Back to Journals » Cancer Management and Research » Volume 11
The lncRNA HOXA11-AS regulates Rab3D expression by sponging miR-125a-5p promoting metastasis of osteosarcoma
Authors Cao K, Fang Y, Wang H, Jiang Z, Guo L , Hu Y
Received 26 November 2018
Accepted for publication 22 April 2019
Published 16 May 2019 Volume 2019:11 Pages 4505—4518
DOI https://doi.org/10.2147/CMAR.S196025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alexandra R. Fernandes
Kun Cao, 1 Yueyang Fang, 1 Hao Wang, 1 Zheng Jiang, 1 Li Guo, 2 Yong Hu 1
1Department of Orthopaedics, The First Hospital Of Anhui Medical University, Hefei, People’s Republic of China; 2Department of Orthopaedics, The Second Hospital Of Shanxi Medical University, Taiyuan, People’s Republic of China
Objective: Many studies have shown that long non-coding RNAs (lncRNAs) are closely related to various cancers. This study aims to explore the roles of lncRNA HOXA11-AS in the development and progression of osteosarcoma (OS).
Methods: The expression levels of HOXA11-AS and miR-125a-5p in tumor tissues and the adjacent tissues were detected by RT-PCR method. The proliferation, migration and invasion of MG-63 and KHOS cells were determined.
Results: It was found that HOXA11-AS expression levels in OS tissues and OS cell lines were higher than those in OS adjacent tissues and normal human osteoblast cell lines. The higher expression level of HOXA11-AS was positively correlated with more severe clinical stage, distant metastasis and poor prognosis of OS. Inhibition of HOXA11-AS expression could reduce metastasis and invasion of OS cell lines. In addition, HOXA11-AS was found to be an endogenous inhibitor of miR-125a-5p, it down regulated the expression level of miR-125a-5p, and this process could promote the expression of Rab3D, the target gene of miR-125a-5p.
Conclusion: Our study elucidated the role of a new HOXA11-AS/miR-125a-5p/Rab3D regulatory pathway in promoting OS metastasis.
Keywords: osteosarcoma (OS), long non-coding RNAs (lncRNAs), HOXA11-AS, miR-125a-5p, Rab3D
Corrigendum for this paper has been published
Introduction
Osteosarcoma (OS) is the most common primary malignant tumor in childhood, and it is also the second major pediatric tumor that causes death.1 Half of the patients will eventually have lung metastases, although there are many treatments, such as chemotherapy, radiotherapy and surgical treatment.2 The five-year survival rate of patients with OS is not more than 30%, and lung metastasis is the main cause of death.3 Therefore, it is particularly important to study the pathogenesis of OS and find new diagnostic markers and therapeutic targets.
Long non-coding RNAs (IncRNA) are a class of non-coding RNA with a length larger than 200 bases, and they participate in X chromosome inactivation, splicing, epigenetic control and gene transcription regulation.4 Recent studies have shown that IncRNA can be used as competing endogenous RNAs (ceRNA) or molecular sponges to regulate the expression of microRNAs, little is known about its other functions.5,6 The interaction between ceRNA and miRNA represents a new form of gene regulation, which plays a role in a variety of pathophysiological processes including tumorigenesis.7 Homeobox A11 antisense (HOXA11-AS), a newly identified lncRNA, locates on the HOXA gene cluster.8 It has been reported to be up-regulated in a wide variety of carcinomas and is generally associated with poor prognosis.9–13
miR-125a-5p is an anti-oncogene, which can inhibit the proliferation and invasion of liver cancer, gastric cancer, breast cancer, lung cancer, glioma and melanoma. It has been confirmed that SIRT7, PI3K, E2F3, ERBB2, TSTA3, HDAC4, HDAC5, EGFR, Gab2 and Lin28B are target genes of miR-125a-5p.14–24 However, the role of miR-125a-5p in OS has not been reported, and it needs further study.
