meat in human subjects need to be conducted.
Alpha-gal sensitization has also been linked to coronary artery disease (CAD) with increased atherosclerotic burden and plaque instability.98 Wilson et al found that individuals 65 years or younger were more likely to possess higher levels of atheromatous plaques with unstable features within their coronary arteries if they had detectable circulating alpha-gal IgE.98 Although the association was not shown to be causal, this study highlights a possible link between allergic sensitization to alpha-gal and CAD, which may be driven by activated, alpha-gal sensitized allergic effector cells releasing inflammatory chemicals that promote blood vessel wall damage.98,99
Gastroenterology
Since Mabelane et al described a subset of patients with AGS with a GI-predominant phenotype, consisting of severe, persistent abdominal cramping, diarrhea, and/or gastroesophageal reflux, there has been a growing understanding that alpha-gal allergy is an underlying driver for gastrointestinal symptoms.79 For example, a retrospective review of four years of alpha-gal IgE testing in a single community gastroenterology practice in the southeastern US found that of 1112 adult patients with gastrointestinal symptoms, 32.3% (359) had detectable circulating alpha-gal IgE.100 Of the 122 patients with follow-up data available for analysis, 82% reported improvement of symptoms reminiscent of irritable bowel syndrome following removal of red (mammalian) meat from the diet.100 In another retrospective cohort study, Croglio et al studied 16 patients from two gastroenterology clinics in the southeastern US with elevated serum IgE to alpha-gal and gastrointestinal complaints without associated rash or anaphylaxis to determine if elimination of mammalian meat would improve their GI symptoms. In this study, follow-up ranged from two months to three years, and all patients reported symptom improvement within this time frame, with a majority following a strict elimination diet.83 Although it has a small sample size, this study demonstrates that AGS can be responsible for isolated gastrointestinal symptoms and that dietary elimination may lead to resolution.83
Adverse Reactions to Biological Products
Pharmaceutical products containing alpha-gal also have the potential to cause fatal reactions in AGS sufferers. Classically, therapy with the chimeric, monoclonal antibody cetuximab, a drug used primarily to treat head and neck and colorectal cancers, can cause severe anaphylaxis and death if administered to patients with AGS.101 While globally, the immediate hypersensitivity response rate to cetuximab was less than 3%, studies of patients in the southeastern US who received this biological product revealed hypersensitivity reactions as high as 22%.52,101,102 Cetuximab is synthesized in a mouse-derived myeloma SP2/0 cell line that glycosylates the monoclonal antibody with alpha-gal.103 In the southern US, areas with large tick populations have been associated with higher rates of cetuximab anaphylaxis,67,104 since lone star tick exposure is linked to the development of the preformed alpha-gal specific IgE driving immediate hypersensitivity reactions to the alpha-gal on cetuximab. Patients experiencing anaphylaxis upon the first administration of cetuximab have been described outside the US as well.105–107 These reports illustrate the importance of screening for a history of adverse reactions to mammalian meat prior to using cetuximab and to develop protocols to screen patients for sensitization to alpha-gal or gelatin (which contains alpha-gal) with allergen-specific IgE serum testing before administering cetuximab.67,104,108 Others have described the utility of cetuximab binding to IgE on basophils to predict alpha-gal sensitized patients with high likelihood of hypersensitivity responses to cetuximab,109 but this test is not available outside research settings.
There is one case report of anaphylaxis to infliximab, an anti-tumor necrosis factor monoclonal antibody also synthesized in the SP2/0 murine-derived cell line, in a patient with inflammatory bowel disease and AGS.103 Notably, the index patient, and two other patients with alpha-gal syndrome who had never received any monoclonal antibody infusions, had detectable IgE not only to cetuximab and infliximab but also to natalizumab, another biological product synthesized in mouse-myeloma derived SP2/0 cell line, used to treat multiple sclerosis and Crohn’s disease.103 Presumably, the serum alpha-gal specific IgE from these patients bound to the alpha-gal moieties common across these three biological agents. This finding points to the potential for IgE-mediated hypersensitivity responses to occur in AGS patients upon first-time infusion of infliximab and natalizumab as well as cetuximab. Potential solutions to this include the synthesis of cetuximab, infliximab, natalizumab, and other biological products in cell lines with minimal to no protein glycosylation with galactose-alpha-1,3-galactose, like the Chinese hamster ovary (CHO) cell line.110 Although CHO cells can glycosylate synthesized glycoproteins with alpha-gal moieties, cetuximab produced by CHO cells had no detectable alpha-gal moieties.110 In addition, several other monoclonal antibodies synthesized by CHO cells, including rituximab, mepolizumab, and omalizumab, contain no detectable alpha-gal.103 To date, there are no reports of anaphylaxis on first infusion of these CHO cell-line synthesized medications in patients with AGS. This suggests that the risk of anaphylaxis on first infusion to biological products produced without alpha-gal moieties would be comparable in patients with and without AGS.
