Back to Journals » OncoTargets and Therapy » Volume 11
The lncRNA TUG1 promotes epithelial ovarian cancer cell proliferation and invasion via the WNT/β-catenin pathway
Authors Liu S, Liu Y, Lu Q, Zhou X, Chen L, Liang W
Received 11 March 2018
Accepted for publication 30 May 2018
Published 12 October 2018 Volume 2018:11 Pages 6845—6851
DOI https://doi.org/10.2147/OTT.S167900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
This paper has been retracted
Shankun Liu,1 Ying Liu,1 Qiang Lu,2 Xiao Zhou,2 Li Chen,2 Weifeng Liang3
1Department of Gynecology, Tai’an City Center Hospital, Tai’an, Shandong, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Affiliated Qingdao Hiser Hospital of Qingdao University (Qingdao Hospital of Traditional Chinese Medicine), Qingdao, Shandong, People’s Republic of China; 3Department of Obstetrics and Gynecology, Qilu Hospital, Shandong University, Qingdao, Shandong, People’s Republic of China
Purpose: Epithelial ovarian cancer (EOC) is among the most common malignant tumors of the endocrine system. Numerous studies have shown that genetic factors are important in the development of EOC, and there is evidence that long noncoding RNA molecules (lncRNAs) can regulate gene expression at the transcription, posttranscription, and epigenetic levels to influence cancer proliferation and invasion, cell differentiation, and apoptosis. However, the roles of lncRNAs in the pathogenesis of EOC remain unclear. Here, we investigated the role of the lncRNA, taurine upregulated gene 1 (TUG1), in EOC.
Patients and methods: TUG1 mRNA levels were evaluated in EOC and matched normal tissue samples and in EOC cell lines by quantitative real-time PCR. Lentiviral vectors expressing the lncRNA, TUG1, and siRNA targeting TUG1 were constructed and transfected into EOC cells. MTT and Transwell assays were used to determine the effects of TUG1 on cell proliferation, migration, and invasion. Western blotting was performed to determine the influence of TUG1 up- or downregulation on WNT/β-catenin signaling, which is involved in the occurrence and development of cancer.
Results: TUG1 expression was clearly elevated in EOC compared with control tissue and cells. Moreover, TUG1 expression was associated with lymphatic metastasis, T stage, and clinical stage in patients with EOC. Downregulation of TUG1 in EOC inhibited cell proliferation, migration, and invasion. In EOC cells, levels of the WNT/β-catenin pathway factors, β-catenin, cyclin D1, and c-Myc, were significantly up- and downregulated in response to TUG1 over- and underexpression, respectively.
Conclusion: Our data suggest that knockdown of TUG1 may represent a novel therapeutic approach for the management of EOC.
Keywords: epithelial ovarian cancer, long noncoding RNA, prognosis, molecular mechanisms
Introduction
Ovarian cancer is one of 3 major gynecological malignant tumors and remains a major cause of death worldwide. There are a wide range of ovarian cancer subtypes, of which 85%–90% are epithelial ovarian cancer (EOC). EOC is thought to originate from undifferentiated cells in the cambium layer of the ovary surface; genetic mutation in these cells increases the likelihood of the development of a malignant tumor.1 Therefore, improved understanding of the pathogenesis of EOC may assist in the development of novel diagnostic, therapeutic, and preventive strategies for this disease.
Long noncoding RNAs (lncRNAs) are a class of RNA transcripts >200 nucleotides in length that have no evidence of protein coding potential.2 lncRNAs can regulate gene expression through diverse mechanisms, including epigenetic silencing, mRNA splicing, and lncRNA–miRNA interaction.3,4 Dysregulation of lncRNAs is involved in the pathophysiological processes underlying various human diseases, including cancers. For example, Liu et al5 demonstrated that upregulation of the lncRNA, CCAT1, correlates with tumor progression and poor prognosis in EOC.