Rab GTPases is a highly conserved intracellular transporter, it is the basic component and the major regulator of vesicular transport signaling pathway.23 Rab GTPases include Rab3A/B/C/D, Rab26, Rab27A/B and Rab37, which control the corresponding transport process. Abnormal expression of Rab GTPases can affect the development and metastasis of tumor.24 Rab3D is one of the most important members of the Rab GTPases family, and it is mainly expressed in the cells of non-nerve tissue and regulates the transport of specific types of cells. Abnormal expression of Rab3D occurred in a variety of tumor tissues, and increased expression of Rab3D in colorectal cancer and esophageal squamous cell carcinoma is associated with increased invasiveness of tumor cells.25 The expression of Rab3D is also increased in OS, which is related to the proliferation and invasion of OS.26 miR-125a-5p acted on the 3’-UTR of Rab3D, and HOXA11-AS has a ponging effect of Mi-125a-5p in intestinal cancer.13 However, the role of Rab3D in OS remains unclear. Therefore, we explored the roles of HOXA11-AS/miR-125a-5p/Rab3D regulatory pathway in the development and progression of OS in this study.
Materials and methods
Subjects
A total of 61 patients with OS who did not receive chemotherapy or radiotherapy but received surgical treatment directly were recruited in this study. They were from the Department of orthopedics of the First Affiliated Hospital of Anhui Medical University during the 2011–2017 years, and their demography and clinical data were collected. Surgically resected tumor tissues para-tumor tissues were collected, some of them were fixed with 4% polyoxymethylene, embedded with paraffin, and tissue sections were prepared for immunohistochemical staining. The others were sheared and put into RNAlater™ Stabilization Solution (Thermo Fisher Scientific, Waltham, MA, USA) for RNA extraction. The clinical data of the patients are shown in Table 1.
 | Table 1 The primers used in this study |
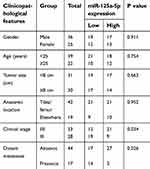 | Table 3 The correlation between the expression level of miR-125a-5p and the clinicopathological features of OS |
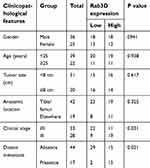 | Table 4 The correlation between the expression level of Rab3D and the clinicopathological features of OS |
This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The informed consent was obtained from patients. The patient consent was written informed consent. These experiments were approved by the Ethics Committee of Anhui medical university.
Experimental animals
BALB/c-nu nude mice (4~6 week old, weighT 16–20 g) were purchased from Anhui experimental animal center. They were bred in the SPF class barrier system of Anhui experimental animal center and were allowed to eat and drink freely. All animal experiments were conducted according to the Principles of Laboratory Animal Care (National Society for Medical Research). These experiments were approved by the Ethics Committee of Anhui medical university.
Cell culture
Human OS cell lines U2OS, MG-63 and KHOS, normal human osteoblast cell line Nhost were purchased from American Type Culture Collection (ATCC). The cells were cultured with DMEM medium containing 10% FBS at 37°C in 5% CO2.
siRNA transfection
miR-125a-5p mimics, miR-125a-5p inhibitors and HOXA11-AS-siRNAs (for the knockdown of HOXA11-AS expression) and Rab3D-siRNAs were purchased from Gene Pharma (Shanghai, China). pcDNA3.1-HOXA11-AS plasmid was constructed. miRNA mimics, miRNA inhibitors, PADI2-siRNAs or HOXA11-AS-siRNAs Rab3D-siRNAs (5ʹ- AGGAGATTCTGGGATTGCCAGTTCT-3ʹ; 5ʹ- CCATCTTCTCTTGCCTGGTCCTTGA-3ʹ; 5ʹ-GGATGTCGTGAAGATGGCAAGAAGA-3ʹ) or HOXA11-AS-siRNAs (5ʹ- TCGTCACTCGGTGTTCTCACCGAAA-3ʹ; 5ʹ- GCACGGTGACTTGATTACACTCTCT-3ʹ; 5ʹ-CGGAAACGGCTAACAAGGAGATTTG-3ʹ) were transfected into U2OS, MG-63, KHOS and Nhost cell lines, respectively. All siRNA transfections were performed using Lipofectamine 2000 Reagent (Life Technologies, Carlsbad, CA, USA), according to the manual protocol.