Perioperative and Intraoperative Setting
AGS can present challenges for anesthesiologists when selecting anesthetic agents and other perioperative medications as patients are not always identified or clearly labeled as having AGS prior to procedures and it is difficult to determine which medications may contain alpha-gal.111 In regard to medications, the major concern is inactive ingredients, such as stearic acid, lactic acid, glycerin, and gelatin, rather than the active drug itself. Some inactive ingredients are derived from animal or plant sources; however, this information is not always readily available to be able to identify safe products.111 There have also been case reports of intraoperative anaphylaxis to gelatin-based hemostatic agents in patients with AGS.112–114
Vaccines
There have been case reports of individuals with AGS experiencing anaphylaxis following administration of gelatin-containing vaccines115–117 and reports of patients with AGS tolerating vaccines with gelatin in the excipients.118 Schmidle et al demonstrated increased basophil activation in patients with AGS receiving mumps measles rubella (MMR) vaccine containing gelatin in comparison to those that received a non-gelatin containing MMR vaccine, where basophil activation was not seen.119 Based on these findings, immunizations containing gelatin products should be administered with caution or even potentially avoided in patients with alpha-gal syndrome (Figure 1).
COVID-19 and Alpha-Gal Syndrome
As introduced above, anti-gal antibodies may modulate coronavirus disease 2019 (COVID-19) infection caused by SARS-CoV2,11,12 though this hypothesis remains controversial.13 In the beginning of the COVID-19 pandemic, the prevalence and mortality rates of this viral illness varied widely across the world and were found to be lower in Africa and Asia than in Europe and North America.12 One hypothesis to explain this discrepancy was that the production of anti-gal antibodies, especially IgG and IgM, in individuals living in Africa and Asia, is able to confer protection by interfering with SARS-CoV-2 virus attachment to the host cell.120 In support of this theory, Urra et al conducted a retrospective case–control study (25–29 patients per group) and observed that critically ill patients with COVID-19 requiring the intensive care unit (ICU) had lower amounts of alpha-gal specific IgG, IgM, and IgE, but comparable amounts of IgA compared to healthy, uninfected controls. They also found that asymptomatic COVID-19 patients seemed to have the highest amounts of alpha-gal specific IgE.11 By contrast, Keshavarz et al did not observe similar patterns in alpha-gal specific IgG levels when comparing individuals with severe COVID-19 (n = 60) and samples from healthy, uninfected individuals banked prior to the pandemic (n = 109).13,121 Further, in five critically ill patients with COVID-19, alpha-gal IgG levels stayed about the same from acute symptom onset through recovery and very little alpha-gal specific IgE were detected in these patients.13 The discrepancies in the findings from these two groups are likely related to differences in sample size and in methods used to measure alpha-gal specific Ig – colorimetric ELISA techniques by Urra et al and a solid phase ImmunoCAP by Keshavarz et al.11,13,121
Despite these conflicting results, there is a growing interest in designing COVID-19 vaccines that exploit anti-gal antibody responses to strengthen the protective effects of a COVID-19 vaccine.122,123 Some have also proposed improving immunity and decreasing transmission of COVID-19 by introducing alpha-gal via oral or mucosal routes with probiotics and postbiotics.120 Given the protective role of anti-gal antibodies in other infectious diseases, and earlier proof-of-principle studies demonstrating that incorporating engineered alpha-gal expressing glycan shields into inactivated influenza vaccines can enhance the immunogenicity of the vaccine,124 such an approach seems reasonable. However, as discussed by Wilson et al, a potential problem with this strategy is the increased risk for IgE-mediated hypersensitivity responses to any COVID-19 vaccine that explicitly incorporates alpha-gal into its formulation, especially in regions of the globe where sensitization to alpha-gal is common13.
Endocrinology
Thyroid
Alpha-gal is present on mammalian thyroglobulin125 and medications that include natural, non-primate thyroid extracts are at risk for contributing to allergic reactions in alpha-gal sensitized individuals. Specific inactive ingredients that may result in reaction are magnesium stearate, which can be derived from fatty acids of bovine origin, as well as gelatin.126 As a result, the possibility of an adverse reaction to mammalian-derived fillers and excipients in thyroid hormone drugs, such as levothyroxine, should be considered when prescribing thyroid hormone replacement to individuals with hypothyroidism and AGS.127
Type 1 Diabetes Mellitus
Engineered porcine islet cells are being developed and deployed as a potential treatment for type 1 diabetes. Because of the origin from pig, one study assessed whether or not engineered porcine islet cells express alpha-gal. In 57 porcine and 34 human N-glycans, as well as 21 porcine and 14 human O-glycans detected in cultured islets, there was no evidence of an alpha gal epitope detected on any of the islets,128 suggesting this may not be an issue.
