Back to Journals » OncoTargets and Therapy » Volume 12
The Long Non-Coding RNA-14327.1 Promotes Migration and Invasion Potential of Endometrial Carcinoma Cells by Stabilizing the Potassium Channel Kca3.1
Authors Zhang Y, Zhang P, Chen L, Zhao L, Zhu J , Zhu T
Received 10 August 2019
Accepted for publication 5 November 2019
Published 27 November 2019 Volume 2019:12 Pages 10287—10297
DOI https://doi.org/10.2147/OTT.S226737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yingli Zhang, Ping Zhang, Lu Chen, Lingqin Zhao, Jianqing Zhu, Tao Zhu
Department of Gynecologic Oncology, Zhejiang Cancer Hospital, Hangzhou 310022, People’s Republic of China
Correspondence: Tao Zhu; Jianqing Zhu
Department of Gynecologic Oncology, Zhejiang Cancer Hospital, 1 Banshan East Road, Hangzhou 310022, People’s Republic of China
Email [email protected]; [email protected]
Background: The intermediate-conductance Ca2+-activated potassium channel (Kca3.1) plays a key role in maintaining intracellular Ca2+ homeostasis and is involved with the carcinogenesis of many human tumors including endometrial carcinoma. However, the underlying mechanism is still remained to be further elucidated.
Methods: The relationship between Kca3.1 and the clinicopathological characteristics of endometrial carcinoma was analyzed using UALCAN cancer database, and its expression was determined by immunohistochemistry. The Kca3.1 binding candidate lncRNAs were screened using RNA immunoprecipitation sequencing assay in the endometrial carcinoma cell line. MTT assay and transwell assay were used to confirm the cell proliferation migration and invasion, respectively. FACS was used to determine the cell cycle distribution. The overexpression efficiency of the lncRNAs was detected by qRT-PCR. The expression of EMT related proteins and the stability of Kca3.1 were analyzed by Western blot assay.
Results: Kca3.1 is related to clinicopathological characteristics of endometrial carcinoma, such as tumor stages. Several Kca3.1 binding lncRNAs were obtained from RNA immunoprecipitation sequencing assay. Stable expression of lncRNA-14327.1, one of the candidate lncRNAs, led to significant upregulation of Kca3.1 protein level, cell migration and invasion abilities, but suppressed cell proliferation and induced cell cycle arrest. Additionally, our data also demonstrated that Lenti-lncRNA-14327.1 could stabilize the protein of Kca3.1 and subsequently increase intracellular Ca2+ concentration. Transfection of siRNA-Kca3.1 significantly inhibited cell migration and invasion, and attenuated the EMT in Lenti-lncRNA-14327.1 stably expressed endometrial carcinoma cells.
Conclusion: Taken together, our results demonstrated that the lncRNA-14327.1 promoted cell migration and invasion potential of endometrial carcinoma cells by stabilizing Kca3.1 protein, implying that the lncRNA-14327.1/Kca3.1 might be a promising therapeutic target in endometrial carcinoma, particularly the metastatic one.
Keywords: endometrial carcinoma Kca3.1, lncRNA-14327.1, metastasis, EMT
Introduction
Endometrial carcinoma is the most common malignancy of the female genital tract and is a growing number of in incidence, especially a high incidence rate in China.1,2 The mortality of endometrial carcinoma is only inferior to ovarian and cervical cancers and is presently increasing annually.3 In recent years, The Cancer Genome Atlas (TCGA) database issued a comprehensive analytical report of genome, transcriptome, and proteome profiles in 373 patients diagnosed with endometrial carcinoma.4 Additionally, this alliance decided four prognostic categories (from good to poor) for classification of endometrial carcinoma: (I) polymerase ɛ (POLE) hypermutation; (II) microsatellite instability (MSI) ultra-mutation; (III) lower copy number and (IV) higher copy number. However, in spite of advancements on early detection and better treatment options in the last few years, palindromia and metastasis can still occur leading to poor prognosis.5 Therefore, it is desirable to further elucidate the molecular mechanisms that mediate endometrial carcinoma progression, especially metastasis.