RNA extraction and qRT-PCR
Total RNA was extracted using a Trizol reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA concentration and purity were detected using a Qubit Fluorometer (Thermo Fisher Scientific, Inc.). A total of 1 μg RNA was subjected to reverse transcription using a Prime Script Kit (Takara Bio Inc., Otsu, Japan). qPCR was performed using a SYBR Premix Ex Taq™ Kit (Takara Bio Inc.). The quantification method used was the 2-ΔΔCT method. The thermocycling conditions were as follows: pre-degeneration at 95°C for 10 mins, followed by 40 cycles of 95°C for 12 s and 62°C for 40 s; U6 and β-actin genes were used as an internal control. The primers used in this study are shown in Table 1.
Cell proliferation ability
The cell proliferation was detected with Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the kit’s manual protocol. The cells in the logarithmic growth phase were digested with trypsin and inoculated into 96-well plates (2×104cell/well), and they were cultured at 37°C with 5% CO2 overnight. Each well was added with 10 μLCCK-8 solution before detection and incubation for 1 hr, and A450 values were detected by Epoch Microplate Spectrophotometer (Bio Tek, Winooski, VT, USA) after culture for 24 hrs, 48 hrs, 72 hrs and 96 hrs, respectively, to evaluate the proliferation of cells.
Cell migration and invasion assay
Cell migration and invasion were determined using a Transwell system (Corning, Inc., Corning, NY, USA). Cells were trypsinized and suspended with serum-free medium and cells (50,000 each well) were added to the upper chamber of the 24-well Transwell system with or without Matrigel (BD Biosciences, San Jose, CA, USA). A 0.6 ml medium containing 10% FBS was added to the lower chamber. Cells were incubated at 37°C for 12 hrs for migration test and continued to culture at 37°C for 24 hes for invasion test. The cells were fixed and stained with 0.1% crystal violet dye (Richard-Allan Scientific, San Diego, CA, USA). They were washed twice with PBS. Stained cells were observed under an inverted microscope.
Xenograft mouse model
Each nude mouse was inoculated 5×106 cells of different groups. Tumor volume was measured every three days (0.5× length × width2) after inoculation for 12 d. The mice were sacrificed after transfection for 36 d, and the tumor was removed for weight measurement.
Dual-luciferase reporter gene analysis
The luciferase reporter gene plasmid WT HOXA11- AS (luc-HOXA11-AS-WT) and point mutation of luciferase reporter gene plasmid Mut HOXA11-AS (luc-HOXA11-AS-MU) were constructed. The 293T cells were inoculated into 24-well plates and cultured overnight, and luciferase reporter plasmid, Renilla luciferase and miR-125a-5p mimic or control were transfected into 293T cells simultaneously. The cells were split using Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manual after culture for 48 hrs. The results were detected using Panomics Luminometer (Affymetrix, Santa Clara, CA, USA) after the luminescence was added. The sea renin fluorescence was used as an internal reference.
Western blotting method
Cells in the logarithmic growth period were lysed with cell lysis solution (Sigma-Aldrich, St. Louis, MO, USA). The supernatant was collected after they were centrifuged at 4°C (1000 rpm) for 5 mins. Total proteins were extracted and protein concentration was determined using BCA. Proteins (50 μg per lane) were separated using 12% SDS-PAGE. Proteins were then electrotransferred to a PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA). The PVDF membrane was rinsed with TBS for 10–15 mins, placed in TBS/T blocking buffer containing 5% (w/v) skimmed milk powder. It was incubated at room temperature for 2 hrs following the addition of an appropriate dilution of primary antibodies (1:1000 Rab3D, Proteintech, Rosemont, IL, USA; 1:2000 β-actin, Proteintech, Rosemont, IL, USA). The membrane was then rinsed with TBST three times (5–10 mins/wash) and then incubated at room temperature for 1 hr with horseradish peroxidase-labeled secondary antibody (1:50,000; Abcam, Cambridge, UK; diluted with TBST containing 0.05% (w/v) skimmed milk powder). The membrane was then rinsed three times with TBST (5–10 mins/wash). Protein bands were detected using an enhanced chemiluminescence kit (Perkin-Elmer Inc.) and quantified as the ratio to β-actin. Quantification was performed using Imagequant LAS4000 (GE Healthcare, Japan).