Taurine upregulated gene 1 (TUG1), a 7.1-kb lncRNA mapping to chromosome 22q12.2, was initially identified as a transcript upregulated in retinal cells treated with taurine.6 Subsequently, TUG1 was found to be dysregulated in various tumors and was found to participate in the progression of diverse malignancies, possibly by tumor suppressor or oncogenic activity.7,8 TUG1 is aberrantly overexpressed in osteosarcoma tissues and cells and acts as a possible oncogene in osteosarcoma development;9 however, the relationship between TUG1 expression and EOC development is unknown.
In the present study, the expression of TUG1 lncRNA was determined in EOC tissues and cell lines by quantitative real-time PCR (qRT-PCR). Associations between TUG1 expression and the clinicopathological features of EOC were also investigated. Moreover, we determined the effects of TUG1 on EOC cell proliferation, migration, and invasion in vitro and evaluated the effect of TUG1 on the WNT/β-catenin pathway.
Materials and methods
Tissue samples
Paired human EOC and adjacent normal tissues from 80 patients with EOC were obtained at the Qilu Hospital of Shandong University from 2014 to 2017. All diagnoses of EOC were confirmed by histology. Patients were excluded from the study if they received chemotherapy or radiotherapy prior to surgery. All patients provided written informed consent prior to surgery. The study protocol was approved by the Ethics Committee of Qilu Hospital of Shandong University and complied with the Declaration of the Helsinki. Samples were immediately snap-frozen and stored in liquid nitrogen until use.
Cell culture and transfection
The human EOC cell lines, HO8910, SKOV3, and CAOV3, and human normal ovarian surface epithelium cells (IOSE80) were obtained from American Type Culture Collection and cultured in humidified air at 37°C with 5% CO2 in DMEM culture media supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 U/mL).
Human EOC cell lines were transfected with 50 nM TUG1 Lentiviral Vector (ABM Inc., Richmond, BC, Canada), TUG1 siRNA (si-TUG1) (5′-GTACGTGTCTTGGAAAGTCT-3′), and negative control siRNA (Scrambled control shRNA: 5′-CCGGTTTCTCCGAACGTGTC-3′) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Forty-eight hours after transfection, cells were harvested for total RNA extraction and qRT-PCR analysis.
Cell proliferation assay
Proliferation of EOC cells was measured using an MTT kit (Sigma, St Louis, MO, USA). Briefly, EOC cells in the logarithmic growth phase were detached from culture dishes by trypsinization, seeded in 96-well plates at a density of 2×104 cells/well, and transfected with 50 nM si-TUG1 or si-NC. Cell proliferation was assessed daily for 4 days after transfection. Absorbance at 492 nm was measured after incubation with 20 μL of MTT (Thermo Fisher Scientific, Waltham, MA, USA) for 4 h. A cell proliferation curve was then drawn and proliferation efficiency determined. Experiments were repeated 3 times, independently.
Cell migration and invasion assay
The migration and invasion potentials of cells were measured using Transwell chambers. For migration assays, 5×104 cells were seeded into the upper chambers of Transwell plates (BD Bioscience, San Jose, CA, USA). For invasion assays, 1×105 cells were added into the upper chambers precoated with Matrigel (BD Bioscience). In both assays, cells were maintained in DMEM medium without serum in the upper chamber, and DMEM medium containing 10% fetal bovine serum was added to the lower chamber as a chemoattractant. After incubation for 24 h, nonmigrated or noninvading cells that remained on the upper surface were removed using cotton swabs. Then, membranes were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100 at room temperature for 15 min, and stained with 0.1% crystal violet for 5 min. Three random fields were counted per chamber using an inverted microscope (Olympus, Tokyo, Japan). Experiments were repeated 3 times independently.
RNA extraction and qRT-PCR
Total RNA was extracted from cells using TRIzol® reagent (Invitrogen) or from tissues samples with a RecoverAll™ Total Nucleic Acid Isolation kit (Ambion, Foster City, CA, USA), according to the manufacturer’s instructions. For qRT-PCR, RNA was reverse transcribed into cDNA using a Reverse Transcription Kit (Takara, Dalian, People’s Republic of China). qRT-PCR analyses were performed using SYBR Premix Ex Taq (Takara). Results were normalized to the expression of GAPDH. The primers for TUG1 were as follows: forward, 5′-TAGCAGTTCCCCAATCCTTG-3′ and reverse, 5′-CACAAATTCCCATCATTCCC-3′. The primers for GAPDH were: forward, 5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse, 5′-AGTCTTCTGGGTGGCAGTGAT-3′. Data were analyzed using the 2−ΔΔCt method, and each experiment was performed in triplicate.
