Back to Journals » OncoTargets and Therapy » Volume 13
The lncRNA NORAD/miR-520a-3p Facilitates Malignancy in Non-Small Cell Lung Cancer via PI3k/Akt/mTOR Signaling Pathway
Authors Wan Y, Yao Z, Chen W, Li D
Received 13 September 2019
Accepted for publication 16 January 2020
Published 19 February 2020 Volume 2020:13 Pages 1533—1544
DOI https://doi.org/10.2147/OTT.S230954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Yunyan Wan, Zhouhong Yao, Weijuan Chen, Dezhi Li
Department of Respiratory and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong 250021, People’s Republic of China
Correspondence: Dezhi Li
Department of Respiratory and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong University, No. 324 Jingwuweiqi Road, Jinan, Shandong 250021, People’s Republic of China
Email [email protected]
Background/Aims: The effects of lncRNA-NORAD/mir-520a-3p on proliferation and invasion of non-small cell lung cancer (NSCLC) were studied, and its potential molecular mechanism was discussed.
Methods: qRT-PCR was used to detect the expression of lncRNA NORAD and miR-520a-3p in non-small cell lung cancer tissues and cell lines. CCK-8 method and Transwell test were used to identify the effects of lncRNA NORAD on the proliferation and invasion in NSCLC. Target gene prediction and screening and luciferase reporter assay was used to verify downstream target genes of lncRNA NORAD. The expressions of PI3K, AKT, and mTOR proteins were detected by Western blot.
Results: Compared with normal tissues and cells, the expressions of lncRNA NORAD in cancer tissues and cells were significantly higher. Compared with normal cells, the expression of miR-520a-3p in cells was considerably lower. LncRNA NORAD could accelerate the growth and metastasis of NSCLC in vitro and in vivo. Luciferase reporter assay results indicated that miR-520a-3p was a downstream target gene of lncRNA NORAD. Further findings showed that lncRNA NORAD might bind to miR-520a-3p, thereby affecting the PI3k/Akt/mTOR signaling pathway.
Conclusion: LncRNA NORAD can regulate the proliferation of NSCLC by regulating miR-520a-3p/PI3k/Akt/mTOR signaling pathway, thus promoting the occurrence and development of NSCLC.
Keywords: non-small cell lung cancer, lncRNA NORAD, miR-520a-3p, PI3k/Akt/mTOR, proliferation
Background
Lung cancer has high morbidity and mortality, poor prognosis, which poses a severe threat to human health and life.1,2 According to different degrees of differentiation and morphological characteristics, lung cancer is divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), in which NSCLC accounts for about 85% of all lung cancer patients. When clinical symptoms appear, it has developed to the middle and late stage.3,4 Therefore, finding useful and sensitive early diagnostic indicators, therapeutic evaluation indicators, drug resistance monitoring indicators. Prognostic evaluation indicators has become the main direction of lung cancer. This mainly depends on molecular biology and through the study of lung cancer in the occurrence, development, invasion, metastasis, and other transformation processes of detailed mechanisms.
Current studies have shown that the occurrence and development of lung cancer are related to abnormal gene expression and regulatory function.5,6 It was found that lncRNAs have higher intracellular transcription ratio than miRNA.7,8 In the development of tumors, lncRNAs can regulate cancer cell growth, differentiation, and metabolism.9,10 Accumulative evidence shows that lncRNA is involved in the progression of NSCLC.11 It was shown that lncRNA CCAT2 is up-regulated in NSCLC, and silencing of lncRNA CCAT2 by siRNA inhibits proliferation of NSCLC cell lines in vitro.12 The current study has found that lncRNA NORAD can participate in the regulation of the occurrence and development of a variety of cancers, including proliferation, apoptosis, migration, invasion, metabolism, epithelial-mesenchymal transition etc. It could thereby inhibit tumor growth and metastasis.13,14 However, there are few studies on lncRNA NORAD in NSCLC.