Toxicology
Adverse reactions to snake antivenom are very common in several parts of the world, with acute reaction rates as high as 75%.129 Since antivenom, the lynchpin of therapeutics for treating poisonous snakebites, is typically produced in non-primate mammals, there have been studies to determine whether sensitization to alpha-gal could be the underlying cause of the frequent, infusion-induced, hypersensitivity reactions. One study of 55 people presenting for emergency care for snake bite in Laos reported that 15 of 55 (27%) experienced early acute reactions after snake antivenom infusion, and alpha-gal IgE was detected in the serum of 17 (31%) of these patients. However, there was no relationship between alpha-gal IgE positivity and development or severity of acute adverse reactions to antivenom.130 The detection of alpha-gal specific IgE in one-third of this cohort, irrespective of early adverse reaction status to antivenom, is not fully understood, but it does suggest that alpha-gal specific IgE was not the major driver for hypersensitivity reactions to antivenom in this population. However, there are case reports of patients in the southeastern US with anaphylaxis to sheep-derived, crotalidae polyvalent immune Fab antivenom (CroFab) as a first presentation of alpha-gal syndrome.131 Subsequent reports demonstrated that galactose-alpha-1,3-galactose is detectable in CroFab132 as well as in equine antivenom and can bind to alpha-gal allergic serum causing degranulation of alpha-gal sensitized basophils.133
Occupational Health/Medicine
Alpha-gal present in reagents or fluids that workers may come into contact with can present an occupational hazard and needs to be taken into account in a certain subset of patients with AGS. Our cohort of study participants with alpha-gal includes a 32-year-old woman with AGS and asthma who experienced anaphylaxis while working at a leather tanning factory and required an emergency department visit. A published case series describes three cattle workers with alpha-gal syndrome who developed symptoms (urticaria, pruritus, and/or dyspnea) following direct contact with bovine amniotic fluid during calving.134
Quality of Life in Patients with Alpha-Gal Syndrome
Although there is increasing awareness of AGS, it still appears to go underdiagnosed. Flaherty et al interviewed twenty-eight patients seen at the University of North Carolina-Chapel Hill for AGS from March to June 2016 to assess time to diagnosis and healthcare utilization. They found that the time to diagnosis was delayed in a majority of these patients, with a mean diagnosis time of 7.1 years. Over half of these patients experienced anaphylaxis requiring treatment in the emergency department, with two patients requiring multiple hospitalizations for symptoms. In all but one of these cases, individuals were treated for idiopathic anaphylaxis and discharged.135 The findings suggest that there may be substantial healthcare utilization secondary to symptoms by patients with undiagnosed AGS and that delayed diagnosis results in increased cost.135 A majority of these patients did not obtain information regarding AGS from their healthcare provider and felt that their primary care physician had little to no information about AGS, suggesting that reaching the AGS diagnosis and obtaining appropriate counseling on living with the condition is often patient driven.136 As with conventional IgE-mediated food allergies,137 other considerations for quality of life include the impact of continued food avoidance on eating out and social interactions with family and friends and the anxiety associated with potentially life-threatening anaphylactic reactions. Additional studies are warranted to understand the psychological and financial impacts of the AGS diagnosis for patients.
Conclusion
Alpha-gal syndrome is an unusual food allergy. IgE-mediated hypersensitivity responses to the glycan alpha-gal (and not to a food-protein) reframe our current conception of the mechanisms of pathogenesis of food allergy. Alpha-gal IgE rises after exposure to parasites, with hard-bodied ticks considered the leading agents of sensitization to alpha-gal. However, this does not exclude the possibility of internal endoparasites promoting sensitization to alpha-gal, a line of investigation under active study. While allergic sensitization likely involves the same cell populations involved in food-protein allergies, because the allergen is found not just on protein but also lipid, there may be roles for unconventional cellular players like invariant natural killer T cells or plasmablasts specific for alpha-gal IgE. Moreover, the delay in the development of allergic symptoms in alpha-gal syndrome contrasts with the immediate hypersensitivity responses of traditional IgE-mediated allergies. It may be due to extensive digestion and metabolism of alpha-gal glycoproteins and the processing of alpha-gal glycolipids into lipid particles like chylomicrons that must leave the digestive system and spread systemically before activating allergic effector cells at distant sites.
While the mainstay of management for AGS remains avoidance, emerging supplementary therapies include complementary and alternative acupuncture, oral immunotherapy using mammalian meat or dairy products, and the use of antihistamines, mast cell stabilizers, and biologicals that target IgE and stabilize mast cells. The impact of alpha-gal syndrome moves beyond food choices. It can also affect the safety and tolerability of cosmetics, pharmaceuticals, and medical devices, present unique occupational health considerations, and significantly increase healthcare utilization and impact quality of life. Continued study of the mechanisms driving this alpha-gal syndrome, psychosocial and financial impacts, and potential therapeutics will have implications for our general conception of food allergy.
Acknowledgments
We thank Jada Suber, Audrey Carson, Yugen Zhang, Shailesh Choudhary, and Scott Commins for their helpful discussions. All figures were designed with Biorender.com.
Funding
This work was supported by the NIH/NIAID K08AI141691 (OII); a 2020 American Association of Allergy, Asthma, and Immunology Foundation Faculty Development Award (OII); and the National Institutes of Health 1UM2AI30836-01. The funders had no role in the analysis, decision to prepare or to publish the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
OII is a consultant for Blueprint Medicines and Novartis and is a co-author on an industry sponsored abstract for Genentech. The other authors have no financial conflicts of interest.
References
1. Patel C, Iweala OI. ‘Doc, will I ever eat steak again?’: diagnosis and management of alpha-gal syndrome. Curr Opin Pediatr. 2020;32(6):816–824. doi:10.1097/MOP.0000000000000955
2. Hilger C, Fischer J, Swiontek K, et al. Two galactose-alpha-1,3-galactose carrying peptidases from pork kidney mediate anaphylactogenic responses in delayed meat allergy. Allergy. 2016;71(5):711–719.
