Back to Journals » OncoTargets and Therapy » Volume 7
The long-term survival of a thymic carcinoma patient treated with S-1: a case report and literature review
Authors Tanaka H, Morimoto T, Taima K, Tanaka Y, Nakamura K, Hayashi A, Kurose A, Okumura K, Takanashi S
Received 23 September 2013
Accepted for publication 21 November 2013
Published 27 December 2013 Volume 2014:7 Pages 87—90
DOI https://doi.org/10.2147/OTT.S54843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Hisashi Tanaka,1 Takeshi Morimoto,1 Kageaki Taima,1 Yoshihito Tanaka,1 Kunihiko Nakamura,1 Akihito Hayashi,3 Akira Kurose,2 Ken Okumura,1 Shingo Takanashi4
1Department of Cardiology, Respiratory Medicine and Nephrology, Hirosaki University Graduate School of Medicine, Zaifu-cho, Hirosaki, Japan; 2Department of Anatomic Pathology, Hirosaki University Graduate School of Medicine, Zaifu-cho, Hirosaki, Japan; 3Respiratory Medicine, Hachinohe City Hospital, Tamukaibishamontai, Hachinohe, Japan; 4Hirosaki University, Health Administration Center, Bunkyo, Hirosaki, Japan
Background: Thymic carcinoma is a rare neoplasm of the thymus. Systemic chemotherapy is an important therapeutic modality for thymic carcinoma. However, no standard chemotherapy for this carcinoma has yet been established. The usefulness of second-line or later-line chemotherapy has remained unclear. A case of relapsed thymic carcinoma that was successfully treated by S-1 as second-line chemotherapy is reported herein.
Case presentation: A 73-year-old man diagnosed as having thymic carcinoma was treated with three cycles of first-line chemotherapy with ADOC (cisplatin, doxorubicin, vincristine, and cyclophosphamide) and additional radiotherapy (50 Gy). Since his serum cytokeratin 19 fragment level increased suddenly after 3 months of stable disease, he was considered to have progressive disease, and was given S-1 as chemotherapy. Two months later, he had partial response, and the S-1 treatment has been continued since July 2009. Progression-free survival of greater than 4 years was obtained with S-1.
Conclusion: A case of relapsed thymic carcinoma that was treated with S-1, and continues to show a long progression-free survival with good quality of life on treatment is described. S-1 might be an active agent against relapsed thymic carcinoma.
Keywords: thymic carcinoma, S-1, thymidylate synthase
Introduction
Thymic carcinoma is a rare cancer of the thymus arising from the thymic epithelium, which differs from thymoma with respect to its malignant nature. It is characterized histologically by clear-cut cytological atypia and a set of cytoarchitectural features similar to those of carcinoma arising from any other organ. It has been a distinct entity in the World Health Organization (WHO) classification since 2004.1 The prognosis of patients with thymic carcinoma is poor: 5-year survival rates are reported to be about 30%.2 Since approximately 50% of patients with thymic carcinoma have Stage IVa or IVb disease according to the Masaoka–Koga Staging system at the initial presentation, systemic chemotherapy is an important therapeutic modality for thymic carcinoma. However, even an optimal first-line chemotherapy regimen for thymic carcinoma has not been established, because of the rarity of the disease and the difficulty of conducting prospective clinical trials.
S-1 (Taiho Pharmaceutical Co, Ltd, Tokyo, Japan) is an orally active combination of tegafur (a prodrug of 5-fluorouracil [5-FU]), gimeracil (an inhibitor of dihydropyrimidine dehydrogenase, which degrades fluorouracil), and oteracil (an inhibitor of the phosphorylation of fluorouracil in the gastrointestinal tract resulting in a reduction of the gastrointestinal toxic effects of fluorouracil) in a molar ratio of 1:0.4:1. S-1 is now used in the treatment of various solid tumors such as gastric cancer, colon cancer, non-small-cell lung cancer, head and neck cancer, pancreatic cancer, and esophageal cancer in Japan. In relapsed thymic carcinoma, there are few case reports indicating the novel efficacy of S-1 therapy.3–7 The present case showed a novel effect of S-1 as second-line therapy.
