Back to Journals » Cancer Management and Research » Volume 12
The Landscape of COVID-19 in Cancer Patients: Prevalence, Impacts, and Recommendations
Authors Abdihamid O, Cai C, Kapesa L , Zeng S
Received 13 July 2020
Accepted for publication 17 August 2020
Published 23 September 2020 Volume 2020:12 Pages 8923—8933
DOI https://doi.org/10.2147/CMAR.S272008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Omar Abdihamid,1 Changjing Cai,1 Linda Kapesa,2 Shan Zeng1,3,4
1Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan 410008, People’s Republic of China; 2Department of Oncology, Muhimbili National Hospital, Dar-es-Salaam, Tanzania; 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan 410008, People’s Republic of China; 4Key Laboratory for Molecular Radiation Oncology of Hunan Province, Xiangya Hospital, Central South University, Changsha, Hunan 410008, People’s Republic of China
Correspondence: Shan Zeng
Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan 410008, People’s Republic of China
Tel +86-13574846576
Email [email protected]
Abstract: Cancer patients are susceptible groups to COVID-19, and risk-adjusted models show that most cancer patients have a 25– 39% mortality risk if infected with COVID-19. The infection rate of SARS-CoV-2 in cancer patients in China was 0.79% (12 of 1524 patients; 95% CI, 0.31.2%). The case fatality rate of COVID-19 in the overall population ranges from 2.3 to 8.0%; among these, the case fatality rate for cancer patients is at 5.6%. In a retrospective cohort study of 28 COVID-19-infected cancer patients, a total of 15 (53.6%) patients had severe outcomes with a mortality rate of 28.6%. In a pooled analysis by Aakash et al, a 2% cancer prevalence was found among admitted patients with COVID-19. In Italy, a report shows that among the 3200 patients who died of SARS-CoV-2, 19.4% were patients with cancer. In New York, 61 (28%) cancer patients succumbed to COVID-19 with a case fatality rate of 37% (20/54) and 25% (41/164) for hematologic and solid malignancies, respectively. Impacts of COVID-19 in cancer care include interruptions of life-saving therapies, distraction effects, and diagnostic overshadowing that involve diverting attention to the pandemic rather than to cancer patients and disruptions of primary palliative care to patients due to forced quarantine. Herein, we review the landscape of COVID-19 in cancer care. We also briefly share our experience and the measures in place to protect cancer patients against COVID-19 in our center.
Keywords: SARS-CoV-2, cancer care, high-risk, mortality
Introduction
As of December 2019, the world has grappled with a novel strain of virus caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes the disease COVID-19. The first case was identified in the city of Wuhan, Hubei province, China, and rapidly spread globally.1
The origin of the virus is believed to be from bats and later transmitted to humans through an unknown intermediary in Wuhan wet market, Hubei province, China, in December 2019.2
Similar incidents of transmission happened in the past two decades, where animals to human crossover were witnessed. The first instance of such was in 2002–2003, when a crossover transmission of a new coronavirus was identified in Guangdong province of China.3,4
The case fatality rate of COVID-19 is estimated to range from 2 to 3%. This novel virus is more virulent but genetically different from SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), albeit with lower fatality.5,6 The universal impact of this new pandemic is exponential with countries like the USA, Italy, Spain, and the UK reporting staggering numbers of cases and deaths.7
Following the outbreak in Wuhan on 31st December 2019, China officially notified the World Health Organization, and on 1st January 2020, the epicenter market was closed.6
As of August 2020, worldwide confirmed cases stand at 19.6 million, with over 727,258 deaths according to the latest World Health Organization (W.H.O) situation reports.8
Coronaviruses are a type of enveloped RNA viruses with a size of 60 nm to 140 nm in diameter with crown-like projections when viewed microscopically; hence the name coronavirus.9
On 7th January 2020, the genome sequence of virus was identified by the Chinese scientists to be coronavirus, which showed a striking similarity of > 95% with the bat coronavirus and >70% with the SARS- CoV. Samples from the wet market tested positive, confirming the epicenter of the virus and patient zero.10
Based on this, there was clear evidence that human-to-human transmission was happening among close contacts of patient zero since the middle of December 2019. Concerted efforts by the Chinese authorities leading up to the total shutdown of Wuhan city lead to excellent control of transmission in China, albeit with over 3000 deaths.11 The decline in the number of cases in China depicts, strict public health measures can be successful in containing epidemics.1
Cancer patients are caught on the crossfire of this pandemic as a unique group with increased risks of contracting COVID-19. Their weakened immunity is resulting from a myriad of factors including underlying malignancy burden and active cancer treatments like cytotoxic chemotherapy, radiotherapy, or even due to patients undergoing transplant and use of immunosuppressants to avoid rejection.12
However, recent evidence shows that mortality in cancer patients from COVID-19 is mainly driven by old age and comorbidities and less likely by the use of cytotoxic drugs.13
Risk-adjusted models show that most cancer patients have a 2528% percent mortality risk if infected with COVID-19.14 Also, most cancer patients are frail and of older age with comorbidities compared to the healthy population; hence they tend to have poor outcomes.15
This pandemic has caused a huge, unprecedented impact on cancer patients’ care continuum in terms of diagnosis, treatment schedules, and follow up visits. New and old patients are losing valuable clinic visits due to quarantine.
