Back to Journals » International Journal of General Medicine » Volume 15
The Mean Platelet Volume Combined with Procalcitonin as an Early Accessible Marker Helps to Predict the Severity of Necrotizing Enterocolitis in Preterm Infants
Authors Cai N, Liao W, Chen Z, Tao M, Chen S
Received 28 October 2021
Accepted for publication 10 January 2022
Published 8 April 2022 Volume 2022:15 Pages 3789—3795
DOI https://doi.org/10.2147/IJGM.S346665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Na Cai, Wei Liao, Zhiqiang Chen, Min Tao, Sheng Chen
Department of Pediatrics, The First Hospital Affiliated to Army Medical University, Chongqing, 400038, People’s Republic of China
Correspondence: Min Tao; Sheng Chen, Department of Pediatrics, The First Hospital Affiliated to Army Medical University, No. 30, Gaotanyan Street, Chongqing, 400038, People’s Republic of China, Tel +86-23-68766215 ; +86-23-68766213, Email [email protected]; [email protected]
Purpose: This study aims to evaluate the value of the mean platelet volume (MPV) combined with procalcitonin (PCT) in predicting the severity of necrotizing enterocolitis (NEC) in preterm infants.
Methods: This was a retrospective cohort study conducted in a neonatal intensive care unit from January 2014 to July 2020. Premature neonates with NEC were enrolled. In this study, mild-moderate NEC was defined as Bell’s stage I and II, and severe NEC was defined as Bell’s stage III. The demographic data, blood cell count analysis, C-reactive protein and PCT were compared between the severe and mild-moderate groups.
Results: A total of 18 premature infants with NEC in the severe group and 57 infants in the mild-moderate group were enrolled. The MPV and PCT were all significantly higher in the severe group than in the mild-moderate group (P < 0.01), and white blood cells were lower in the severe group (P < 0.05). The results of logistic regression suggested that the MPV (OR = 6.194, P = 0.000) and PCT (OR = 1.093, P = 0.006) were independent predictive factors for the severity of NEC. A receiver operating characteristic analysis showed that the areas under the curve (AUCs) were 0.829 for MPV alone, 0.706 for PCT alone, and 0.895 for MPV combined with PCT.
Conclusion: The combination of MPV with PCT had the highest overall AUC of the investigated parameters, and their combination can be considered an early marker for predicting the severity of NEC in preterm infants.
Keywords: premature infants, necrotizing enterocolitis, mean platelet volume, procalcitonin
Introduction
Necrotizing enterocolitis (NEC) is considered to be the most common gastrointestinal emergency among neonates and mostly occurs in premature and low birth weight neonates. The morbidity of NEC is 2% to 5% in the neonatal intensive care unit (NICU),1,2 and it is one of the main causes of neonatal death.3–7 The mortality rate in extremely low birth weight (<1000 grams) infants is 30–50%, and for infants with a very low birth weight (VLBW) (<1500 grams), it is 10–30%.8 In cases of perforation or severe clinical deterioration on Medical treatment, resection of the affected bowel is often the treatment of choice.3 This group carries the highest mortality.9 Even surviving neonates often have short-term and long-term complications, such as intestinal stenosis, short bowel syndrome and neurological sequelae.2,10–12 These complications seriously affect quality of life. It is therefore essential to detect these severe cases early enough to reduce mortality and improve quality of life and outcomes.
However, currently, the factors associated with the severity of NEC are not well defined, and research has shown that serum amyloid A (SAA), intestinal fatty acid binding protein (I-FABP), E-selectin, C5a, interleukin-6, fecal calprotectin (FC) and mitochondrial deoxyribonucleic acid (mtDNA) may be useful markers for predicting the severity of NEC.13–21 However, these testing methods have not been popularized in hospitals, especially in developing countries, and it also takes a long time to obtain results, so they are not suitable for clinical work. Furthermore, venous puncture is a delicate and unfavorable procedure in neonates and a primary cause of anemia among preterm infants. Therefore, it is highly desirable to discriminate neonates with severe NEC from those that can be treated conservatively by using methods that inflict minimal damage to neonates. To examine this, we identified premature infants with proven NEC in our hospital databases and sought statistical associations with the severity of NEC among commonly used clinical indicators, such as blood cell count analysis, C-reactive protein (CRP) and procalcitonin, which have the advantages of a short-duration assay and the use of a small sample volume.