In recent years, the regulatory relationship of lncRNA-miRNAs is currently a research hotspot. Abnormal expression of small RNAs is associated with lung cancer. Recently, it has been reported that miR-520a-3p can inhibit apoptosis of NSCLC. It has been found that miRNA-520a-3p can inhibit proliferation and reverse gefitinib resistance by targeting HOXD8 and miRNA-520a-3p in non-small cell lung cancer cells.15 We will also explore the mechanism of the role of the lncRNA NORAD/miR-520a-3p axis in promoting the development of NSCLC. It will provide a reliable basis for clinical diagnosis and targeted therapy of NSCLC.
Methods and Materials
Tissue Samples
The 26 pairs of clinical lung cancer and para-cancerous tissue samples used in this study were from Shandong Provincial Hospital Affiliated to Shandong University. All lung cancer and adjacent tissue samples were determined by histopathological examination after surgical resection of the tumors. Tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C until use. All patients had not received preoperative treatment for cancer. All patients signed a written informed consent form. The study was approved by the Ethics Committee of the Shandong Provincial Hospital Affiliated to Shandong University. The detailed characteristics of the 26 patients were shown in Table 1.
 |
Table 1 The Detailed Characteristics of the 26 Patients |
Cell Culture
Normal human bronchial epithelial cells (NHBE), HEK-293T cells and NSCLC cell lines (A549, H1299, H460, SK-MES-1 and Calu3) were obtained from the Central Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM medium (Gibco, Carlsbad, CA, USA). Subculture was carried out in a 37 ° C, 5% CO2 incubator.
Transfection
The miR-520a-3p mimic, the miR-520a-3p inhibitor anti-miR-520a-3p) and the corresponding negative control miR (miR-NC, anti-miR-NC) were obtained from RiboBio (Guangzhou, China). Lipofectamine 2000 reagent (Invitrogen) was used for transfection. Cells were prepared 48 hrs after transfection for further analysis.
Overexpression or Knockdown of NORAD
The full-length human NORAD gene was subcloned into the lentiviral vector pLV (Add-gene) for NORAD overexpression vector construction. pLV-NORAD, psPAX2, and pMD2.G were transiently transfected into HEK-293T cells. NORAD shRNA was inserted into the lentiviral vector pLKO.1 (Addgene) to knock down NORAD. Lentiviruses were collected 48 hrs after transfection.
RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
RNA was extracted from synovial tissues and cells and synthesized by using Quantitact reverse transcription kit (QIAGEN, Valencia, CA). The expression level of mature miR-22 was detected using TaqMan Micro-RNA Assays. The standard internal control of miRNA was U6. qRT-PCR analysis refers to the literature for detailed steps.16
Cell Proliferation Detection
The cells were seeded into 96-well plates at a density of 1 × 103 cells/well. Three wells were repeated for each group, and ten μL/well of CCK8 was added to each well of the cells for the last 2 hrs of incubation. Finally, the OD value of the cell liquid was measured by an enzyme-linked immunosorbent assay.
Transwell Assays
The cells were inoculated into the upper chamber of the Corning chamber. The medium was inoculated into the lower chamber of the Corning chamber. The cells were cultured for 48 hrs under normal or hypoxic conditions, and the migrated cells were fixed and stained with crystal violet.
The invasion assay was performed by seeding the cells in medium and laying them on top of a Matrigel-coated polycarbonate Transwell filter. The medium was inoculated into the lower chamber of the Corning chamber. The cells were cultured for 48 hrs under normal or hypoxic conditions, and then non-invasive free cells were removed from the apical chamber using a cotton swab. The invading cells in Matrigel were fixed with 20% methanol and stained with crystal violet. The cells were observed under a microscope (Jingtong, Suzhou, China) and photographed. Eight low-power fields (×l00) were randomly selected for cell counting, and the average value was calculated.