Likewise, new patients could potentially miss early diagnosis opportunities and patients with advanced cancer experience delays or interruptions of their routine treatment leading to risks of disease progression.16
Additionally, patients who develop adverse side-effects from cancer treatments could lose valuable time to come in for an intervention. These include; immunotherapy related adverse effects, tumor lysis syndromes, differentiation syndromes, and other severe treatment-related events. Also critical is oncological emergencies commonly seen, such as; spinal cord compression, need for acute pain relief, electrolyte imbalances, need for blood transfusions, febrile neutropenia, among other sudden detrimental events.
Moreover, distraction effects that involve diverting attention to the pandemic as compared to cancer patients cause collateral loss and unfavorable outcomes in oncology care. Instances, where even oncology care clinicians are in the frontlines to care for COVID −19 patients, are reported in many institutions due to shortages of staff.17
In the middle- and low-income countries with the ailing healthcare system and sub-optimal oncology care, these distraction effects are far worse.18
The inability to deliver palliative care or primary palliative care to patients is another significant concern due to forced quarantine.19
There could be a double wave of cancer patients’ deaths: a surge in immunocompromised patients who contract COVID-19 or those whose treatments were de-escalated, canceled, or delayed. As one medical oncologist described in a perspective article: “to survive SARS-CoV-2 only to then succumb to an undertreated cancer would be a Pyrrhic victory”.20
This article gives an overview of the prevalence and impacts of COVID-19 in cancer patients and institutional recommendations put in place to mitigate the virus in this patient population. We also briefly share our proposed organizational model in our institution on the measures taken to protect cancer patients.
Incidence of COVID-19 in Cancer Patients
The spread of the disease is exponential in immunocompromised populations like cancer patients during outbreaks, and eventually, immunity limits transmission.21
Similar to cases seen during the MERS-outbreak where having cancer was identified as a risk factor for MERS-CoV mortality, the COVID-19 pandemic also poses threats to cancer patients.12
The burden and risk of developing COVID-19 in cancer patients are compounded by their age and litany of other comorbidities. A systematic review that looked at the prevalence of underlining conditions of already hospitalized COVID-19 patients showed cardiovascular diseases and malignancy to be among the most prevalent conditions.22
As the world population continues to live longer, age becomes an overall risk factor for cancer. In 2018 alone, 6.6 million cancer cases were diagnosed globally in persons aged ≥70. Many of the latest COVID-19 studies also show advanced age and comorbidities to be associated with poor outcomes. Therefore, age-stratified data in the continuum of cancer care during this pandemic and beyond is paramount.23
In one of the early data by Yu et al published in JAMA Oncology, the infection rate of SARS-CoV-2 in cancer patients from Wuhan, China, was at 0.79% (12 of 1524 patients; 95% CI, 0.31.2%). And in their subgroup analysis, they found that the prevalence was higher in lung cancer patients older than age 60 compared to those younger than 60 years (4.3% vs 1.8%).24
Liang et al,15 also looked at the prevalence of cancer in COVID-19 cases in a nationwide analysis. They analyzed a total of 1590 cases and found a prevalence of 1% (95% CI, 0.61% to 1.65%), which was higher than the overall cancer risks in the Chinese population.25
Lung cancer was similarly the most common type of disease from this cohort, accounting for 28%. Their subgroup analysis also reported lung cancer patients with COVID-19 to be at a higher risk of pulmonary complications and poor outcomes from the use of chemotherapy.15
In a retrospective cohort study of 28 COVID-19-infected cancer patients with laboratory-confirmed COVID-19 from three hospitals in China, a total of 15 (53.6%) patients had severe outcomes with a mortality rate of 28.6%. Also, tumor treatment within last 14 days seemed to significantly increase the risk of severe outcome [hazard ratio (HR) = 4.079, 95% confidence interval (CI) 1.086–15.322, P = 0.037.26
However, according to the data from the Chinese Center for Disease Control and Prevention, case fatality was higher in patients with comorbidities compared to those without. Among these, the case fatality rate for cancer patients was at 5.6%.27
A review of 355 COVID-19 patients who died in Italy showed old age (mean 79.5yrs) and comorbidities as the main contributing factors. Among these, 72 patients (20%) had active cancer.28
In a multicenter study, patients with hematological and metastatic cancer were found to carry a high risk for SARS-CoV-2, which was similar to those who recently received surgery. Receiving radiotherapy was not found to have any significant differences in severe outcomes when compared to non-cancer patients.29
In a retrospective analysis of the perioperative outcomes of six lung cancer patients with COVID-19, Cai et al, found that three patients died from COVID-19 pneumonia, indicating surgery might be a risk factor for death in these patients.30