As the most various classes of ion channels, the potassium channel can regulate numerous cellular functions in both stressful and non-stressful cells. Furthermore, due to its cell surface approachability, the potassium channel offers great therapeutic potential in many human diseases, including cancers.6 The intermediate conductance Ca2+-activated potassium channel Kca3.1, also known as SK4 or IK (encoded by the KCNN4 gene), is indispensable to maintain intracellular calcium homeostasis.7,8 The abnormal expression of Kca3.1 has been demonstrated to be associated with malignancy in most kinds of human cancers, such as pancreatic cancer, kidney cancer, ovarian cancer, colorectal cancer, breast cancer, lung cancer and glioblastoma.9–16 However, the role of Kca3.1 in the regulation in endometrial carcinoma remains to be further illustrated.
Long non-coding RNAs (lncRNAs) belong to a subgroup of noncoding RNAs that are at lowest 200 nucleotides in length and without protein-coding capacity.17,18 Across the previous studies, there are mounting evidences that the abnormal expression of long non-coding RNAs (lncRNAs) plays key roles in tumorigenesis and cancer metastasis.19–22 Moreover, the expression profilings of lncRNAs may facilitate the diagnosis and prognosis of human cancer, implying that lncRNAs may serve as efficacious therapeutic targets for intervention. Previous evidence also shows that several lncRNAs are important in the pathogenesis of endometrial carcinoma, including MALAT1, HOTAIR and H19.23–26 To be better understanding of molecular-level information provided by lncRNAs, we can inform diagnosis and therapy in endometrial carcinoma.
In the present study, the expression levels of Kca3.1 were found to be significantly up-regulated in endometrial carcinoma tissues compared to normal tissues, and correlations between Kca3.1 expression and different grades, stage were obtained from the datasets of TCGA (The Cancer Genome Atlas) project. Moreover, using RNA immunoprecipitation sequencing assay, we found that Kca3.1 may be highest targeted by lncRNA-14327.1, a part of an uncharacterized noncoding RNA LOC105378179. However, whether lncRNA-14327.1 is involved in the aberrant expression of Kca3.1, and the activation of EMT in endometrial carcinoma remains undiscovered. This study aimed to explore the function of lncRNA-14327.1 on Kca3.1regulation in endometrial carcinoma. We also investigated the relationship between lncRNA-14327.1, Kca3.1 and EMT in endometrial carcinoma, to seek a potential therapeutic target for endometrial carcinoma, particularly the metastatic one.
Materials and Methods
Clinical Specimens and Cell Culture
Twenty-five endometrial carcinoma biopsy specimens were collected from patients at the Zhejiang Cancer Hospital at the time of surgery and fixed in buffered formaldehyde solution, transferred to 70% ethanol, embedded in paraffin, sectioned into 5um sections and kept in -20°C. All patients provided a written informed consent for the use of these clinical materials in research, and the project was approved by the Institutional Ethics Committee of Zhejiang Cancer Hospital. The human endometrial carcinoma cell line HEC-1A was purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and cultured in DMEM medium supplemented with 10% FBS. All cell lines were cultured in a humidified incubator in an atmosphere of 5% (v/v) CO2 at 37 °C.
Immunohistochemistry
Tissue slices were deparaffinage and dehydration. The activity of endogenous peroxidase was blocked with 3% hydrogen peroxide. Heat-induced antigen retrieval (HIER) was accomplished all slices in 0.01 M citrate buffer by using a steamer at 95 °C. All primary antibodies were diluted with BSA to a concentration of 1:100 and applied to the slices. Incubation for 45 mins at room temperature followed by incubation with a DakoEnVision+System-HRP Labelled Polymer for 30 mins at room temperature. Diaminobenzidine was then applied for 10 mins. The slices were redyed with hematoxylin and then were dehydrated, covers lipped and visualized.
RNA Immunoprecipitation
The RIP assay was performed using a Thermo Fisher RIP kit (Thermo Fisher Scientific, MA, U.S.A) following the manufacturer’s instructions. Briefly, the cell lysates in RIP lysis buffer and RNAs were incubated with the magnetic beads coupled with IgG or anti-Kca3.1 antibody at 4 °C overnight. After the RNA was purified from the RIP production, lncRNAs were analyzed by high-throughput sequencing assay and validation was performed by qPCR assay.