3. Commins SP, James HR, Kelly LA, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–1293.
4. Platts-Mills TA, Hilger C, Jappe U, et al. Carbohydrate epitopes currently recognized as targets for IgE antibodies. Allergy. 2021;76(8):2383–2394.
5. Chiang WC, Huang CH, Llanora GV, et al. Anaphylaxis to cow’s milk formula containing short-chain galacto-oligosaccharide. J Allergy Clin Immunol. 2012;130(6):1361–1367.
6. Lee L, Zhong Y, Leow SY, et al. Allergy to prebiotic galacto-oligosaccharides: house dust mites – the putative primary sensitizer. J Allergy Clin Immunol. 2020;145(2):707–710.
7. Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of alpha-gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy. 2018;73(7):1525–1531.
8. Yilmaz B, Portugal S, Tran TM, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159(6):1277–1289.
9. Cabezas-Cruz A, de la Fuente J. Immunity to alpha-gal: the opportunity for malaria and tuberculosis control. Front Immunol. 2017;8:1733.
10. Cabezas-Cruz A, Mateos-Hernandez L, Alberdi P, et al. Effect of blood type on anti-alpha-gal immunity and the incidence of infectious diseases. Exp Mol Med. 2017;49(3):e301.
11. Urra JM, Ferreras-Colino E, Contreras M, et al. The antibody response to the glycan alpha-gal correlates with COVID-19 disease symptoms. J Med Virol. 2021;93(4):2065–2075.
12. Hodzic A, de la Fuente J, Cabezas-Cruz A. COVID-19 in the developing world: is the immune response to alpha-gal an overlooked factor mitigating the severity of infection? ACS Infect Dis. 2020;6(12):3104–3108.
13. Wilson JM, Platts-Mills TAE, Keshavarz B. Reply to: the antibody response to the glycan alpha-gal correlates with COVID-19 symptoms. J Med Virol. 2021;93(9):5219–5220.
14. Lee EJ, Lee H, Park EM, Kang HJ, Kim SJ, Park CG. Immunoglobulin M and Immunoglobulin G subclass distribution of anti-galactose-alpha-1,3-galactose and anti-N-glycolylneuraminic acid antibodies in healthy Korean adults. Transplant Proc. 2021;53:1762–1770.
15. Wilson JM, Keshavarz B, James HR, et al. alpha-gal specific-IgE prevalence and levels in Ecuador and Kenya: relation to diet, parasites, and IgG4. J Allergy Clin Immunol. 2021;147(4):1393–1401.
16. Kollmann D, Nagl B, Ebner C, et al. The quantity and quality of alpha-gal-specific antibodies differ in individuals with and without delayed red meat allergy. Allergy. 2017;72(2):266–273.
17. Platts-Mills TAE, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol. 2016;137(6):1662–1670.
18. Barshow SM, Kulis MD, Burks AW, Kim EH. Mechanisms of oral immunotherapy. Clin Exp Allergy. 2021;51(4):527–535.
19. Carson AS, Gardner A, Iweala OI. Where’s the beef? Understanding allergic responses to red meat in alpha-gal syndrome. J Immunol. 2022;208(2):267–277.
20. Crispell G, Commins SP, Archer-Hartman SA, et al. Discovery of alpha-gal-containing antigens in north American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056.
21. Choudhary SK, Karim S, Iweala OI, et al. Tick salivary gland extract induces alpha-gal syndrome in alpha-gal deficient mice. Immun Inflamm Dis. 2021;93:984–990.
22. Park Y, Kim D, Boorgula GD, et al. Alpha-gal and cross-reactive carbohydrate determinants in the N-glycans of salivary glands in the lone star tick, Amblyomma americanum. Vaccines. 2020;8(1):18.
23. Skallova A, Iezzi G, Ampenberger F, Kopf M, Kopecky J. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J Immunol. 2008;180(9):6186–6192.
24. Iweala OI, Burks AW. Food allergy: our evolving understanding of its pathogenesis, prevention, and treatment. Curr Allergy Asthma Rep. 2016;16(5):37.
25. Ristivojevic MK, Grundstrom J, Tran TAT, et al. Alpha-gal on the protein surface affects uptake and degradation in immature monocyte derived dendritic cells. Sci Rep. 2018;8(1):12684.
26. Wilson JM, Schuyler AJ, Schroeder N, Platts-Mills TA. Galactose-alpha-1,3-galactose: atypical food allergen or model ige hypersensitivity? Curr Allergy Asthma Rep. 2017;17(1):8.
27. Iweala OI, Choudhary SK, Addison CT, Commins SP. T and B lymphocyte transcriptional states differentiate between sensitized and unsensitized individuals in alpha-gal syndrome. Int J Mol Sci. 2021;22(6):3185.
28. Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous exposure to clinically relevant lone star ticks promotes ige production and hypersensitivity through CD4(+) T cell- and MyD88-dependent pathways in mice. J Immunol. 2019;203(4):813–824.
29. Pattarabanjird T, Wilson JM, Erickson LD, et al. Chemokine receptor activation enhances memory B cell class switching linked to IgE sensitization to alpha gal and cardiovascular disease. Front Cardiovasc Med. 2021;8:791028.