Immunohistochemical test
Briefly, the osteosarcoma resection tissues were embedded with paraffin using a conventional method. They were cut into 5-μm slices and incubated with 0.3% endogenous peroxidase blocking solution for 20 mins after dewaxing and hydrating. Then, they were incubated at room temperature for 10 mins with 3% hydrogen peroxide methanol solution and washed with PBS for 3 times (3 mins/time). Antigen retrieval was performed using citrate buffer (pH 6.0) at 121°C for 2 mins. After blocking with 5% BSA (Gibco; Thermo Fisher Scientific, Inc), the cells were incubated with a primary monoclonal antibody anti-Rab3D (abcam, ab128997; 1:500) overnight at 4°C. The cells were then incubated with goat anti-rabbit non-biotinylated regents (Zhongshanjinqiao, Beijing, China) according to the manual and mounted with epoxy resin. They were observed using Photo and Image Auto Analysis System (Image-Pro-Plus, China). Five visual fields were randomly selected for each slice, and the integrated optical density (IOD) was calculated by using Image J 6.0 software. The relative expression levels of Rab3D in different tissues were analyzed by comparing IOD.
Statistical analysis
The data were analyzed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). All results are presented as the mean ± SD. Student’s t test, the Wilcoxon signed-rank test and Pearson’s chi-square test were used to evaluate the differences among groups. The correlation between HOXA11-AS and miR-125a-5p and the expression level of miR-125a-5p and Rab3D were analyzed by Pearson’s correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
The expression level of HOXA11-AS increased in OS cases
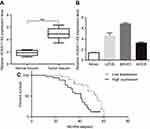
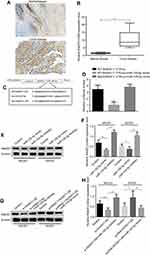
The expression levels of lncRNA HOXA11-AS in 61 OS patients’ resected tumors and para-tumor tissues were detected by fluorescence quantitative PCR method. The results showed that the expression level of HOXA11-AS in tumor tissues was significantly higher than that in para-tumor tissues (P<0.01, Figure 1A), and its expression level in OS cell lines was significantly higher than that in normal human osteoblasts (P<0.01, Figure 1B). The 61 cases were divided into high-expression group and low-expression group according to the median expression level of HOXA11-AS detected by fluorescence quantitative PCR. Kaplan–Meier analysis showed that increased expression of HOXA11-AS was associated with patients’ poor survival (P=0.002, Figure 1C), decreased expression level of microRNA-125a-5p was associated with patients’ poor survival (P=0.002, Figure 1D) and increased Rab3D expression was associated with patients’ poor survival (P=0.007, Figure 1E). Analysis of the correlation between clinicopathological features and expression levels of HOXA11-AS, microRNA-125a-5p and Rab3D showed that the higher expression level of HOXA11-AS was positively correlated with clinical staging, distal metastasis and poor prognosis of OS patients (P<0.05, Tables 2–4).
 | Table 2 Correlation between the expression level of HOXA11-AS and the clinicopathological features of OS |
HOXA11-AS promotes the proliferation of OS
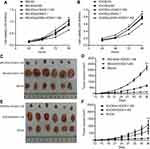
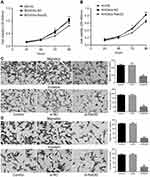
In vitro experiments showed that down-regulation of HOXA11-AS expression in MG-63 and KHOS cells could inhibit cell proliferation, while up-regulation of HOXA11-AS expression could promote cell proliferation (Figure 2A and B). In vivo experiments showed that down-regulation of HOXA11-AS expression in MG-63 and KHOS cells could inhibit the growth of subcutaneous solid tumors in nude mice, while up-regulation of HOXA11-AS expression could promote the growth of subcutaneous solid tumors in nude mice (Figure 2C–F).
HOXA11-AS promotes the migration and invasion of OS
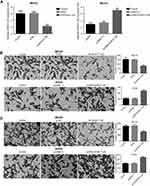
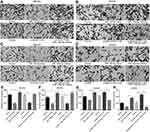
The invasion and migration ability by up-regulating and down-regulating HOXA11-AS gene in both MG-63 and KHOS cell lines were detected, respectively. The results showed that the migration and invasion ability of MG-63 and KHOS cells decreased significantly after HOXA11-AS siRNA inhibited HOXA11-AS expression, the migration and invasion of MG-63, and KHOS cells increased significantly after overexpressed HOXA11-AS (P<0.01, Figure 3). These results suggested that HOXA11-AS could promote the migration and invasion of OS cells.