Materials and Methods
Participants
Premature neonates with NEC diagnosed by a neonatal specialist at the First Hospital Affiliated with Army Medical University, China, between January 2014 and July 2020 according to the Bell staging criteria for necrotizing enterocolitis were enrolled.23 In this study, mild-moderate NEC was defined as Bell’s stage I and II, and severe NEC was defined as Bell’s stage III. None of the included cases used probiotics. The exclusion criteria were as follows: (1) gestational age ≥ 37 weeks; (2) neonates with amniotic fluid meconium contamination, asphyxia and spontaneous intestinal perforation; (3) age of onset < 3 days; (4) those with genetic metabolic diseases (neonates were screened for genetic metabolic diseases 3 days after birth) and congenital deformity; (5) the use of drugs that can cause changes in red blood cell morphology; and (6) cardiovascular diseases.
This study was approved by the Ethics Committee of the First Hospital Affiliated with Army Medical University. All procedures were carried out according to the Declaration of Helsinki.
Data Collection
The data for all the study patients were obtained from the electronic medical records, including comorbidities during pregnancy, sex, gestational age, birth weight, age of onset, blood cell count analysis, C-reactive protein (CRP) and procalcitonin (PCT). Blood samples were collected within the first few hours after the onset of clinical manifestations of NEC (such as bloating, bloody stools, and vomiting). Laboratory parameters such as the white blood cell (WBC) count, platelet count (PLT), mean platelet volume (MPV), red blood cell distribution width (RDW), hemoglobin (Hb), CRP and PCT were measured.
Statistical Analysis
Categorical variables are expressed as proportions and were compared using the chi-square test and Fisher’s exact test. Continuous variables with a non normal distribution are presented as the median and interquartile range (IQR) percentiles, and variables with a normal distribution are presented as the mean value ± standard deviation. The differences in continuous variables were assessed for significance using t-tests or Mann–Whitney U-tests. Logistic regression analysis was applied to assess the independent risk factors for the severity of NEC. The receiver operating characteristic (ROC) method was conducted to evaluate the utility of different variables in predicting the severity of NEC. The areas under the ROC curve (AUCs) and optimal cut‐off points based on maximizing the sum of the sensitivity and specificity were calculated for each of the variables. The statistical analyses were performed with SPSS software (20.0). P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 75 patients were included in the study. We analyzed the baseline data of infants with NEC, including their sex, gestational age, birth weight, age of onset, breastfeeding and perinatal situation. No significant differences were observed regarding these data between the severe group and the mild-moderate group (P > 0.05, Table 1).
 |
Table 1 The Baseline Clinical Characteristics of the Severe, Mild-Moderate Groups |
Significant Differences in the MPV and PCT
Significant differences were observed in the WBC, MPV and PCT between the two groups (P < 0.05, Table 2). The MPV and PCT were significantly higher in severe group than mild-moderate group, but the WBC count was lower in the former. No significant differences in PLT, RDW, Hb and CRP were observed between the two groups (P > 0.05, Table 2).
 |
Table 2 Comparison of Complete Blood Count Analysis, CRP and PCT Between the Two Groups |
MPV and PCT Were Independent Predictive Factors for the Severity of NEC
Logistic regression was used. The severity of NEC was taken as the dependent variable, and P <0.05 was used as the screening criterion. The above statistically significant indicators, including WBC, MPV and PCT, were used as independent variables to test whether each factor had a significant effect on the severity of NEC. The Hosmer–Lemeshow test showed that the regression model fit well (P = 0.962 > 0.05). The results suggested that the MPV (OR = 6.194, P = 0.000 <0.05) and PCT (OR = 1.093, P = 0.006 <0.05) were independent predictive factors for the severity of NEC in preterm infants (Table 3).