Case presentation
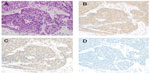
A 73-year-old Japanese man was referred to our hospital in October 2008 because of an abnormal shadow in the mediastinum on chest X-ray. His medical history was unremarkable, and he had taken no medications. He had smoked 50 pack-years. Physical examination revealed no significant abnormalities except for his clubbed fingers. Laboratory findings were within normal ranges, except the serum cytokeratin 19 fragment (CYFRA) level of 16.6 ng/mL (normal range, 0–3.5 ng/mL). A subsequent computed tomography (CT) scan revealed a mass lesion in the anterior mediastinum involving the superior vena cava, with multiple pleural dissemination. Whole body 18F-fluorodeoxy glucose positron emission tomography (FDG-PET) showed strong activity in the anterior mediastinal lesions (7.1 maximum standardized uptake values) and the multiple pleural ones. A CT-guided needle biopsy of the main lesion in the anterior mediastinum was performed. The pathological diagnosis was squamous cell thymic carcinoma. Immunohistochemically, the tumor cells showed positive staining for CD117 (c-kit) and CD5, and negative staining for terminal deoxynucleotidyl transferase (TdT) in the lymphocytes in the tumor. The Ki-67 (MIB-1) index was more than 30%. The results of immunohistochemical analysis supported a diagnosis of thymic carcinoma. Immunohistochemical staining for the enzymes related to 5-FU metabolism was also performed: thymidylate synthase (TS) was strongly positive, dihydropyrimidine dehydrogenase (DPD) was weakly positive, and orotate phosphoribosyltransferase (OPRT) was not stained (Figure 1).
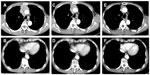
Since he had advanced stage disease (Masaoka–Koga staging system, IVa), he was treated with three cycles of firstline chemotherapy, including cisplatin (50 mg/m2) on day 1, doxorubicin (40 mg/m2) on day 1, vincristine (0.6 mg/m2) on day 3, and cyclophosphamide (700 mg/m2) on day 4, every 4 weeks from December 2008 to February 2009. After three cycles of chemotherapy, stable disease (SD) was observed. Additional radiotherapy (50 Gy) was given as multimodality treatment for the main anterior mediastinal lesion from March to April 2009. He had SD after the radiotherapy, and the serum CYFRA level was 14.0 ng/mL. In July 2009, the serum CYFRA level increased suddenly (34.7 ng/mL). Therefore, second-line chemotherapy with S-1 (50 mg/m2) twice a day was given. The administration of S-1 was conducted as follows: 2 weeks of administration and 1 week of withdrawal of S-1. The S-1 therapy was effective, since the main tumor and the pleural dissemination showed remarkable reduction in their sizes, and the serum CYFRA level became 1.7 ng/mL after two cycles of chemotherapy in September 2009 (Figures 2 and 3). The hematologic adverse events (AEs) were as follows: leukopenia (grade 3), neutropenia (grade 2), anemia (grade 2), and thrombocytopenia (grade 1), with no febrile neutropenia. There were no non-hematologic grade 3 or 4 AEs. The patient has been continued on S-1 without disease progression or impairment of his quality of life (QOL) for more than 4 years.
Discussion
The optimal chemotherapy for advanced thymic carcinoma is still controversial. In the National Comprehensive Cancer Network (NCCN) guidelines for thymoma and thymic carcinoma, carboplatin and paclitaxel are recommended. The ADOC regimen (cisplatin, doxorubicin, vincristine, and cyclophosphamide) is also effective, but it is more toxic than carboplatin and paclitaxel.8 There have been reports showing that anthracycline-based chemotherapy or non-anthracycline-based chemotherapy produces an objective response rate of 24%–55% and progression-free survival (PFS) of 5.0–7.9 months.9–12 Grade 3/4 hematological toxicities occurred in more than 76% of cases treated with anthracycline-based chemotherapy.9,10
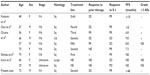
In the present case, S-1 showed a remarkable effect against the relapsed thymic carcinoma. To the best of our knowledge, there have been no cases with long progression-free survival for more than 4 years with relapsed thymic carcinoma treated by S-1. No serious toxicities have been observed in the present case. There have been a few case reports that evaluated the efficacy of S-1 for advanced thymic carcinoma.3–7 Ten patients, including the present case have been reported (Table 1); three of them, who had no response to prior platinum chemotherapy, achieved partial response (PR) with S-1. Okuma et al reported that the response to first-line chemotherapy may be a potential surrogate for survival in advanced thymic carcinoma.13 However, in the present case, the patient had long PFS, and therefore S-1 might be active for relapsed thymic carcinoma or non-responders to prior platinum chemotherapy.