Xenograft Model
The A549 stably transfected cells were constructed by lentiviral infection, and the A549 cells were stably expressed with lncRNA NORAD. According to the construction method of stable transfection, Plenti empty vector and Plenti-lncRNA NORAD vector were transfected. Cells were counted according to the specifications of 1×106 cells/100 μL (subcutaneous tumor) and 1×106 cells/200 μL (metastatic tumors) per mouse. Tumor growth of mice was detected every other week and measured with calipers. After the tumors grew to a certain volume, mice were executed in vertebral dislocation way. Tumor volume(V)=(length × width2)/2. Metastatic tumors (lungs) were taken out, weighed and photographed. The experimental data were processed, and results were analyzed, and then H. E staining was carried out. All experimental procedures were approved by the Animal Ethics Committee of the First Affiliated Shandong Provincial Hospital Affiliated to Shandong University. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shandong University and approved by the Animal Ethics Committee of “Animal Ethical and Welfare Committee (AEWC)”.
RNA Immunoprecipitation (RIP)
A549 cells were co-transfected with pCMV-MS2, pCMV- NORAD -MS2 or pCMV- NORAD -mut-MS2 and pMS2-GFP (Addgene) for 48 hrs. After that, RIP was conducted according to the literature method.17
Luciferase Reporter Gene Assay
The wild-type or mutant NORAD was amplified and subcloned into the pm-irGLO vector. The miR-520a-3p mimetic was co-transfected with pmirGLO, pmirGLO- NORAD or pmirGLO- NORAD -mut into cells in each well using Lipofectamine 2000 (Invitrogen). After transfection, cells were harvested, and the activity of Firefly Luciferase and Renilla Luciferase was measured using a dual-luciferase reporter system (Promega) and normalized to the activity of Renilla luciferase. The average value of the results of the microRNA-control transfected cells was set to 1.0.
Western Blot
The cells were weighed, and the total protein was extracted. Then it was transferred to the PVDF membrane by electrophoresis. First Antibody was added. After incubated overnight, it was incubated with 1:5000 labeled anti-rabbit secondary antibody for 1 h. After that, the gray values of the target bands and the internal reference bands were recorded by ECL chemiluminescence. The experiment was conducted according to the literature method.18
Statistical Method
The monitoring data were analyzed by SPSS19.0 statistical software. The results of data analysis were shown as mean ± standard deviation (mean ±SD). Multigroup data analysis was based on one-way ANOVA. LSD test is used for subsequent analysis. P < 0.05, the difference was significant.
Results
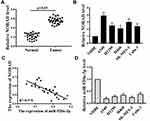
Expression of NORAD and miR-520a-3p in NSCLC
The level of NORAD in NSCLC was analyzed by qRT-PCR. As shown in Figure 1A, compared with adjacent normal tissues, NORAD expression in 26 pairs of NSCLC tissues was significantly increased(p <0.01). The results of Figure 1B and D showed that compared with that in normal human bronchial epithelial cells, the expression of (NHBE)NORAD was significantly increased in NSCLC cell lines (A549, H1299, H460, SK-MES-1, and Calu-3), while the expression of miR-520a-3p was reduced considerably (p < 0.01). In addition, NORAD and miR-520a-3p expression were negatively correlated in NSCLC tissues (Figure 1C). The results indicated that NORAD and miR-520a-3p might play a significant role in NSCLC development.
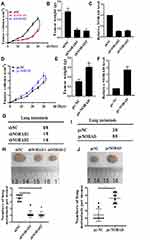
NORAD Promoted NSCLC Cell Tumorigenicity and Metastasis in vivo
The effect of NORAD in vivo was further analyzed. A xenograft mouse model were performed using A549 cells. As shown in Figure 2A and B, compared with the control group, the tumor volume was significantly smaller, and the tumor weight was significantly lower in the NORAD knockdown group (p < 0.01). Compared with the control group, the expression of NORAD was significantly reduced in the NORAD knockdown group (Figure 2C) (p < 0.01). Compared with the control group, the tumor volume in the overexpressed NORAD was significantly increased, and the tumor weight was also significantly higher (Figure 2D and E) (p < 0.01). NORAD expression was significantly increased in the NORAD overexpression group compared with that in the control group (Figure 2F) (p < 0.01).