 |
Table 3 Logistic Regression Analysis Results |
 |
Table 4 The AUC of MPV Combined with PCT |
The High Predictive Value of the MPV Combined with PCT for the Severity of NEC in Preterm Infants
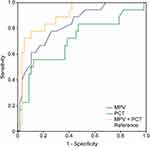
The receiver operating characteristic (ROC) analysis showed that the area under the curve (AUC) was 0.829 for MPV alone, 0.706 for PCT alone, and 0.895 for MPV combined with PCT, suggesting that MPV combined with PCT was better for predicting the severity of NEC in preterm infants (Table 4, Figure 1).
Discussion
NEC is often very aggressive, which means that determining the severity of NEC in time and administering appropriate treatment is of utmost importance to researchers, physicians, parents and infants. In addition to clinical signs and symptoms, various biomarkers, including serum amyloid A, intestinal fatty acid binding protein, E-selectin, C5a, IL-6, fecal calprotectin and mitochondrial deoxyribonucleic acid (mtDNA), have been described to correlate with the severity of NEC.13–22 However, due to limited resources, these testing methods have not been popularized in the vast majority of hospitals. To date, there is a lack of effective indicators available to clinicians to predict the severity of NEC, and hence, physicians cannot make timely judgments, which may lead to delayed treatment. Therefore, our research focuses on searching for effective indicators that can predict the severity of NEC among commonly used clinical indicators. Complete blood count analysis, CRP and PCT are test items that are widely carried out in hospitals at all levels that have the advantages of a small blood demand, economical convenience and quick results. Our study found that compared to the mild-moderate group, the MPV and PCT were higher in the severe group. There were significant differences between the two groups, suggesting that a high MPV and PCT might be risk factors for severe NEC. Further logistic regression analysis indicated that MPV and PCT were independent predictors for the severity of NEC. Therefore, we speculate that MPV and PCT can represent relevant predictive markers for the severity of NEC in premature infants. To date, no studies have evaluated the significance of MPV and PCT in jointly predicting the severity of NEC. As such, our current study is unique in that it mainly focused on a predictive approach for the severity of NEC, which has the potential to change the current recommendation. Our results support the use of these easily available parameters for the prediction of the severity of NEC.
The MPV describes the average size of platelets in a blood sample and is a simple, economical and useful diagnostic marker for children. Many studies have shown that elevated MPV during hospitalization is a predictor of mortality in critically ill adults.24,25 High MPV was suggested to be associated with overall survival in various diseases, such as cancer, cardiac disease, septic shock, and kidney injury.26–29 Go et al30 found that MPV ≥ 10.2 fl correlates with mortality among infants born at < 32 weeks of gestation. A possible explanation for the relationship between MPV and mortality is the inflammatory response.31,32 Currently, there are few studies on the relationship between MPV and NEC in neonates, especially preterm infants. Cekmez et al33 revealed that a high MPV in the first hours of life may reflect the presence of a risk factor for the development of NEC. Namachivayam et al34 found that moderate-severe intestinal injury was associated with increased MPV in a neonatal mouse NEC model. Our study found that MPV in the severe group was higher than that in the mild-moderate group, which was similar to the findings of Namachivayam. While precise pathophysiologic mechanisms remain elusive, a possible explanation is that in the case of premature delivery, infection and hypoxic ischemia, stimulate enhanced release of platelet activating factor, which can cause platelets to aggregate and form microthrombi, resulting in mesenteric artery ischemia and necrosis. Furthermore, a reduction in the number of platelets in the circulation promotes the feedback activation of megakaryocytes to produce a larger volume of platelets. A large volume of platelets contains more active substances, produces more thromboxane, releases dense particles and inflammatory factors, and is more likely to cause thrombosis. At the same time, it promotes the inflammatory reaction. The above process forms a vicious cycle, aggravating intestinal damage and ultimately triggering NEC.