Western blot analysis
Cell proteins were extracted using a General Protein Kit (Beyotime, Haimen, People’s Republic of China). All protein samples were adjusted to equal concentrations, followed by addition of bromophenol blue. After removal of bubbles from the wells of acrylamide gels, equal amounts of proteins were loaded, along with 6 μL of GAPDH protein marker. Protein samples were separated using a predetermined voltage (150 V). Next, proteins were transferred to nitrocellulose membranes and blotted with rabbit polycolonal anti-E-cadherin, anti-Vimentin, anti-β-catenin, anti-Cyclin D1, anti-c-Myc, anti-GAPDH primary antibodies (Cell Signaling Technology, Danvers, MA, USA) at a dilution of 1:1000, followed by horse-radish peroxidase-conjugated secondary antibodies (Cell Signaling Technology). Detection was performed using a LI-COR Odyssey Scanning Infrared Fluorescence Imaging System (LI-COR, Lincoln, NE, USA).
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). All data are presented as means ± SD. Differences between groups were evaluated using the Student’s t-test, χ2 test, or Mann–Whitney analysis. P-values <0.05 were considered significant.
Results
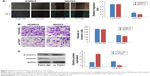
TUG1 is upregulated in EOC tissues and cell lines
We first evaluated lncRNA TUG1 expression in the EOC cell lines HO8910, SKOV3, and CAOV3 and the normal human ovarian surface epithelium cell line, IOSE80, by qRT-PCR. Compared with that in IOSE80, lncRNA TUG1 was upregulated in the HO8910, SKOV3, and CAOV3 cell lines (Figure 1A). We also examined the expression of TUG1 in 80 paired EOC and adjacent nontumor tissue samples. TUG1 was significantly upregulated in EOC compared with healthy tissue (Figure 1B). For the clinicopathological correlation analysis, 80 EOC patients were divided into two groups: high TUG1 group and low TUG1 group by adopting the median of TUG1 expression in EOC tissues as a cut-off value. Elevated TUG1 expression was associated with various clinicopathological characteristics, including lymphatic metastasis, T stage, and clinical stage (Table 1). These results indicate that TUG1 lncRNA may have a role in the progression of EOC.
  | Table 1 Correlation between TUG1 expression and clinicopathologic characteristics of EOC patients |
Downregulation of TUG1 lncRNA inhibits EOC cell proliferation
We transfected HO8910 and SKOV3 cells with TUG1 siRNA to explore the effect of TUG1 on cell proliferation. The proliferation rate of EOC cells transfected with si-TUG1 was significantly decreased compared with negative controls (Figure 2).
Downregulation of TUG1 lncRNA suppresses EOC cell migration and invasion in vitro
We next investigated the effect of TUG1 lncRNA on EOC cell migration and invasion. Transwell migration assays demonstrated that downregulation of TUG1 dramatically suppressed HO8910 and SKOV3 cell migration and invasion relative to negative controls (Figure 3A and B). Furthermore, expression of E-cadherin was upregulated and that of Vimentin downregulated in HO8910 cells (Figure 3C). These findings indicate that TUG1 lncRNA can induce the migration and invasion of EOC cells.
TUG1 lncRNA induces EOC cell proliferation, migration, and invasion via the WNT/β-catenin pathway
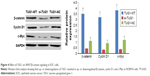
To determine the possible mechanism underlying TUG1 lncRNA regulation of the progression of EOC cells, Western blot analysis was used to explore the effects of TUG1 overexpression (TUG1-WT) or knockdown (si-TUG1) on the WNT/β-catenin pathway, which is often aberrantly activated in human cancers and contributes to enhanced cell proliferation, migration, and invasion. TUG1 overexpression significantly increased the levels of β-catenin, cyclin D1, and c-Myc in EOC cells, while TUG1 inhibition led to significant decreases in expression of the same proteins (Figure 4; P<0.05). These data suggest that the WNT/β-catenin pathway may participate in TUG1-induced proliferation, migration, and invasion of EOC cells.