Plasmid Construction and Transfection
The sequences of lncRNA-14327.1, lncRNA-14324.1, and lncRNA-14327.3 were synthesized and then subcloned into the vector pcDNA3.1 (Invitrogen, CA, USA), generating the overexpression vectors for pcDNA-14327.1, pcDNA-14324.1 and pcDNA-14327.3. The integrity of the respective plasmid constructs was confirmed by DNA sequencing. The plasmid formed a complex with Lipofectamine 2000 (Invitrogen) for 20 min at room temperature, and transfection was applied at 37 °C for 24 h. The empty pcDNA3.1 vector was used as a contral. The expression of the lncRNAs was then determined by qPCR.
Generation of Stable Cell Lines with Overexpression of lncRNA-14327.1
For stably overexpressing lncRNA-14327.1, the lncRNA-14327.1 sequence was subcloned into the LV-13 (pLenti-EF1a-LUC-F2A-Puro-CMV) vector (GenePharma, Shanghai, China), and endometrial carcinoma cells were infected with the concentrated virus. Subsequently, cells were treated with 2 μg/mL puromycin for 2 weeks to select for stable cell line, in which the expression of lncRNA-14327.1 was validated by qPCR analysis.
RNA Interference of Kca3.1
Short interfering RNAs for Kca3.1 and non-targeting siRNA-negative control were obtained from GenePharma. Cells were transfected with siRNA using Lipofectamine 2000 reagent according to the manufacturer’s instructions. Before any treatment, cells were incubated for 24 h or 48h and the silence efficiency of the siRNA was determined by Western blotting.
In vitro Migration and Invasion Assays
For the migration assay, a transwell system (24 wells, 8 μm pore size with a poly-carbonate membrane, Corning) was applied according to the manufacturer’s protocols. Cells were seeded into the upper chambers and cultured in serum-free DMEM medium. The lower compartment was stuffed with 10% FBS in DMEM as a chemotactic factor. After incubation for 24 h, the cells that remained in the upper chamber were removed, and the cells at the bottom of the insert were fixed, stained in 0.5% crystal violet and counted under a microscope (Olympus Corp., Tokyo, Japan). The results were averaged over three independent experiments. For invasion assay, the invasion chambers were pre-coated with Matrigel (1:3 dilution; BD Biosciences) at 37°C for 30 min. The following steps were described as same as migration assay.
Western Blotting
Cells were lysed in RIPA buffer containing protease inhibitor cocktail (Sigma). After separation by electrophoresis on a 10% or 12% SDS-PAGE gel, proteins were transferred onto PVDF (polyvinylidene difluoride) membranes. Then, the membranes were blocked with 5% milk. After that, the membranes incubated with primary antibodies against Kca3.1, E-cadherin, N-cadherin, TIMP3 (all four from Abcam) at 4 °C overnight. The corresponding HRP (horseradish peroxidase)-conjugated secondary antibody was incubated for 1 h at room temperature. Signals were visualized after chemiluminescence reaction with the HRP substrate. β-actin or GAPDH (CST) was applied as a loading control.
Cell Viability Assay
The cell viability was assayed in 96-well plates of endometrial carcinoma cell lines using the MTT assay. In brief, the pcDNA3.1, pcDNA-14327.1, pcDNA-14324.1 or pcDNA-14327.3 transfected cells were seeded into 96-well plates at a density of 5000 cells/well and allowed to incubate for 24 h and 48 h. After that, the cells followed by the MTT assay to determine cell survival rates. The absorbance of each sample was read at 570 nm using a microplate reader. For the cell viability of stable cell lines with overexpression of lncRNA-14327.1 was the same progresses as above.
Cell Cycle Analysis
After treated, cells were harvested and washed with 1×PBS (phosphate-buffered saline) and fixed in 75% ethanol at −20 °C. The fixed cells were washed with 1×PBS, incubated for 30 min at 37 °C with RNase A (100 µg/mL) and 0.1% TritonX-100, stained with propidium iodide (30 μg/mL), and analyzed by flow cytometry (FACS Calibur, BD Biosciences). The percentages of cells at G1, S, and G2/M were calculated by the FlowJo software program (Flowjo, LLC) based on the DNA content.
Intracellular Ca2+ Levels
To measure intracellular Ca2+ levels, the Calcium Assay Kit from Abcam was used according to the manufacturer’s protocols.