HOXA11-AS is the molecular sponge of miR-125a-5p
RT-PCR results showed that the expression level of miR-125a-5p in OS tissues was significantly lower than that in their adjacent tissues (P<0.01, Figure 4B). Luciferase reporter gene test also confirmed that HOXA11-AS could directly regulate the expression of miR-125a-5p (P<0.01, Figure 4A). Inhibiting the expression of HOXA11-AS in MG-63 and KHOS cells could significantly increase the expression level of miR-125a-5p (P<0.01, Figure 4C). Overexpression of HOXA11-AS in MG-63 and KHOS cells could significantly inhibit the expression of miR-125a-5p (P<0.01, Figure 4D).
HOXA11-AS promotes the invasion and migration of OS through competitive binding with miR-125a-5p
Compared with the control group, it was found that miR-125a-5p mimic could inhibit the migration and invasion of OS, while miR-125a-5p inhibitors could promote the migration and invasion of OS (P<0.05, Figure 5). When HOXA11-AS- siRNAs and miR-125a-5p inhibitors were transfected into MG-63 cells at the same time, and the ability of HOXA11-AS- siRNAs to inhibit cell migration and invasion was counteracted. When pcDNA-HOXA11-AS and miR-125a-5p mimic were transfected into KHOS cells at the same time, it could inhibit the ability of pcDNA-HOXA11-AS to promote cell migration and invasion (Figure 5).
HOXA11-AS can regulate the expression of its target gene Rab3D by regulating miR-125a-5p expression
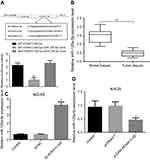
Immunohistochemical and RT-PCR results showed that the expression level of Rab3D in tumor tissues was significantly higher than that in their adjacent tissues (Figure 6A–C). The online software TargetScan analysis showed that miR-125a-5p may be combined with 3 ‘UTR of Rab3D. Luciferase reporter gene analysis showed that miR-125a-5 could target the 3’UTR of Rab3D (Figure 6D and E). miR-125a-5p could inhibit the expression of Rab3D (Figure 6D and E), while HOXA11-AS could promote the expression of Rab3D by inhibiting the expression of miR-125a-5p (Figure 6H and I).
Rab3D can promote the migration and invasion of OS
To further determine whether Rab3D is related to the proliferation, migration and invasiveness of osteosarcoma cells, we transfected Rab3D siRNA into MG-63 and KHOS cells, and it was found that the proliferation of MG-63 and KHOS decreased after down-regulation of Rab3D expression (Figure 7A and B), and the migration and invasion of MG-63 and KHOS cells also decreased significantly after down-regulation of Rab3D expression (Figure 7C and D). These results suggested that HOXA11-AS/miR-125a-5p/Rab3D was involved in the invasion and metastasis of OS (Figure 8).
 | Figure 8 Schematic diagram of HOXA11-AS/miR-125a-5p/Rab3D signal axis regulating OS invasion. |
Discussion
OS is common in children and young people. The incidence rate peaked around the age of 20, and it is about 5 million cases per year.1 The current treatment strategy is limited in the treatment of metastatic and recurrent OS, and new strategies and innovative therapies are urgently needed to improve the survival rate of patients with OS. Understanding the pathogenesis, progression and prognosis of OS at the molecular level will enable molecular targeted therapy to become a new method for the treatment of OS. In this study, we explored the role of lncRNAs in the genesis and development of OS.
lncRNA-HOXA11-AS locates on the chromosome 7p15.2; it contains 3885 nucleotides and is one of the members of the homeobox (HOX) family.6 The HOXA11-AS expression is associated with a variety of cancers. It was up-regulated in glioma and promoted gliomagenesis through regulating cell cycle.7 Sun et al reported that HOXA11-AS/miR-1297/EZH2 cascade played an important role in the occurrence and development of gastric cancer.27 HOXA11-AS expression was up-regulated in colon cancer tissues, which was closely related to the hepatic metastasis of colon cancer.11 Lung metastasis of OS is one of the important causes of death, whether there is a link between HOXA11-AS and lung metastasis of OS remains unclear.