Compared with that in the control group, the number of lung metastases in the NORAD knockdown group was significantly reduced (Figure 2G and H). In contrast, Compared with that in the control group, mice in the NORAD overexpressing group experienced a significant increase in the number of lung metastases (Figure 2I and J) (p < 0.01). The above results indicated that NORAD could promote the tumorigenicity and metastasis of NSCLC cells in vivo.
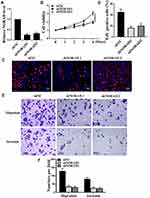
NORAD Promoted Proliferation, Migration, and Invasion of NSCLC Cells in vitro
Cellular assays were used to analyze the effects of NORAD in vitro further. qRT-PCR results showed a significant decrease in cell line expression after NORAD knockdown compared with that in the control group, and a significant increase in cell line expression after over-expression NORAD compared with that in the control group (Figure 3A and Figure S1A) (p < 0.01). Compared with the control group, the cell proliferation rate was significantly decreased after NORAD knockdown, the cell proliferation rate was significantly increased after pcNORAD (Figure 3B and Figure S1B), and Edu staining further confirmed that NORAD knockdown could inhibit cell proliferation. (Figure 3C and D) (p < 0.01). Compared with the control group, the migration and invasion of cells decreased significantly after the knockout of NORAD, and the migration and invasion of cells increased significantly after the over-expression NORAD (Figure 3E and F and Figure S1C).
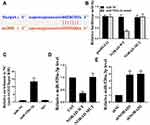
miR-520a-3p Was the Target of NORAD
The mechanism of action of NORAD in the progression of NSCLC was analyzed. We predicted by online tools, and miR-520a-3p was identified as a potential target for NORAD (Figure 4A). Next, the wild type NORAD transcript or its mutation sequence (a mutant in the miR-520a-3p binding site) was cloned into a luciferase reporter construct and then transiently co-transfected into cells with miR-520a-3p or a negative control mimic (miR-NC). As shown in Figure 4B, miR-520a-3p was able to significantly inhibit luciferase activity in wild-type construct transfected A549 cells but did not affect luciferase activity in cells transfected with mutant constructs (P < 0.01).
As a result, in Figure 4C, endogenous NORAD was significantly increased in A549 cells transfected with miR-520a-3p mimics compared with that in the control group. Furthermore, wild-type NORAD significantly reduced miR-520a-3p expression in H1299 cells compared with that in the control group but did not affect miR-520a-3p expression in cells transfected with the mutant construct (Figure 4D) (p < 0.01). Compared with the control group, the NORAD knockdown was able to significantly up-regulate the level of miR-520a-3p in A549 cells (Figure 4E). The result indicated that NORAD can target miR-520a-3p by targeting and that NORAD can act as a ceRNA.
NORAD Positively Regulated PI3K/AKT/mTOR via Sponging miR-520
Whether the NORAD/miR520 can regulate the PI3K/AKT/mTOR signaling pathway was analyzed. As shown in Figure 5A, wild-type NORAD increased expression of the PI3K/AKT/mTOR pathway-associated protein compared with that in the control group and did not affect the expression of PI3K/AKT/mTOR pathway-related proteins in NORAD mutant-transfected cells. The expression of PI3K/AKT/mTOR pathway-related proteins was not significantly affected by the mimic of miR-520a-3p. The co-transfection of NORAD with mimic microRNA-520 reversed the expression of PI3K/AKT/mTOR pathway-related proteins in wild-type NORAD. Compared with the control group, the NORAD silencing group had no significant effect on the expression of PI3K/AKT/mTOR pathway-related proteins, and anti-miR-520 significantly increased the expression of PI3K/AKT/mTOR pathway-related proteins. Anti-miR-520 NORAD silencing co-transfection reversed the expression of the anti-miR-520 against the PI3K/AKT/mTOR pathway-associated protein (Figure 5B). The above results indicated that NORAD could activate the PI3K/AKT/mTOR signaling pathway via the sponge miR-520.