PCT is a protein that is synthesized during sepsis and inflammation. In these states, its production is stimulated by bacterial toxins and inflammatory mediators, such as tumor necrosis factor-α (TNF-α), IL-6 and IL-1β. It reaches a physiological peak from 24 h to 30 h after birth, and from the third day, the normal reference value is the same as that of adults (< 0.1 μg/L). Therefore, our research subjects were neonates aged more than three days. Routine determination of the PCT concentration is useful in the rapid detection and monitoring of bacterial and fungal infections with high sensitivity and specificity. Compared to other proinflammatory proteins, such as CRP, IL-6, and TNF-α, PCT is characterized by a rapid response time, especially compared with the classic acute phase protein CRP. PCT rises rapidly within 2–6 h and reaches a peak at 12–48 h after infection. Therefore, PCT can be used as a diagnostic standard for early infection. Likewise, the concentration of PCT is closely related to the severity of infection.35–37 In severely infected patients, PCT increased significantly. Conversely, when the infection was mild, PCT increased slightly. In addition, some studies have reported that compared to other commonly used indicators, PCT has higher value in predicting the severity of infection and organ involvement.36,38 Neonates with NEC are often accompanied by varying degrees of infection, and severe cases may even develop sepsis. Therefore, can PCT be used to predict the severity and prognosis of NEC? Unfortunately, there are few studies on the relationship between PCT and NEC. Only a few studies have revealed that PCT can be used for the diagnosis of NEC,39,40 but no research has focused on the use of PCT for the early prediction of the severity and prognosis of NEC in preterm infants. Our study found that in the early onset of NEC, PCT in the severe group was significantly higher than that in the mild-moderate group, suggesting that elevated PCT might be a risk factor for stage III NEC in preterm infants. We look forward to more studies exploring the relationship between PCT and the severity of NEC.
By comparing the ROC curves of the MPV and PCT, the results showed that the AUCs were 0.829 for MPV alone, 0.706 for PCT alone, and 0.895 for MPV combined with PCT, which suggests that MPV (cutoff value: 10.8 fL) combined with PCT (cutoff value: 18.57 ng/mL) was better at predicting the severity of NEC in preterm infants.
Limitations
This study has some limitations. First, this study was retrospective, and that data were not available for all variables and were reliant on what was captured by the treating physician. Second, the incidence of NEC in preterm infants in our hospital was not as high as that reported in the literature, so the sample size of the severe group was small, definitively limiting the conclusions.
Conclusion
In conclusion, the combination of the MPV with PCT, which is easily accessible, fast, and affordable, can be considered an early marker for predicting the severity of NEC in preterm infants and represents a valuable tool to highlight the severity of NEC and thus to quickly start appropriate therapy.
Data Sharing Statement
The anonymized datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant/Award Number: 82170565).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moschopoulos C, Kratimenos P, Koutroulis I, et al. The neurodevelopmental perspective of surgical necrotizing enterocolitis: the role of the gut-brain axis. Mediators Inflamm. 2018;2018:7456857.
2. Neu J, Pammi M. Pathogenesis of NEC: impact of an altered intestinal microbiome. Semin Perinatol. 2017;41(1):29–35. doi:10.1053/j.semperi.2016.09.015
3. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–264. doi:10.1056/NEJMra1005408
4. Overman RE
5. Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40(1):27–51. doi:10.1016/j.clp.2012.12.012
6. Niño DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. 2016;13(10):590–600. doi:10.1038/nrgastro.2016.119
7. Yu L, Sun B, Miao P, Feng X. Risk factors for prognosis of neonatal necrotizing enterocolitis: an analysis of 82 cases. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15(12):1082–1085.