30. Kim MS, Straesser MD, Keshavarz B, et al. IgE to galactose-alpha-1,3-galactose wanes over time in patients who avoid tick bites. J Allergy Clin Immunol Pract. 2020;8(1):364–367.
31. Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2500 patients. Expert Rev Clin Immunol. 2020;16(7):667–677.
32. Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433.
33. Commins SP, James HR, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134(1):108–115.
34. Iweala OI, Choudhary SK, Addison CT, et al. Glycolipid-mediated basophil activation in alpha-gal allergy. J Allergy Clin Immunol. 2020;146(2):450–452.
35. Roman-Carrasco P, Lieder B, Somoza V, et al. Only alpha-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy. 2019;74(10):1956–1968.
36. Michel S, Scherer K, Heijnen IA, Bircher AJ. Skin prick test and basophil reactivity to cetuximab in patients with IgE to alpha-gal and allergy to red meat. Allergy. 2014;69(3):403–405.
37. Steinke JW, Pochan SL, James HR, Platts-Mills TAE, Commins SP. Altered metabolic profile in patients with IgE to galactose-alpha-1,3-galactose following in vivo food challenge. J Allergy Clin Immunol. 2016;138(5):1465–1467.
38. Krstic Ristivojevic M, Grundstrom J, Apostolovic D, et al. Alpha-gal on the protein surface hampers transcytosis through the caco-2 monolayer. Int J Mol Sci. 2020;21(16):1.
39. Line J, Thomson P, Naisbitt DJ. Pathology of T-cell-mediated drug hypersensitivity reactions and impact of tolerance mechanisms on patient susceptibility. Curr Opin Allergy Clin Immunol. 2022;22(4):226–233.
40. Dua S, Dowey J, Foley L, et al. Diagnostic value of tryptase in food allergic reactions: a prospective study of 160 adult peanut challenges. J Allergy Clin Immunol Pract. 2018;6(5):1692–1698.
41. Diaz JH. Red meat allergies after lone star tick (Amblyomma americanum) bites. South Med J. 2020;113(6):267–274.
42. Iglesia EGA, Stone CA
43. Murangi T, Prakash P, Moreira BP, et al. Ascaris lumbricoides and ticks associated with sensitization to galactose alpha1,3-galactose and elicitation of the alpha-gal syndrome. J Allergy Clin Immunol. 2022;149(2):698–707.
44. Mabelane T, Ogunbanjo GA. Ingestion of mammalian meat and alpha-gal allergy: clinical relevance in primary care. Afr J Prim Health Care Fam Med. 2019;11(1):e1–e5.
45. Fischer J, Lupberger E, Hebsaker J, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy. 2017;72(10):1540–1547.
46. Fischer J, Huynh HN, Hebsaker J, Forchhammer S, Yazdi AS. Prevalence and impact of type I sensitization to alpha-gal in patients consulting an allergy unit. Int Arch Allergy Immunol. 2020;181(2):119–127.
47. Gonzalez-Quintela A, Dam Laursen AS, Vidal C, Skaaby T, Gude F, Linneberg A. IgE antibodies to alpha-gal in the general adult population: relationship with tick bites, atopy, and cat ownership. Clin Exp Allergy. 2014;44(8):1061–1068.
48. Westman M, Asarnoj A, Ballardini N, et al. Alpha-gal sensitization among young adults is associated with male sex and polysensitization. J Allergy Clin Immunol Pract. 2022;10(1):333–335.
49. Villalta D, Pantarotto L, Da Re M, et al. High prevalence of sIgE to Galactose-alpha-1,3-galactose in rural pre-Alps area: a cross-sectional study. Clin Exp Allergy. 2016;46(2):377–380.
50. Mittermann I, Dzoro S, Gattinger P, et al. Molecular IgE sensitization profiles of urban and rural children in South Africa. Pediatr Allergy Immunol. 2021;32(2):234–241.
51. Arkestal K, Sibanda E, Thors C, et al. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-alpha 1,3-galactose. J Allergy Clin Immunol. 2011;127(4):1024–1028.
52. O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–3648.
53. Burk CM, Beitia R, Lund PK, Dellon ES. High rate of galactose-alpha-1,3-galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis Esophagus. 2016;29(6):558–562.
54. Binder AM, Commins SP, Altrich ML, et al. Diagnostic testing for galactose-alpha-1,3-galactose, United States, 2010 to 2018. Ann Allergy Asthma Immunol. 2021;126(4):411–416.
55. Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018;121(5):594–597.
56. Mateo Borrega MB, Garcia B, Larramendi CH, et al. IgE-mediated sensitization to galactose-alpha-1,3- galactose (alpha-gal) in urticaria and anaphylaxis in Spain: geographical variations and risk factors. J Investig Allergol Clin Immunol. 2019;29(6):436–443.
57. Jacquenet S, Moneret-Vautrin DA, Bihain BE. Mammalian meat-induced anaphylaxis: clinical relevance of anti-galactose-alpha-1,3-galactose IgE confirmed by means of skin tests to cetuximab. J Allergy Clin Immunol. 2009;124(3):603–605.
58. Berg EA, Platts-Mills TA, Commins SP. Drug allergens and food – the cetuximab and galactose-alpha-1,3-galactose story. Ann Allergy Asthma Immunol. 2014;112(2):97–101.