Discussion
EOC is the most aggressive subtype of ovarian cancer, with significantly higher recurrence and mortality rates than other subtypes.10 EOC is characterized by late clinical manifestation, subtle symptomatology, and rapid disease progression. As a consequence, up to 75% of patients with EOC have already developed metastases at first diagnosis. Despite the great advances achieved in surgery and chemotherapy over the last few decades, the prognosis of EOC patients remains poor, with a 5-year survival rate of only 30%.11 In light of these observations, new biomarkers are still needed to overcome the diagnostic and therapeutic obstacles in a subset of patients with EOC.12
lncRNAs are involved in cellular processes including apoptosis, cell proliferation, migration, and invasion,13–15 and there is also evidence that lncRNAs are crucial determinants of gastric cancer metastasis.16–18 Identification of lncRNAs involved in cancer progression will improve understanding of cancer; these molecules can exert their regulatory functions through a variety of mechanisms, including chromatin remodeling, RNA processing, localization, translation, and modification of mRNA stability, and they can even function as competing endogenous RNA.19–21 Despite growing evidence that aberrant lncRNA expression is key to carcinogenesis and cancer progression,22 the biological and molecular mechanisms underlying lncRNA functions in diverse tumors are yet to be fully elucidated.
Emerging evidence indicates that TUG1 is frequently upregulated and has an oncogenic role in the development and progression of multiple tumors. TUG1 is upregulated in cervical cancer cells, and its downregulation suppresses cell proliferation and activates apoptosis, partially through regulating the expression of BCL2 and caspase-3, and inhibits cell invasion and migration via modulation of epithelial–mesenchymal transition (EMT).23 TUG1 is also highly expressed in renal cell carcinoma, and its knockdown suppresses cell migration, invasion, and proliferation, while inducing apoptosis.24 In esophageal squamous cell carcinoma, TUG1 was reported to be overexpressed, and its silencing using siRNA inhibited cell proliferation and migration and blocked cell cycle progression;25 however, to the best of our knowledge, the expression of TUG1 and its function in EOC progression have not previously been described.
In the present study, we identified TUG1 lncRNA as apparently having a significant role in the promotion of EOC tumors. Our data demonstrate that TUG1 is considerably overexpressed in EOC tissue samples compared with matched normal tissue. The expression profile of lncRNA in different tissues has only been reported in a few cases.28 In cell lines, TUG1 expression profiles were consistent with those in EOC tissue. Furthermore, downregulation of TUG1 resulted in various changes which are generally associated with curbing of tumor development, including inhibition of cell proliferation, invasion, and migration, in contrast to the effects of TUG1 upregulation. Down- or upregulation of TUG1 led to variation in the expression of numerous cancer-associated mRNA molecules. These results suggest that TUG1 is necessary for the maintenance of the basal activities of cells and has an oncogenic role in the development and progression of multiple tumors.
WNT/β-catenin signaling is critical in controlling the balance between cell survival and apoptosis. This pathway is usually activated in a wide range of cancers and promotes tumor invasion and metastasis through upregulation of factors regulating EMT.26 Therefore, inhibition of WNT/β-catenin decreases cell survival and enhances the effects of chemotherapeutic drugs in many types of cancer cell.27 Recent studies have highlighted the cross-talk between lncRNAs and WNT/β-catenin signaling in various cancers; however, whether lncRNAs participate in processes involving WNT/β-catenin and EMT in EOC has not previously been determined.29 In the present study, we found that inhibition of TUG1 significantly decreased the expression levels of β-catenin, cyclin D1, and c-Myc, indicating the WNT/β-catenin pathway might participate in TUG1-induced progression of EOC. Our findings expand the known function of WNT/β-catenin signaling in EOC progression.