Statistical Analysis
All data are expressed as the means ± S.D. from at least three independent experiments. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) software packages. Statistical significance was determined using two-sided Student’s t-test, and P < 0.05 (*) was considered significant.
Results
Relationship Between Kca3.1 Expression and Characteristics of Clinical Pathological in Endometrial Carcinoma Patients
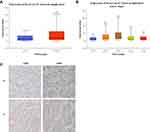
To investigate the clinical relevance of Kca3.1 in endometrial carcinoma, we first analyzed the TCGA database and found that the expression level of Kca3.1 was much higher in endometrial carcinoma tissue than in adjacent normal tissues (Figure 1A). Moreover, high Kca3.1 expression was associated with advanced tumor-node-metastasis (TNM) stage (Figure 1B).
To explore whether the Kca3.1 expression profiling in clinical specimens was consistent with the database, the Kca3.1 protein level in 25 paired normal tissues and endometrial carcinoma tissues was detected by Immunohistochemistry. These analyses revealed that the protein level of Kca3.1 was significantly upregulated in endometrial carcinoma tissues compared with the normal counterparts (Figure 1C, representative results were shown). Taking together, these results indicated that the upregulation of Kca3.1 might play a crucial role in endometrial carcinoma development and progression.
The lncRNA-14327.1 Might Directly Bind to Kca3.1 to Promote Cell Migration in Endometrial Carcinoma Cells
To determine the association between lncRNA and Kca3.1 in endometrial carcinoma, RNA immunoprecipitation (RIP) was carried out in HEC-1A cells. Using a specifically Kca3.1 targeted antibody to pull down the complex, followed by RNA seq and qPCR validation. In the Kca3.1 antibody group, the content of lncRNA ranked in the top three are presented (Table 1).
 |
Table 1 The Result of RIP Sequencing |
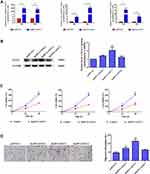
To further explore the biological role of these three lncRNAs in endometrial carcinoma, we first constructed their overexpression plasmids and examined the transfection efficiency in HEC-1A cells and observed that pcDNA-14327.1, pcDNA-14324.1 and pcDNA-14327.3 were highly expressed after transfection for both 24h and 48h compared with the empty vector control (Figure 2A). The sequences for the primers used are listed in Table 2. Next, Western blot assay was used to analyze the protein levels of Kca3.1 in the HEC-1A cells that transfected with pcDNA-14327.1, pcDNA-14324.1, pcDNA-14327.3 and pcDNA3.1, respectively. As shown in Figure 2B, all three lncRNAs could upregulate the protein expression of Kca3.1, but the cells transfected with pcDNA-14327.1 expressed the highest level of Kca3.1. However, the results of the MTT assay revealed that the lncRNA-14327.1, lncRNA-14324.1and lncRNA-14327.3 overexpression significantly inhibited cell proliferation (Figure 2C).
 |
Table 2 The Sequence of Primers |
To further examine the effect on the tumor behavior of lncRNA-14327.1, lncRNA-14324.1 and lncRNA-14327.3 overexpression in endometrial carcinoma cells, migration ability was measured by the transwell assays. As shown in Figure 2D, lncRNA-14327.1 overexpressed cells migrated fastest, the lncRNA-14324.1 overexpressed cells were the second, while the lncRNA-14327.3 overexpressed cells were the last one. These data confirmed that HEC-1A cells with a higher expression level of lncRNA-14327.3 were more aggressive. Taken all together, these results indicated that up-regulation of lncRNA-14327.3 increased Kca3.1 protein level might be related to the onset of endometrial carcinoma cell migration.
The lncRNA-14327.1 Stabilizes Kca3.1 Protein to Increase Ca2+ Inward Transport in Lenti-14327.1 HEC-1A Cells
To further elucidate the function of lncRNA-14327.1 in endometrial carcinoma cells in vitro, we constructed the lncRNA-14327.1 stably overexpressed HEC-1A cell line and its control using the lentivirus system, and the lncRNA-14327.1 expression level in stable HEC-1A cells was determined by qPCR assay (Figure 3A). Next, the stable HEC-1A cells were analyzed by Western blot. As expected, a significant increase in protein level of Kca3.1 in the lenti-14327.1 group compared with those observed in the lenti-control group (Figure 3B).