In this study, we found that Rab3D was highly expressed in OS tissues and OS cell lines, but it was relatively low in OS adjacent tissues and human osteoblasts NHost cell line, and its expression level was highly correlated with the expression level of HOXA11-AS. Rab3D is one of the ras-related GTP-binding protein Rab family members. Rab GTPases are highly conservative, and they could regulate a variety of biological processes, which include the skeleton changes, cell movement and invasion of tumor cells.25 miR-125a-5p had tumor suppressor effect in a variety of tumors; HOXA11-AS could competitively combine with miR-125a-5p as a molecular sponge.14,24,28,29 Therefore, we speculated that HOXA11-AS could competitively combine with miR-125a-5p in OS, which leads to the increase of Rab3D expression and promotes the metastasis and invasion of tumor cells.
In this study, we found that the expression levels of HOXA11-AS, miR-125a-5p and Rab3D in OS tissues and OS cell lines were significantly different from those in OS adjacent tissues and human osteoblasts NHost cell line. The expression level of miR-125a-5p was negatively correlated with that of HOXA11-AS and Rab3D. The expression level of HOXA11-AS was positively correlated with more severe clinical stage and distant metastasis. Kaplan–Meier analysis showed that patients with high expression of HOXA11-AS had a poorer prognosis than those with low expression of HOXA11-AS. HOXA11-AS and Rab3D had a positive effect on the invasiveness of OS cell lines, while miR-125a-5p had a negative regulatory effect on the invasiveness of OS cell lines. Luciferase reporter gene test showed that HOXA11-AS could competitively combine with miR-125a-5p and inhibit the regulation effect of miR-125a-5p on Rab3D mRNA. These results suggested that HOXA11-AS could competitively inhibit the binding of miR-125a-5p to Rab3D mRNA and result in a high level of Rab3D expression in OS, which may be one of the mechanisms of high metastasis of OS. Therefore, HOXA11-AS may be a potential therapeutic target in OS. Designing antisense oligonucleotides targeting HOXA11-AS and reducing the expression of HOXA11-AS may provide a new therapeutic strategy for OS.
Conclusions
In a word, in this study, we found that HOXA11-AS could promote the expression of Rab3D by combining with miR-125a-5p competitively as its ceRNA. The HOXA11-AS/miR-125a-5p/Rab3D regulatory network was involved in the metastasis of OS. HOXA11-AS may be an important molecular marker and therapeutic target for OS prognosis.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi:10.1007/978-1-4419-0284-9_1
2. Meazza C, Scanagatta P. Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther. 2016;16(5):543–545. doi:10.1586/14737140.2016.1168697
3. Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47(1):283–292. doi:10.1016/j.ocl.2015.08.022
4. Wang J, Samuels DC, Zhao S, Xiang Y, Zhao YY, Guo Y. Current research on non-coding ribonucleic acid (RNA). Genes (Basel). 2017;8(12):366. doi:10.3390/genes8120366
5. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi:10.1038/nature12986
6. Cesana M, Daley GQ. Deciphering the rules of ceRNA networks. Proc Natl Acad Sci U S A. 2013;110(18):7112–7113. doi:10.1073/pnas.1305322110
7. Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3(10):1113–1121. doi:10.1158/2159-8290.CD-13-0202
8. Nunes FD, de Almeida FC, Tucci R, de Sousa SC. Homeobox genes: a molecular link between development and cancer. Pesqui Odontol Bras. 2003;17(1):94–98.
9. Wang Q, Zhang J, Liu Y, et al. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259. doi:10.1016/j.canlet.2016.01.039
10. Chen J, Fu Z, Ji C, et al. Systematic gene microarray analysis of the lncRNA expression profiles in human uterine cervix carcinoma. Biomed Pharmacother. 2015;72:83–90. doi:10.1016/j.biopha.2015.04.010
11. Richards EJ, Permuth-Wey J, Li Y, et al. A functional variant in HOXA11-AS, a novel long noncoding RNA, inhibits the oncogenic phenotype of epithelial ovarian cancer. Oncotarget. 2015;6:34745–34757.