Similarly, cancer patients with COVID-19 were found to have a higher risk of severe events (for example, need for intensive care unit and invasive ventilation, or even death) compared with patients without cancer.31
Quantifying the risks of COVID-19 for cancer patients hinges on; the absolute risk of COVID-19 during anticancer therapy and the additional risk of mortality should the patient become infected. Because of resulting neutropenia and lymphopenia and interference with both innate and adaptive immunity from cancer drugs, cancer patients can have a worse disease course.32
In a pooled analysis by Aakash et al, a 2% cancer prevalence was found among admitted patients with COVID-19.33 Alarmingly, even a past medical history of cancer in the setting of cancer survivors seemed to increase the risk for poor outcomes due to possible immune status. However, this could be a confounding factor related to old age.19
A case report from Wenzhou, China of a 39-year-old gentleman previously treated for non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL), was found to have COVID-19, after presenting with atypical symptoms. The authors conclude that the patient’s compromised weak immune system and CLL masked the infection.34
Another case report from Wuhan of a multiple myeloma patient who presented with solitary chest tightness was diagnosed with COVID and successfully treated with tocilizumab.35
A lung cancer case on long-term Nivolumab therapy, a sudden severe interstitial pneumonitis during treatment was noticed, and the patient later tested positive for SARS-CoV-2. Immunotherapy related pneumonitis usually occurs during the first 3 months of therapy, and the sudden onset could be attributed to the negative synergy of the concomitant use of Nivolumab and SARS-CoV-2 infection.36
A pan-cancer analysis by Cai et al that looked at cancer types with the highest risk for COVID-19 found that endometrial carcinoma expressed both Transmembrane Protease Serine 2 (TMPRSS2) and Angiotensin-Converting Enzyme 2 (ACE2) receptors which are launching pads for SARS-CoV-2. Therefore, these subgroups of cancer patients could be at higher risk.37
In New York, a total of 61 cancer patients succumbed to COVID-19 with a case fatality rate of 37% (20/54) and 25% (41/164) for hematologic and solid malignancies, respectively. Using multivariate analysis, older age, comorbidities, increased levels of lactate, and need for intensive care were associated with worse outcomes.38
Report from the Superior Institute of Health in Italy shows that 3200 patients who died of SARS-CoV-2, 19.4% were patients with cancer. The report further indicates, old age and other comorbidities to have contributed to these adverse outcomes.39 Therefore, a risk-benefit analysis of systemic treatment should be considered in this patient group, and factors like age >70, comorbidities and the number of treatment visits required should inform decisions.40
Immunotherapy, as one of the armamentariums of cancer treatments, usually comes with immune-related adverse events in a subset of patients. Immune checkpoint inhibitors (ICI) are commonly used to treat solid tumors such as melanoma, lung cancer, renal carcinoma, urothelial cancers, and head and neck tumors.41 However, the susceptibility of this subset of patients to contract COVID-19 or other bacterial, viral infections has not been well studied. Granted that immunotherapy restores the cellular immunocompetence of these patients, there could be a plausible theory that they are maybe more immunocompetent as compared with patients undergoing radiotherapy or chemotherapy.42
In an analysis that sort to look for the correlation between the history of immunotherapy drugs in lung cancer patients, the authors did not find any evidence even when adjusted for smoking history status which Is common in these patient group.43
A new hypothesis on the role of androgen hormone in COVID-19, data from Italy shows prostate cancer patients receiving androgen deprivation therapy had a significant fourfold reduced risk of COVID-19 infections compared to their counterparts.44
Currently, through the cyclic media news, all cancer and potential cancer patients are more oriented toward COVID-19 symptoms and could potentially ignore any ominous symptoms like breast lump or rectal bleeding that would normally lead them to see a doctor.45
The differential diagnosis of COVID-19, especially in cancer patients, includes all types of respiratory viral infections and bacterial infections, which often present atypically. Therefore, travel history is essential, and oncologists should have a high index of suspicion.46
Above all, there is the possibility of an actual higher prevalence of SARS-CoV-2 in cancer patients than it is reported. This is further confirmed by the higher number of young and healthy young population who are probably carriers of COVID-19 with mild or no symptoms and who do not require hospital admission, thus escaping the rapid laboratory testing.47
In addition, SARS-CoV-2 could as well present with genetic drift and mutation; hence should be closely monitored to avoid a second wave which will further the current effects on cancer care.5
More data from COVID-19 studies on the clinical characteristics and outcomes showed cancer to be a common comorbidity with varying types, as shown in Table 1 below.