To confirm the effect of increasing endogenous lncRNA-14327.1 expression on Kca3.1 protein degradation, we compared the half life time of Kca3.1 protein between lncRNA-14327.1 stably overexpressed HEC-1A cell line and its control. The stability of Kca3.1 protein was found to be upregulated more than twofold after overexpression of lncRNA-14327.1 compared with the control cells in a CHX pulse-chase assay (Figure 3C). Since Kca3.1 is a regulator of intracellular Ca2+, we next tested whether upregulation of Kca3.1 following stable lenti-14327.1 expression was associated with intracellular Ca2+ homeostasis. As expected, a remarkable enhancement of intracellular concentration of Ca2+ in the lenti-14327.1 group compared with those in the lenti-control group was observed (Figure 3D). Taken together, our results suggested that the lncRNA-14327.1 could indeed stabilize Kca3.1 protein and finally increase the intracellular concentration of Ca2+ in HEC-1A cells.
The lncRNA-14327.1 Promotes Endometrial Carcinoma Cell Migration and Invasion
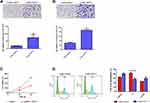
Next, we detected the function of lncRNA-14327.1 in cell behavior in HEC-1A cells with stable expression of lncRNA-14327.1. As shown in Figure 4A and B, stable expression of lncRNA-14327 significantly increased cells migration and invasion abilities compared to the control group.
Subsequently, the results of the MTT assay proved that the stable expression of lncRNA-14327.1 significantly inhibited cell proliferation, which was consistent with the result from the experiments with transient transfection (Figure 4C). Inhibition of tumor cell growth is frequently related to cell cycle arrest. Therefore, we investigated the influence of lncRNA-14327.1 stable expression on the cell cycle distribution in endometrial carcinoma cell lines by Flow cytometry. The results showed that stable expression of lncRNA-14327 reduced cell cycle distribution in the S/M fraction and no significant difference in G0/G1 and G2/M (Figure 4D). Combined results suggested that stable expression of lncRNA-14327 promoted endometrial carcinoma cell migration and invasion, but suppressed cell proliferation by inducing cell cycle.
The Couple of lncRNA-14327.1 and Kca3.1 Promoted Endometrial Carcinoma Cells Migration and Invasion by Inducing EMT
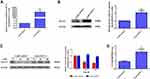
To define the role of lncRNA-14327.1 and Kca3.1 in the progression of cell metastasis in endometrial carcinoma, we treated the lenti-14327.1 HEC-1A cells with siRNA of Kca3.1. Firstly, lenti-14327.1 HEC-1A cells were associated with the decrease of the epithelium marker E-cadherin and TIMP3, an inhibitor of metalloproteinases, but the increase of mesenchymal markers N-cadherin compared with those observed in the lenti-control group (Figure 5A). As shown in Figure 5B, Kca3.1 was effectively down-regulated in Kca3.1-siRNA-2 transfected cells. Next, to determine whether lncRNA-14327.1 induced endometrial carcinoma cells EMT, cell migration and invasion via coupling with Kca3.1, we made use of a Kca3.1 silence model. As shown in Figure 5C and D, we found that inhibition of Kca3.1 not only significantly decreased cell migration and invasion in the lenti-control group but also attenuated the pro-effects of lncRNA-14327.1 on cell migration and invasion. In addition, the silence of Kca3.1 inhibited the expression of two classical mesenchymal cell markers (N-cadherin) in lenti-14327.1 cells, whereas the epithelial cell marker E-cadherin and metalloproteinases inhibitor TIMP3 was elevated (Figure 5E). Taken together, all these results suggested that the lncRNA-14327.1 promoted the migration and invasion potential of endometrial carcinoma cells by upregulating Kca3.1 at least partially.
Discussion
The data from TCGA dataset revealed that the expression of Kca3.1 mRNA was upregulated in endometrial carcinoma tissues compared to adjacent noncancerous tissues and positively associated with the tumor stages. Our data from immunohistochemistry analysis also showed that the expression of Kca3.1 protein was higher expressed in endometrial carcinoma tissues compared to adjacent noncancerous tissues. Although the roles of Kca3.1 in several cancer types have been documented, the regulation mechanism for Kca3.1 expression in endometrial carcinoma remains to be illustrated, especially the role of long non-coding RNA.