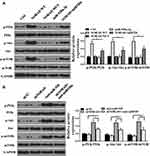 |
Figure 5 NORAD regulated PI3K/AKT/mTOR by targeting miR-520. (A and B). Western blot assay for PI3K/AKT/mTOR expression levels. n = 3, *P < 0.5. |
NORAD Promoted NSCLC Progression Partly Through miR-520- PI3K/AKT/mTOR
Whether NORAD regulates NSCLC progression via miR-520-PI3K/AKT/mTOR was investigated. The results were shown in Figure 6A–C. Compared with the control group, NORAD silencing can significantly reduce the proliferation, migration, and invasion of A549 cells, but this trend was significantly inhibited by anti-miR-520 (p < 0.01). As shown in Figure 6D–F, overexpression of NORAD promoted cell proliferation, migration, and invasion of H1299 cells. Ectopic expression of miR-520 or inhibition of PI3K/AKT/mTOR inhibited the enhanced cell proliferation, migration, and invasion of H1299 cells induced by NORAD.
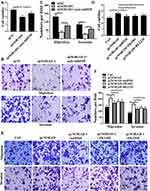 |
Figure 6 The NORAD moiety promoted NSCLC progression via miR-520-PI3K/AKT/mTOR. (A and D). Cell viability assay; (B, C, E, and F). Cell migration and invasion assay. n = 3, *P < 0.5. |
Discussion
Lung cancer is a highly malignant tumor.19,20 The mortality of lung cancer is at the forefront of all kinds of malignant tumors.21 Overall, the current treatment of lung cancer is not satisfactory. The main reason is that the specific pathogenesis of lung cancer is not clear. At the same time, there is a lack of useful, sensitive and reproducible early diagnostic indicators, therapeutic evaluation indicators, drug resistance monitoring indicators, and prognostic evaluation indicators.
As a significant component of non-coding protein genes, lncRNA plays a critical role in lung cancer.11 More and more evidence has showed that lncRNA is involved in the progress of the NSCLC. The study of tumor-suppressing lncRNA provides a new way to elucidate the pathogenesis and development of NSCLC and offers a unique platform for seeking more effective treatment of NSCLC.11,22 The primary function of NORAD is to regulate cell proliferation, cell cycle, and cell aging. In tumors, ectopic expression of NORAD reduces tumor cell doubling time. This study found that the expression level of NORAD in tumor tissues and cell lines was significantly higher, indicating that NORAD acted as an oncogene.23 In addition, the study showed that after NORAD silencing in vivo and in vitro and, the cell proliferation rate and the number of cell invasion and migration were significantly lower. The volume of tumors and the number of lung metastases were also significantly reduced in mice. These results indicated that NORAD had important implications for NSCLC.