59. Martinez Arcediano A, Audicana Berasategui MT, Longo Areso N, et al. Allergy to galactose-alpha-1,3-galactose: clinical features and the diagnostic value of cetuximab. J Investig Allergol Clin Immunol. 2014;24(6):450–452.
60. Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2012;129(5):1334–1342.
61. Uyttebroek A, Sabato V, Bridts CH, De Clerck LS, Ebo DG. Anaphylaxis to succinylated gelatin in a patient with a meat allergy: galactose-alpha(1, 3)-galactose (alpha-gal) as antigenic determinant. J Clin Anesth. 2014;26(7):574–576.
62. Serrier J, Khoy K, Ollivier Y, et al. Recurrent anaphylaxis to a gelatin-based colloid plasma substitute and to cetuximab following sensitisation to galactose-alpha-1,3-galactose. Br J Anaesth. 2021;126(6):e200–e202.
63. Hamsten C, Tran TAT, Starkhammar M, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. J Allergy Clin Immunol. 2013;132(6):1431–1434.
64. Brestoff JR, Tesfazghi MT, Zaydman MA, et al. The B antigen protects against the development of red meat allergy. J Allergy Clin Immunol Pract. 2018;6(5):1790–1791.
65. Carter MC, Ruiz-Esteves KN, Workman L, Lieberman P, Platts-Mills TAE, Metcalfe DD. Identification of alpha-gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy. 2018;73(5):1131–1134.
66. Bellamy P, Sanderson WT, Winter K, Stringer JW, Kussainov N, Commins SP. Prevalence of alpha-gal sensitization among Kentucky timber harvesters and forestry and wildlife practitioners. J Allergy Clin Immunol Pract. 2021;9(5):2113–2116.
67. Weiss J, Grilley Olson J, Deal AM, et al. Using the galactose-alpha-1,3-galactose enzyme-linked immunosorbent assay to predict anaphylaxis in response to cetuximab. Cancer. 2016;122(11):1697–1701.
68. Wilson JM, Schuyler AJ, Workman L, et al. Investigation into the alpha-gal syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348–2358.
69. Kutlu A, Unal D. Mammalian meat allergy accompanied by venom allergy: a review of 12 cases. Iran J Allergy Asthma Immunol. 2019;18(5):584–588.
70. Kiewiet MBG, Apostolovic D, Starkhammar M, Grundstrom J, Hamsten C, van Hage M. Clinical and serological characterization of the alpha-gal syndrome-importance of atopy for symptom severity in a European cohort. J Allergy Clin Immunol Pract. 2020;8(6):2027–2034.
71. Platts-Mills TAE, Commins SP, Biedermann T, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: a report from the National Institute of Allergy and Infectious Diseases workshop on understanding ige-mediated mammalian meat allergy. J Allergy Clin Immunol. 2020;145(4):1061–1071.
72. Bircher AJ, Hofmeier KS, Link S, Heijnen I. Food allergy to the carbohydrate galactose-alpha-1,3-galactose (alpha-gal): four case reports and a review. Eur J Dermatol. 2017;27(1):3–9.
73. Sim DW, Lee JS, Park KH, et al. Accurate assessment of alpha-gal syndrome using cetuximab and bovine thyroglobulin-specific IgE. Mol Nutr Food Res. 2017;61(10):1601046.
74. Jappe U, Minge S, Kreft B, et al. Meat allergy associated with galactosyl-alpha-(1,3)-galactose (alpha-Gal)-closing diagnostic gaps by anti-alpha-gal IgE immune profiling. Allergy. 2018;73(1):93–105.
75. Platts-Mills TAE, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the alpha-gal syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23 e11.
76. Li J, Fulton RB, O’Connell R, Jang HS, Fernando SL. Specific-IgE to galactose-alpha-1,3-galactose (alpha-gal) has limited utility in diagnosing meat allergy in a tick-endemic population. Ann Allergy Asthma Immunol. 2018;121(4):509–511.
77. Levin M, Apostolovic D, Biedermann T, et al. Galactose alpha-1,3-galactose phenotypes: lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598–602.
78. Gulen T, Akin C. Idiopathic anaphylaxis: a perplexing diagnostic challenge for allergists. Curr Allergy Asthma Rep. 2021;21(2):11.
79. Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29(8):841–849.
80. Fischer J, Yazdi AS, Biedermann T. Clinical spectrum of alpha-gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo J Int. 2016;25:55–62.
81. Morisset M, Richard C, Astier C, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67(5):699–704.
82. Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J Allergy Clin Immunol. 2014;134(3):755–759.
83. Croglio MP, Commins SP, McGill SK. Isolated gastrointestinal alpha-gal meat allergy is a cause for gastrointestinal distress without anaphylaxis. Gastroenterology. 2021;160(6):2178–2180.
84. Raji K, Baucom M. Alpha-gal (mammalian meat) allergy: implications for dermatologic surgeons. Dermatol Surg. 2022;48(4):466–468.
85. Borsani B, De Santis R, Perico V, et al. The role of carrageenan in inflammatory bowel diseases and allergic reactions: where do we stand? Nutrients. 2021;13(10):3402.
86. Tarlo SM, Dolovich J, Listgarten C. Anaphylaxis to carrageenan: a pseudo-latex allergy. J Allergy Clin Immunol. 1995;95(5 Pt 1):933–936.