The discovery of lncRNA, do not exhibit protein-coding potential, was a breakthrough in regulating the expression of eukaryotic genome and inducing the anomaly growth and metastasis of cancer.27,28 Operation of the expression of lncRNA could affect the cell migration and invasion, cell proliferation, cell cycle and so on of cell behavior in various cancers.29 First of all, we verified three lncRNAs, including lncRNA-14327.1, lncRNA-14324.1 and lncRNA-14327.3 might directly interact with Kca3.1by RNA immunoprecipitation seq assay and PCR validation. The overexpression of all three lncRNAs could significantly promote the expression of Kca3.1 and the cell migration of HEC-1A cells, but with the cell proliferation inhibited. Furthermore, it seemed that lncRNA-14327.1 was the most efficient one. Thus, we speculated lncRNA-14327.1 might act as a molecular couple of Kca3.1 and thereby regulated Kca3.1 function. For further elucidation for the underlying mechanism, HEC-1A cell line with stable expression of lncRNA-14327 and its control cell line were constructed with lentivirus.
Our results showed that stably high expression of lncRNA-14327.1 could effectively induce endometrial carcinoma cell migration and invasion with Kca3.1 upregulated. Moreover, knockdown of Kca3.1 could partially reverse the biological response to the overexpression of lncRNA-14327.1. Therefore, our data indicated lncRNA-14327.1 might promote endometrial carcinoma progression through targeting Kca3.1. Additionally, the stability of Kca3.1 protein was found to be upregulated more than twofold after overexpression of lncRNA-14327.1 in a CHX pulse-chase assay. Noticeably, a remarkable enhancement in the concentration of intracellular concentration of Ca2+ in the lenti-14327.1 group was observed compared with those in the lenti-control group. Overall, these data clearly demonstrated that lncRNA-14327.1 could stabilize Kca3.1 to increase the intracellular concentration of Ca2+.
One of several steps involved in metastasis is epithelial–mesenchymal transition (EMT).30 As shown in Figure 5, we found that downregulation of Kca3.1 inhibited the expression of classical mesenchymal cell markers N-cadherin in lenti-14327.1 cells, whereas the epithelial cell marker E-cadherin and metalloproteinases inhibitor TIMP3 was elevated. Combined results suggested that the couple of lncRNA-14327.1 and Kca3.1 might induce endometrial carcinoma cells metastasis by promoting the EMT.
Conclusion
In conclusion, our findings suggest that lncRNA-14327.1 functions as a novel oncogene to regulate the cell migration and invasion of endometrial carcinoma cells by stabilizing the Kca3.1protein and subsequently activating the EMT. Therefore, the lncRNA-14327.1/Kca3.1 axis may be a novel molecular therapeutic target for the treatment of endometrial carcinoma, especially the metastatic one.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee of the Zhejiang Cancer Hospital (Zhejiang, China) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data Sharing Statement
All data for this study has been presented.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
We declare that all authors have no conflicts of interest.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
4. Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi:10.1038/nature12113
5. Nie D, Yang E, Li Z. Pretreatment thrombocytosis predict poor prognosis in patients with endometrial carcinoma: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):73. doi:10.1186/s12885-018-5264-y
6. Mohr CJ, Steudel FA, Gross D, et al. Cancer-Associated Intermediate Conductance Ca(2+)-Activated K(+) Channel KCa3.1. Cancers. 2019;11(1):109. doi:10.3390/cancers11010109
7. Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8(3):321–329. doi:10.1016/S0959-4388(98)80056-1
8. D’Alessandro G, Monaco L, Catacuzzeno L, et al. Radiation increases functional KCa3.1 expression and invasiveness in glioblastoma. Cancers. 2019;11(3):279. doi:10.3390/cancers11030279