Studies have found that some lncRNA can act as ceRNA and play a role in disease through mi RNA-m RNA regulation.24 Highly expressed lncRNA is an essential member of ceRNA, which plays an oncogene role in NSCLC cells.25,26 The expression of mi RNA is related to the occurrence of many major diseases such as tumors, cardiovascular and cerebrovascular diseases.27,28 In the NSCLC study, many down-regulated mi RNAs have been shown to regulate cell proliferation, apoptosis.29,30 This study found that the expression of miR-520a-3p was significantly down-regulation in NSCLC cell lines, and there was a negative correlation between miR-520a-3p and NORAD.31 MiR-520a-3p was selected as the target gene of NORAD through the database. Luciferase results indicated that NORAD regulated its expression by targeting the 3ʹUTR of the NORAD gene. Moreover, wild-type NORAD WT reduced miR-520 expression in H1299 cells, while NORAD knockdown up-regulated miR-520 levels in A549 cells. These results indicated that NORAD and miR-520 could bind to each other and play a role as ceRNA.PI3K/Akt/mTOR signaling pathway is one of the critical signal transduction pathways in cells. PI3K/Akt/mTOR can control important cellular biological processes in tumorigenesis and development by affecting the activation of downstream effector molecules.32 Studies have found that sustained Akt activation and mTOR phosphorylation were found in 51% of NSCLC patient samples and 74% of NSCLC cell lines.33 Previous studies demonstrated that miR-3188 interacts with mTOR to inhibit NSCLC cell proliferation through an mTOR-p-PI3K/AKT signaling pathway. Therefore, miRNA/PI3K/Akt/mTOR might be a potential target for the treatment of NSCLC.34 This study found that NORAD overexpression up-regulated PI3K/AKT/mTOR pathway-associated protein levels, and co-transfection with miR-520 mimics can reverse this effect. The results of NORAD overexpression after NORAD silencing were contrary. Furthermore, inhibition of miR-520 or PI3K/AKT/mTOR overexpression reversed the impact of NORAD overexpression on cell proliferation. The above results indicated that NORAD could promote NSCLC progression through miR-520-PI3K/AKT/mTOR.
Conclusion
NORAD promoted cell proliferation and enhanced cell invasion and migration by directly targeting the miR-520-PI3K/AKT/mTOR axis, which provided experimental evidence for the clinical prognosis of the tumor and further targeted intervention therapy.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University. The research has been carried out in accordance with the World Medical Association Declaration of Helsinki. All patients and healthy volunteers provided written informed consent before their inclusion within the study.
Abbreviations
SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work is supported by the Natural Science Foundation of Shandong province (ZR2014HQ050).
Disclosure
The authors declare that they have no competing interests.
References
1. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi:10.1056/NEJMra0802714
2. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380.
3. Ettinger DS, Akerley W, Borghaei H, et al. Non–small cell lung cancer. J Natal Compr Cancer Netw. 2012;10:1236. doi:10.6004/jnccn.2012.0130
4. Borghaei H, Pazares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:123–135.
5. Falleni M, Pellegrini C, Marchetti A, et al. Survivin gene expression in early-stage non-small cell lung cancer. J Pathol. 2003;200:620. doi:10.1002/(ISSN)1096-9896
6. Singh A, Boldin-Adamsky S, Thimmulappa RK, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi:10.1158/0008-5472.CAN-08-1401
7. Wilkinson B, Campbell DB. Contribution of long noncoding RNAs to autism spectrum disorder risk. Int Rev Neurobiol. 2013;113:35–59.
8. Huang GQ, Ke ZP, Hu HB, Gu B. Co-expression network analysis of long noncoding RNAs (IncRNAs) and cancer genes reveals SFTA1P and CASC2 abnormalities in lung squamous cell carcinoma. Cancer Biol Ther. 2017;18:8. doi:10.1080/15384047.2017.1281494
9. Prensner JR, Sahu A, Iyer MK, et al. The lncRNAs PCGEM1 and PRNCR1 are not implicated in castration resistant prostate cancer. Oncotarget. 2014;5:1434–1438. doi:10.18632/oncotarget.v5i6
10. Cheng W, Zhang Z, Wang J. Long noncoding RNAs: new players in prostate cancer. Cancer Lett. 2013;339:8–14. doi:10.1016/j.canlet.2013.07.008
11. Wang L, Chen Z, An L, et al. Analysis of long non-coding RNA expression profiles in non-small cell lung cancer. Cell Physiol Biochem. 2016;38:2389–2400. doi:10.1159/000445591
12. Zhao Z, Wang J, Wang S, Chang H, Zhang T, Qu J. LncRNA CCAT2 promotes tumorigenesis by over-expressed Pokemon in non-small cell lung cancer. Biomed Pharmacother. 2017;87:692–697. doi:10.1016/j.biopha.2016.12.122