8. Alganabi M, Lee C, Bindi E, et al. Recent advances in understanding necrotizing enterocolitis. F1000Res. 2019;8:107. doi:10.12688/f1000research.17228.1
9. Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109(3):423–428. doi:10.1542/peds.109.3.423
10. Frost BL, Modi BP, Jaksic T, Caplan MS. New medical and surgical insights into neonatal necrotizing enterocolitis: a review. JAMA Pediatr. 2017;171(1):83–88. doi:10.1001/jamapediatrics.2016.2708
11. Wadhawan R, Oh W, Hintz SR, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol. 2014;34(1):64–70. doi:10.1038/jp.2013.128
12. Hau EM, Meyer SC, Berger S, et al. Gastrointestinal sequelae after surgery for necrotising enterocolitis: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F265–F273. doi:10.1136/archdischild-2017-314435
13. Cetinkaya M, Ozkan H, Koksal N, Akaci O, Ozgur T. The efficacy of serial serum amyloid A measurements for diagnosis and follow-up of necrotizing enterocolitis in premature infants. Pediatr Surg Int. 2010;26(8):835–841. doi:10.1007/s00383-010-2635-0
14. Edelson MB, Sonnino RE, Bagwell CE, et al. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. J Pediatr Surg. 1999;34(10):1453–1457. doi:10.1016/S0022-3468(99)90102-1
15. Aydemir C, Dilli D, Oguz SS, et al. Serum intestinal fatty acid binding protein level for early diagnosis and prediction of severity of necrotizing enterocolitis. Early Hum Dev. 2011;87(10):659–661. doi:10.1016/j.earlhumdev.2011.05.004
16. Khoo AK, Hall NJ, Alexander N, et al. Plasma soluble e-selectin in necrotising enterocolitis. Eur J Pediatr Surg. 2008;18(6):419–422. doi:10.1055/s-2008-1038908
17. Tayman C, Tonbul A, Kahveci H, et al. C5a, a complement activation product, is a useful marker in predicting the severity of necrotizing enterocolitis. Tohoku J Exp Med. 2011;224(2):143–150. doi:10.1620/tjem.224.143
18. Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Plasma interleukin-6 and tumour necrosis factor levels as predictors of disease severity and outcome in necrotizing enterocolitis. J Pediatr Surg. 1994;29(6):798–800. doi:10.1016/0022-3468(94)90374-3
19. Evennett NJ, Hall NJ, Pierro A, Eaton S. Urinary intestinal fatty acid-binding protein concentration predicts extent of disease in necrotizing enterocolitis. J Pediatr Surg. 2010;45(4):735–740. doi:10.1016/j.jpedsurg.2009.09.024
20. Reisinger KW, Kramer BW, Van der Zee DC, et al. Non-invasive Serum Amyloid A (SAA) measurement and plasma platelets for accurate prediction of surgical intervention in severe Necrotizing Enterocolitis (NEC). PLoS One. 2014;9(3):e90834. doi:10.1371/journal.pone.0090834
21. Aydemir G, Cekmez F, Tanju IA, et al. Increased fecal calprotec-tin in preterm infants with necrotizing enterocolitis. Clin Lab. 2012;58(7–8):841–844.