9. Bonito B, Sauter DR, Schwab A, Djamgoz MB, Novak I. KCa3.1 (IK) modulates pancreatic cancer cell migration, invasion and proliferation: anomalous effects on TRAM-34. Pflugers Arch. 2016;468(11–12):1865–1875. doi:10.1007/s00424-016-1891-9
10. Rabjerg M, Olivan-Viguera A, Hansen LK, et al. High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival. PLoS One. 2015;10(4):e0122992. doi:10.1371/journal.pone.0122992
11. Robles-Martinez L, Garay E, Martel-Gallegos MG, et al. Kca3.1 activation via P2y2 purinergic receptors promotes human ovarian cancer cell (Skov-3) migration. Sci Rep. 2017;7(1):4340. doi:10.1038/s41598-017-04292-6
12. Pillozzi S, D’Amico M, Bartoli G, et al. The combined activation of KCa3.1 and inhibition of Kv11.1/hERG1 currents contribute to overcome cisplatin resistance in colorectal cancer cells. Br J Cancer. 2018;118(2):200–212. doi:10.1038/bjc.2017.392
13. De Marchi U, Sassi N, Fioretti B, et al. Intermediate conductance Ca2+-activated potassium channel (KCa3.1) in the inner mitochondrial membrane of human colon cancer cells. Cell Calcium. 2009;45(5):509–516. doi:10.1016/j.ceca.2009.03.014
14. Faouzi M, Hague F, Geerts D, et al. Functional cooperation between KCa3.1 and TRPC1 channels in human breast cancer: role in cell proliferation and patient prognosis. Oncotarget. 2016;7(24):36419–36435. doi:10.18632/oncotarget.v7i24
15. Bulk E, Kramko N, Liashkovich I, et al. KCa3.1 channel inhibition leads to an ICAM-1 dependent increase of cell-cell adhesion between A549 lung cancer and HMEC-1 endothelial cells. Oncotarget. 2017;8(68):112268–112282. doi:10.18632/oncotarget.22735
16. Ruggieri P, Mangino G, Fioretti B, et al. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PLoS One. 2012;7(10):e47825. doi:10.1371/journal.pone.0047825
17. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
18. Engreitz JM, Ollikainen N, Guttman M. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat Rev Mol Cell Biol. 2016;17(12):756–770. doi:10.1038/nrm.2016.126
19. Aird J, Baird AM, Lim MCJ, McDermott R, Finn SP, Gray SG. Carcinogenesis in prostate cancer: the role of long non-coding RNAs. Noncoding RNA Res. 2018;3(1):29–38. doi:10.1016/j.ncrna.2018.01.001
20. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi:10.1016/j.cell.2013.02.012
21. Yang F, Wen S, Zhang Y, et al. Identifying potential metastasis-related long non-coding RNAs, microRNAs, and message RNAs in the esophageal squamous cell carcinoma. J Cell Biochem. 2019.
22. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi:10.1016/j.ccell.2016.03.010
23. Liu H, Zhang Z, Xiong W, et al. Long non-coding RNA MALAT1 mediates hypoxia-induced pro-survival autophagy of endometrial stromal cells in endometriosis. J Cell Mol Med. 2019;23(1):439–452. doi:10.1111/jcmm.2019.23.issue-1
24. Li Q, Zhang C, Chen R, et al. Disrupting MALAT1/miR-200c sponge decreases invasion and migration in endometrioid endometrial carcinoma. Cancer Lett. 2016;383(1):28–40. doi:10.1016/j.canlet.2016.09.019
25. Chi S, Liu Y, Zhou X, et al. Knockdown of long non-coding HOTAIR enhances the sensitivity to progesterone in endometrial cancer by epigenetic regulation of progesterone receptor isoform B. Cancer Chemother Pharmacol. 2019;83(2):277–287. doi:10.1007/s00280-018-3727-0
26. Zhang L, Wang DL, Yu P. LncRNA H19 regulates the expression of its target gene HOXA10 in endometrial carcinoma through competing with miR-612. Eur Rev Med Pharmacol Sci. 2018;22(15):4820–4827. doi:10.26355/eurrev_201808_15617
27. Serviss JT, Johnsson P, Grander D. An emerging role for long non-coding RNAs in cancer metastasis. Front Genet. 2014;5:234. doi:10.3389/fgene.2014.00234
28. Zhang DM, Lin ZY, Yang ZH, et al. IncRNA H19 promotes tongue squamous cell carcinoma progression through beta-catenin/GSK3beta/EMT signaling via association with EZH2. Am J Transl Res. 2017;9(7):3474–3486.
29. Chatterjee M, Sengupta S. Emerging roles of long non-coding RNAs in cancer. J Biosci. 2019;44(1):22. doi:10.1007/s12038-018-9820-z
30. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi:10.1016/j.cell.2009.11.007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.