13. Zhang J, Li X-Y, Hu P, Ding Y-S. Oncol Res. 2018;26:1411-1418.
14. Zhou K, Ou Q, Wang G, Zhang W, Hao Y, Li W. Cancer Cell Int. 2019;19:63.
15. Liu Y, Miao L, Ni R, et al. microRNA-520a-3p inhibits proliferation and cancer stem cell phenotype by targeting HOXD8 in non-small cell lung cancer. Oncol Rep. 2016;36:3529–3535. doi:10.3892/or.2016.5149
16. Bustin SA, Mueller R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin Sci. 2005;109:365–379. doi:10.1042/CS20050086
17. Gilbert C, Svejstrup JQ. RNA immunoprecipitation for determining RNA-protein associations in vivo. Curr Protoc Mol Biol. 2006;75. doi:10.1002/0471142727.2006.75.issue-1
18. Liu Z, Mahmood T, Yang P. Western blot: technique, theory, and trouble shooting. N Am J Med Sci. 2012;6:160.
19. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376:629–640. doi:10.1056/NEJMoa1612674
20. Soria J-C, Ohe Y, Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113. doi:10.1056/NEJMoa1713137
21. Hirsch FR, Mcelhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12:208–222.
22. Schmidt LH, Spieker T, Koschmieder S, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–1992. doi:10.1097/JTO.0b013e3182307eac
23. Kawasaki N, Miwa T, Hokari S, et al. Long noncoding RNA NORAD regulates transforming growth factor-β signaling and epithelial-to-mesenchymal transition-like phenotype. Cancer Sci. 2018;109:2211–2220. doi:10.1111/cas.13626
24. Song X, Cao G, Jing L, et al. Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J Cell Mol Med. 2014;18:991–1003. doi:10.1111/jcmm.2014.18.issue-6
25. Li DY, Chen WJ, Luo L, et al. Prospective lncRNA-miRNA-mRNA regulatory network of long non-coding RNA LINC00968 in non-small cell lung cancer A549 cells: a miRNA microarray and bioinformatics investigation. Int J Mol Med. 2017;40(6):1895–1906. doi:10.3892/ijmm.2017.3187
26. Zhang Y-L, Li X-B, Hou Y-X, Fang N-Z, You J-C, Zhou Q-H. Erratum: the lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. Acta Pharmacol Sin. 2017;38:443. doi:10.1038/aps.2017.3
27. Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi:10.1016/j.molcel.2007.06.017
28. Meng F, Henson R, Wehbejanek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi:10.1053/j.gastro.2007.05.022
29. Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Chu Y. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci. 2011;7:805–814. doi:10.7150/ijbs.7.805
30. Seol HS, Akiyama Y, Shimada S, et al. Epigenetic silencing of microRNA-373 to epithelial-mesenchymal transition in non-small cell lung cancer through IRAK2 and LAMP1 axes. Cancer Lett. 2014;353:232–241. doi:10.1016/j.canlet.2014.07.019
31. Nie W, Ge H-J, Yang X-Q, et al. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi:10.1016/j.canlet.2015.11.024
32. Babchia N, Araujo AD, Leclere L, et al. Docosahexaenoic acid protects human RPE cells against oxidative stress via PI3K/Akt m-TOR/p70-p85S6K pathways. Acta Ophthalmol (Copenh). 2012;90. doi:10.1111/j.1755-3768.2012.S055.x
33. Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun S. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi:10.1158/0008-5472.CAN-08-1522
34. Wang C, Liu E, Li W, Cui J, Li T. MiR-3188 inhibits non-small cell lung cancer cell proliferation through FOXO1-mediated mTOR-p-PI3K/AKT-c-JUN signaling pathway. Front Pharmacol. 2018;9:1362.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.