87. Bernal M, Huecker M, Shreffler J, Mittel O, Mittel J, Soliman N. Successful treatment for alpha gal mammal product allergy using auricular acupuncture: a case series. Med Acupunct. 2021;33(5):343–348.
88. Unal D, Coskun R, Demir S, Gelincik A, Colakoglu B, Buyukozturk S. Successful beef desensitization in 2 adult patients with a delayed-type reaction to red meat. J Allergy Clin Immunol Pract. 2017;5(2):502–503.
89. Yucel E, Sipahi Cimen S, Varol S, Suleyman A, Ozdemir C, Tamay ZU. Red meat desensitization in a child with delayed anaphylaxis due to alpha-Gal allergy. Pediatr Allergy Immunol. 2019;30(7):771–773.
90. Hawkins RB, Wilson JM, Mehaffey JH, Platts-Mills TAE, Ailawadi G. Safety of intravenous heparin for cardiac surgery in patients with alpha-gal syndrome. Ann Thorac Surg. 2021;111(6):1991–1997.
91. Kuravi KV, Sorrells LT, Nellis JR, et al. Allergic response to medical products in patients with alpha-gal syndrome. J Thorac Cardiovasc Surg. 2021. doi:10.1016/j.jtcvs.2021.03.100
92. Qiao WH, Liu P, Hu D, Al Shirbini M, Zhou XM, Dong NG. Sequential hydrophile and lipophile solubilization as an efficient method for decellularization of porcine aortic valve leaflets: structure, mechanical property and biocompatibility study. J Tissue Eng Regen Med. 2018;12(2):e828–e840.
93. Liu X, Li N, Gong D, Xia C, Xu Z. Comparison of detergent-based decellularization protocols for the removal of antigenic cellular components in porcine aortic valve. Xenotransplantation. 2018;25(2):e12380.
94. Kim YJ, Ahn KS, Kim M, et al. Alpha-1,3-galactosyltransferase-deficient miniature pigs produced by serial cloning using neonatal skin fibroblasts with loss of heterozygosity. Asian-Australas J Anim Sci. 2017;30(3):439–445.
95. McGregor CG, Carpentier A, Lila N, Logan JS, Byrne GW. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2011;141(1):269–275.
96. McGregor C, Byrne G, Rahmani B, Chisari E, Kyriakopoulou K, Burriesci G. Physical equivalency of wild type and galactose alpha 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves. Acta Biomater. 2016;41:204–209.
97. Maldonado-Ruiz LP, Boorgula GD, Kim D, Fleming SD, Park Y. Tick intrastadial feeding and its role on IgE production in the murine model of alpha-gal syndrome: the tick “transmission” hypothesis. Front Immunol. 2022;13:844262.
98. Wilson JM, Nguyen AT, Schuyler AJ, et al. IgE to the mammalian oligosaccharide galactose-alpha-1,3-galactose is associated with increased atheroma volume and plaques with unstable characteristics-brief report. Arterioscler Thromb Vasc Biol. 2018;38(7):1665–1669.
99. Wilson JM, McNamara CA, Platts-Mills TAE. IgE, alpha-gal and atherosclerosis. Aging. 2019;11(7):1900–1902.
100. Richards NE, Richards RD
101. Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117.
102. Waldron JL, Schworer SA, Kwan M. Hypersensitivity and immune-related adverse events in biologic therapy. Clin Rev Allergy Immunol. 2021;62(3):413–431.
103. Chitnavis M, Stein DJ, Commins S, Schuyler AJ, Behm B. First-dose anaphylaxis to infliximab: a case of mammalian meat allergy. J Allergy Clin Immunol Pract. 2017;5(5):1425–1426.
104. Yuile A, Fanuli C, van Nunen S, et al. Increased rates of cetuximab reactions in tick prevalent regions and a proposed protocol for risk mitigation. Asia Pac J Clin Oncol. 2020;17(6):448–453.
105. Wen S, Unuma K, Chinuki Y, Hikino H, Uemura K. Fatal anaphylaxis due to alpha-gal syndrome after initial cetuximab administration: the first forensic case report. Leg Med. 2021;51:101878.
106. Hane A, Ito A, Ishikura K, Imai H, Okugawa Y. Cardiac arrest induced by an anaphylactic reaction associated with the first dose of cetuximab. Cureus. 2022;14(6):e26351.
107. Ohshita N, Ichimaru Y, Gamoh S, et al. Management of infusion reactions associated with cetuximab treatment: a case report. Mol Clin Oncol. 2017;6(6):853–855.
108. Chinuki Y, Morita E. Alpha-gal-containing biologics and anaphylaxis. Allergol Int. 2019;68(3):296–300.
109. Iwamoto T, Okamoto A, Ishinaga H, et al. A novel approach to predict cetuximab-induced hypersensitivity reaction: detection of drug-specific IgE on basophils. Cancer Med. 2016;5(6):1004–1012.
110. Yi CH, Ruan CP, Wang H, et al. Function characterization of a glyco-engineered anti-EGFR monoclonal antibody cetuximab in vitro. Acta Pharmacol Sin. 2014;35(11):1439–1446.