Conclusion
In conclusion, this study is the first to examine the function of TUG1 lncRNA in EOC progression. Our data suggest that TUG1 has potential for use as a diagnostic marker in EOC. TUG1 lncRNA is involved in various aspects of tumor progression, including cell proliferation, migration, and invasion. Downregulation of TUG1 by therapeutic agents may be valuable for the prevention of EOC; thus, our findings indicate that knockdown of TUG1 is a potential novel therapeutic approach for EOC.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. | ||
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. | ||
Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. | ||
Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. | ||
Liu SP, Yang JX, Cao DY, Shen K. Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials. Cancer Biol Med. 2013;10:138–141. | ||
Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. | ||
Zhang Q, Geng PL, Yin P, Wang XL, Jia JP, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14:2311–2315. | ||
Li J, Zhang M, An G, Ma Q. LncRNA TUG1 acts as a tumor suppressor in human glioma by promoting cell apoptosis. Exp Biol Med. 2016;241:644–649. | ||
Wang H, Yu Y, Fan S, Luo L. Knockdown of long noncoding RNA TUG1 inhibits the proliferation and cellular invasion of osteosarcoma cells by sponging MiR-153. Oncol Res. 2018;26:665–673. | ||
Zhang Y, Dun Y, Zhou S, Huang XH. LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2017;96:1216–1221. | ||
Jin Y, Feng SJ, Qiu S, Shao N, Zheng JH. LncRNA MALAT1 promotes proliferation and metastasis in epithelial ovarian cancer via the PI3K-AKT pathway. Eur Rev Med Pharmacol Sci. 2017;21:3176–3184. | ||
Wu Q, Wu X, Ying X, et al. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosome lncRNA. Cancer Cell Int. 2017;17:1–13. | ||
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. | ||
Wang GY, Zhu YY, Zhang YQ. The functional role of long non-coding RNA in digestive system carcinomas. Bull Cancer. 2014;101:E27–E31. | ||
Guttman M, Donaghey J, Carey BW, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. | ||
Wiestler B, Capper D, Hovestadt V, et al. Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro Oncol. 2014;16:1630–1638. | ||
Yang Z, Wang R, Zhang T, Dong X. Hypoxia/lncRNA-AK123072/EGFR pathway induced metastasis and invasion in gastric cancer. Int J Clin Exp Med. 2015;8:19954–19968. | ||
Lai J, Nie W, Zhang W, et al. Transcriptional regulation of the p73 gene by Nrf-2 and promoter CpG methylation in human breast cancer. Oncotarget. 2014;5:6909–6922. | ||
Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. | ||
Xing Z, Lin A, Li C, et al. lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell. 2014;159:1110–1125. | ||
Heo JB, Sung S. Verbalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. | ||
Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356(2 Pt B):357–366. | ||
Hu Y, Sun X, Mao C, et al. Upregulation of long noncoding RNA TUG1 promotes cervical cancer cell proliferation and migration. Cancer Med. 2017;6:471–482. | ||
Zhang M, Lu W, Huang Y, et al. Downregulation of the long noncoding RNA TUG1 inhibits the proliferation, migration, invasion and promotes apoptosis of renal cell carcinoma. J Mol Histol. 2016;47:421–428. | ||
Xu Y, Wang J, Qiu M, et al. Upregulation of the long noncoding RNA TUG1 promotes proliferation and migration of esophageal squamous cell carcinoma. Tumor Biol. 2015;36:1643–1651. | ||
Arend RC, Londono-Joshi AI, Straughn JM, et al. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol Oncol. 2013;131:772–779. | ||
Sun J, Yang X, Zhang R, et al. GOLPH3 induces epithelial-mesenchymal transition via Wnt/β-catenin signaling pathway in epithelial ovarian cancer. Cancer Med. 2017;6:834–844. | ||
Xu J, Bai J, Zhang X, Lv Y, Gong Y, Liu L, et al. A comprehensive overview of lncRNA annotation resources. Brief Bioinform. 2017;18(2):236–249. | ||
Li J, Zhou L. Overexpression of lncRNA DANCR positively affects progression of glioma via activating Wnt/β-catenin signaling. Biomed Pharmacother. 2018;102:602–607. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.