12. Liang J, Lv J, Liu Z. Identification of stage-specific biomarkers in lung adenocarcinoma based on RNA-seq data. Tumour Biol. 2015;36:6391–6399. doi:10.1007/s13277-015-3327-0
13. Chen D, Sun Q, Zhang L, et al. The lncRNA HOXA11-AS functions as a competing endogenous RNA to regulate PADI2 expression by sponging miR-125a-5p in liver metastasis of colorectal cancer. Oncotarget. 2017;8(41):70642–70652. doi:10.18632/oncotarget.19956
14. Zhang Z, Zhang S, Ma P, et al. Lin28B promotes melanoma growth by mediating a microRNA regulatory circuit. Carcinogenesis. 2015;36:937–945. doi:10.1093/carcin/bgv085
15. Sun L, Zhang B, Liu Y, Shi L, Li H, Lu S. MiR125a-5p acting as a novel Gab2 suppressor inhibits invasion of glioma. Mol Carcinog. 2016;55:40–51. doi:10.1002/mc.22256
16. Wang G, Mao W, Zheng S, Ye J. Epidermal growth factor receptor-regulated miR-125a-5p—a metastatic inhibitor of lung cancer. FEBS J. 2009;276:5571–5578. doi:10.1111/j.1742-4658.2009.07238.x
17. Sun Y, Liu X, Zhang Q, et al. Oncogenic potential of TSTA3 in breast cancer and its regulation by the tumor suppressors miR-125a-5p and miR-125b. Tumour Biol. 2016;37:4963–4972. doi:10.1007/s13277-015-4178-4
18. Jiang L, Huang Q, Zhang S, et al. Hsa-miR-125a-3p and hsa-miR-125a-5p are downregulated in non-small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010;10:318. doi:10.1186/1471-2407-10-663
19. Tang H, Li RP, Liang P, Zhou YL, Wang GW. miR-125a inhibits the migration and invasion of liver cancer cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2015;10:681–686. doi:10.3892/ol.2015.3264
20. Xu Y, Huang Z, Liu Y. Reduced miR-125a-5p expression is associated with gastric carcinogenesis through the targeting of E2F3. Mol Med Rep. 2014;10:2601–2608. doi:10.3892/mmr.2014.2567
21. Kim JK, Noh JH, Jung KH, et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology. 2013;57:1055–1067. doi:10.1002/hep.26101
22. Nishida N, Mimori K, Fabbri M, et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725–2733. doi:10.1158/1078-0432.CCR-10-2132
23. Hsieh TH, Hsu CY, Tsai CF, et al. miR-125a-5p is a prognostic biomarker that targets HDAC4 to suppress breast tumorigenesis. Oncotarget. 2015;6:494–509. doi:10.18632/oncotarget.2674
24. Hsieh TH, Hsu CY, Tsai CF, et al. HDAC inhibitors target HDAC5, upregulate microRNA-125a-5p, and induce apoptosis in breast cancer cells. Mol Ther. 2015;23:656–666. doi:10.1038/mt.2014.247
25. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi:10.1038/nrm2728
26. Jiashi W, Chuang Q, Zhenjun Z, Guangbin W, Bin L, Ming H. MicroRNA-506-3p inhibits osteosarcoma cell proliferation and metastasis by suppressing RAB3D expression. Aging (Albany NY). 2018;10:1294–1305. doi:10.18632/aging.101468
27. Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76(21):6299–6310. doi:10.1158/0008-5472.CAN-16-0356
28. Goldenring JR. A central role for vesicle trafficking in epithelial neoplasia: intracellular highways to carcinogenesis. Nat Rev Cancer. 2013;13(11):813–820. doi:10.1038/nrc3601
29. Luo Y, Ye GY, Qin SL, et al. High expression of Rab3D predicts poor prognosis and associates with tumor progression in colorectal cancer. Int J Biochem Cell Biol. 2016;75:53–62. doi:10.1016/j.biocel.2016.03.017
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.