111. Dunkman WJ, Rycek W, Manning MW. What does a red meat allergy have to do with anesthesia? Perioperative management of alpha-gal syndrome. Anesth Analg. 2019;129(5):1242–1248.
112. Spencer HT, Hsu JT, McDonald DR, Karlin LI. Intraoperative anaphylaxis to gelatin in topical hemostatic agents during anterior spinal fusion: a case report. Spine J. 2012;12(8):e1–6.
113. Lied GA, Lund KB, Storaas T. Intraoperative anaphylaxis to gelatin-based hemostatic agents: a case report. J Asthma Allergy. 2019;12:163–167.
114. Holbert SE, Patel D, Rizk T, Dimitri NG, Jones M. Intraoperative anaphylaxis in response to hemostatic agents with protein derivatives. Cureus. 2020;12(8):e9881.
115. Stone CA
116. Retterer MKC, Workman LJ, Bacon JR, Platts-Mills TAE. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. J Allergy Clin Immunol. 2018;141(5):1956–1957.
117. Stone CA
118. Pinson ML, Waibel KH. Safe administration of a gelatin-containing vaccine in an adult with galactose-alpha-1,3-galactose allergy. Vaccine. 2015;33(10):1231–1232.
119. Schmidle P, Mehlich J, Brockow K, Darsow U, Biedermann T, Eberlein B. Gelatin-containing vaccines for varicella, zoster, measles, mumps, and rubella induce basophil activation in patients with alpha-gal syndrome. Int Arch Allergy Immunol. 2021;182:1–7.
120. la Fuente J, Gortazar C, Cabezas-Cruz A. Immunity to glycan alpha-gal and possibilities for the control of COVID-19. Immunotherapy. 2021;13(3):185–188.
121. Keshavarz B, Wiencek JR, Workman LJ, et al. Quantitative measurement of IgG to severe acute respiratory syndrome coronavirus-2 proteins using immuno CAP. Int Arch Allergy Immunol. 2021;182(5):417–424.
122. Galili U. Amplifying immunogenicity of prospective Covid-19 vaccines by glycoengineering the coronavirus glycan-shield to present alpha-gal epitopes. Vaccine. 2020;38(42):6487–6499.
123. Chen JM. SARS-CoV-2 replicating in nonprimate mammalian cells probably have critical advantages for COVID-19 vaccines due to anti-gal antibodies: a mini review and proposals. J Med Virol. 2021;93(1):351–356.
124. Abdel-Motal UM, Guay HM, Wigglesworth K, Welsh RM, Galili U. Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells. J Virol. 2007;81(17):9131–9141.
125. Spiro RG, Bhoyroo VD. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984;259(15):9858–9866.
126. Muglia C, Kar I, Gong M, Hermes-DeSantis ER, Monteleone C. Anaphylaxis to medications containing meat byproducts in an alpha-gal sensitized individual. J Allergy Clin Immunol Pract. 2015;3(5):796–797.
127. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6(3):e101–e104.
128. Nanno Y, Shajahan A, Sonon RN, Azadi P, Hering BJ, Burlak C. High-mannose type N-glycans with core fucosylation and complex-type N-glycans with terminal neuraminic acid residues are unique to porcine islets. PLoS One. 2020;15(11):e0241249.
129. de Silva HA, Ryan NM, de Silva HJ. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol. 2016;81(3):446–452.
130. Blessmann J, Hanlodsomphou S, Santisouk B, et al. Serum IgE against galactose-alpha-1,3-galactose is common in Laotian patients with snakebite envenoming but not the major trigger for early anaphylactic reactions to antivenom. Toxicon X. 2020;7:100054.
131. Rizer J, Brill K, Charlton N, King J. Acute hypersensitivity reaction to Crotalidae polyvalent immune Fab (CroFab) as initial presentation of galactose-alpha-1,3-galactose (alpha-gal) allergy. Clin Toxicol. 2017;55(7):668–669.
132. Straesser M, Keshavarz B, Borish L, et al. Alpha-gal on Crotalidae-polyvalent Fab antivenom (CroFab): investigating the relevance to immediate hypersensitivity reactions. J Allergy Clin Immunol Pract. 2021;9(2):1015–1017.
133. Fischer J, Eberlein B, Hilger C, et al. Alpha-gal is a possible target of IgE-mediated reactivity to antivenom. Allergy. 2017;72(5):764–771.
134. Nunez-Orjales R, Martin-Lazaro J, Lopez-Freire S, Galan-Nieto A, Lombardero-Vega M, Carballada-Gonzalez F. Bovine amniotic fluid: a new and occupational source of galactose-alpha-1,3-galactose. J Investig Allergol Clin Immunol. 2017;27(5):313–314.
135. Flaherty MG, Kaplan SJ, Jerath MR. Diagnosis of life-threatening alpha-gal food allergy appears to be patient driven. J Prim Care Community Health. 2017;8(4):345–348.
136. Flaherty MG, Threats M, Kaplan SJ. Patients’ health information practices and perceptions of provider knowledge in the case of the newly discovered alpha-gal food allergy. J Patient Exp. 2020;7(1):132–139.
137. Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep. 2016;16(5):38.
138. Integrated taxonomic information system [database on the Internet]. Available from: https://www.itis.gov/.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.