22. Bindi E, Li B, Zhou HT, et al. Mitochondrial DNA: a biomarker of disease severity in necrotizing enterocolitis. Eur J Pediatr Surg. 2020;30(1):85–89. doi:10.1055/s-0039-1697910
23. Zani A, Pierro A. Necrotizing enterocolitis: controversies and challenges. F1000Res. 2015;4:1373. doi:10.12688/f1000research.6888.1
24. Gorelik O, Tzur I, Barchel D, et al. A rise in mean platelet volume during hospitalization for community acquired pneumonia predicts poor prognosis: a retrospective observational cohort study. BMC Pulm Med. 2017;17(1):137. doi:10.1186/s12890-017-0483-6
25. Yildiz A, Yigit A, Benli AR. The prognostic role of platelet to lymphocyte ratio and mean platelet volume in critically ill patients. Eur Rev Med Pharmacol Sci. 2018;22:2246–2252. doi:10.26355/eurrev_201804_14811
26. Zhang Z, Xu X, Ni H, Deng H. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. J Crit Care. 2014;29:
27. Gu M, Zhai Z, Huang L, et al. Pre-treatment mean platelet volume associates with worse clinicopathologic features and prognosis of patients with invasive breast cancer. Breast Cancer. 2016;23:752–760. doi:10.1007/s12282-015-0635-6
28. Sun XP, Li BY, Li J, Zhu WW, Hua Q. Impact of mean platelet volume on longterm mortality in Chinese patients with ST-elevation myocardial infarction. Sci Rep. 2016;6:21350. doi:10.1038/srep21350
29. Kim CH, Kim SJ, Lee MJ, et al. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLoS One. 2015;10:e0119437. doi:10.1371/journal.pone.0119437
30. Go H, Ohto H, Nollet KE, et al. Using platelet parameters to anticipate morbidity and mortality among preterm neonates: a retrospective study. Front Pediatr. 2020;8:90. doi:10.3389/fped.2020.00090
31. Ye S, Zhang Y, Zhang C, Xu D. Are platelet volume indices related to mortality in hospitalized children on mechanical ventilation? J Int Med Res. 2018;46:1197–1208. doi:10.1177/0300060517737211
32. Mohsen L, Akmal DM, Ghonaim EKE, Riad NM. Role of mean platelet volume and ischemia modified albumin in evaluation of oxidative stress and its association with postnatal complications in infants of diabetic mothers. J Matern Fetal Neonatal Med. 2018;31:1819–1823. doi:10.1080/14767058.2017.1330329
33. Cekmez F, Tanju IA, Canpolat FE, et al. Mean platelet volume in very preterm infants: a predictor of morbidities? Eur Rev Med Pharmacol Sci. 2013;17(1):134–137.
34. Namachivayam K, MohanKumar K, Garg L, Torres BA, Maheshwari A. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr Res. 2017;81(5):817–824. doi:10.1038/pr.2017.7
35. Wang J, Wang H, Liu W, Zhang D, Guo S. Assessment values of procalcitonin, lactic acid, and disease severity scores in patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(8):938–941. doi:10.3760/cma.j.issn.2095-4352.2019.08.005
36. Zhang J, Qu D, Ren XX, Cui XD, Fu J. Value of procalcitonin in predicting the severity and prognosis of neonates with septicemia. Zhonghua Yi Xue Za Zhi. 2018;98(16):1267–1272. doi:10.3760/cma.j.issn.0376-2491.2018.16.016
37. Cui N, Zhang H, Chen Z, Yu Z. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Med Res. 2019;47(4):1573–1579. doi:10.1177/0300060518822404
38. Jia YF, Wang Y, Yu XH. Relationship between blood lactic acid, blood procalcitonin, C-reactive protein and neonatal sepsis and corresponding prognostic significance in sick children. Exp Ther Med. 2017;14(3):2189–2193. doi:10.3892/etm.2017.4713
39. Elfarargy MS, El Farargy MS, Atef MM, et al. Early biomarkers in neonatal necrotizing enterocolitis: a pilot study. J Popul Ther Clin Pharmacol. 2019;26(3):e1–e8. doi:10.15586/jptcp.v26i3.602
40. Cetinkaya M, Ozkan H, Köksal N, Akaci O, Ozgür T. Comparison of the efficacy of serum amyloid A, C-reactive protein, and procalcitonin in the diagnosis and follow-up of necrotizing enterocolitis in premature infants. J Pediatr Surg. 2011;46(8):1482–1489. doi:10.1016/j.jpedsurg.2011.03.069
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.