PFS of approximately 6.8–18.0 months has been reported in thymic carcinoma.3–7 In the present case, the patient showed PFS of more than 4 years. The reason for this is unclear, but there may be possibilities of anticancer activity of 5-FU and its metabolites. Kaira et al reported that expressions of TS, OPRT, and DPD tend to increase as the grade of thymic epithelial tumors increases from low to high. They also showed that the biomarkers are closely associated with cell cycle control and angiogenesis, and that positive expressions of TS and DPD are useful for predicting poor outcome. In patients with thymic carcinoma treated by S-1, one patient of three who achieved PR had a remarkable expression of TS. On the other hand, no consistent relationship was seen between DPD or OPRT expression and tumor response to S-1.7 In the present case, TS was markedly expressed in the tumor cells. That might be the reason why the S-1 treatment was effective for thymic carcinoma. TS might be a good biomarker to predict response. However, TS, DPD, and OPRT have not yet been fully evaluated in thymic carcinoma. Further studies are needed to confirm the optimal chemotherapy for thymic carcinoma and to find a useful biomarker to predict the tumor response. At present, a prospective Phase II study (UMIN000010736) that evaluates the efficacy of S-1 against relapsed thymic carcinoma is ongoing in Japan.
A relapsed thymic carcinoma patient treated with S-1, who had a long PFS with good QOL was described. In conclusion, S-1 treatment might be effective for relapsed thymic carcinoma.
Disclosure
The authors report no conflicts of interest in this work.
References
Travis W, Brambilla W, Müller-Hermelink H, Harris C. World Health Organization classification of tumors. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004:145–245. | |
Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105(4):546–551. | |
Koizumi T, Agatsuma T, Komatsu Y, Kubo K. Successful S-1 monotherapy for chemorefractory thymic carcinoma. Anticancer Res. 2011;31(1):299–301. | |
Ono A, Naito T, Yamamoto N. S-1 treatment for chemorefractory thymic carcinoma. J Thorac Oncol. 2008;3(9):1076. | |
Okuma Y, Shimokawa T, Takagi Y, et al. S-1 is an active anticancer agent for advanced thymic carcinoma. Lung Cancer. 2010;70(3):357–363. | |
Shimizu Y, Tsunezuka Y, Tanaka N. [A case of unresectable advanced thymic carcinoma in an elderly woman responding to S-1 with good QOL maintained]. Gan To Kagaku Ryoho. 2008;35(11):1977–1979. Japanese. | |
Kaira K, Serizawa M, Koh Y, et al. Expression of thymidylate synthase, orotate phosphoribosyltransferase and dihydropyrimidine dehydrogenase in thymic epithelial tumors. Lung Cancer. 2011;74(3):419–425. | |
National Comprehensive Cancer Network [homepage on the Internet]. Washington: NCCN Clinical Practice Guideline in oncology [updated 2014]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf. Accessed November 7, 2013. | |
Yoh K, Goto K, Ishii G, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide is an effective treatment for advanced thymic carcinoma. Cancer. 2003;98(5):926–931. | |
Agatsuma T, Koizumi T, Kanda S, et al. Combination chemotherapy with doxorubicin, vincristine, cyclophosphamide, and platinum compounds for advanced thymic carcinoma. J Thorac Oncol. 2011;6(12):2130–2134. | |
Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29(15):2060–2065. | |
Okuma Y, Hosomi Y, Takagi Y, et al. Cisplatin and irinotecan combination chemotherapy for advanced thymic carcinoma: evaluation of efficacy and toxicity. Lung Cancer. 2011;74(3):492–496. | |
Okuma Y, Hosomi Y, Takagi Y, et al. Clinical outcomes with chemotherapy for advanced thymic carcinoma. Lung Cancer. 2013;80(1):75–80. |
 © 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.