 |
Table 1 Clinical Characteristics of COVID-19 Patients with Cancer |
Taken all together, cancer patients are at higher chances to contract and die from COVID-19 compared to the general population, and proper measures to address this should be adopted by hospitals and treatment centers.
Impacts of COVID-19 on Cancer Care
The COVID-19 pandemic presents unprecedented challenges and learning opportunities for many cancer care providers. Even though many patients will not contract the virus due to heightened precautions, people with compromised immune systems, including cancer patients and cancer survivors are at increased risk for COVID-19 and other infections.48
The highest risk group in COVID-19 transmission are the healthcare workers. In 2002, healthcare workers accounted for 21% of those affected by the Severe Acute Respiratory Syndrome (SARS) outbreak.49
As of February 2020, 4.4% of the reported cases in China were health care workers, and by April 2020, a total of 23 health care professionals were among the 3387 deaths from COVID-19.50
A study by Yu et al (2020)24 that enrolled 1524 cancer patients at a cancer center in China reported a double-fold risk of COVID-19 in comparison to the general population. They linked hospital visits to be a contributing factor to this increased incidence.
Also, there is no current clear guideline on the care of cancer patients concerning their cancer types, therapy types, or subpopulation of cancer patients (eg, children, elderly).51
Due to the looming shortage of health care resources, together with the increased risk of cancer treatment during this pandemic, informed decisions about how and when to provide cancer treatment, is paramount.48
The American College of Surgeons recommends a delay of life-saving cancer surgeries and the cancellation of elective surgeries to cushion and shift resources to COVID-19 patients. This hugely affects patients and could lead to loss of vital opportunities in many resectable cancers.52
A survey by the American Cancer Society Cancer Action Network shows 24% of cancer patients reported delayed treatments, and 12% were worried about the uncertainty of future therapies.53
In the UK, urgent cancer referrals that are usually eligible for a two-week wait target are now subject to prioritization rules that will cause delays. Also, cancer screening programs have been halted and is only offered to symptomatic patients.54
A similar modeling study from the UK looking at the impacts of an average 2-weeks delay in cancer patients’ referrals shows 84% reductions as a result of a backlog of referrals of about 25% resulting from the lockdown. Therefore, prompt prioritization of patients for whom referral delays would lead in most life-years lost should be considered.55
In the Netherlands, a country of 17 million people, the overall cancer diagnosis for all cancer sites excluding skin cancer dropped by 25% from January to April 2020. Furthermore, skin cancer, excluding basal cell carcinoma, decreased by 60% in the same time frame.56
The pandemic also poses significant risks to specific cancer patients, such as leukemia patients. Approximately 5075% of patients with acute leukemia present with fever and are at risk of misdiagnosis. Similarly, other cancers (mediastinal tumors or lung cancer, for example) who present with respiratory symptoms like cough and are likely to be dismissed after testing negative for COVID-19. Also, stem cell transplantation services are affected in terms of risks of transplantation, the number of procedures done, and the availability of a matched donor. Such situations could have a negative impact on survival.57
Most of the cancer clinical research programs including, clinical trials, have experienced operational changes, including delays in accruals during the COVID-19 pandemic, according to a report by an international collaborative group that looked at the impacts of COVID-19 on cancer care.58,59
Training and education in the oncology department have been equally affected. Medical students’ training programs are not ongoing. Hospital morning rounds, meetings and journal clubs have been suspended, and are only accessible via teleconference.60
Cancer care providers are at increased risk for coronavirus infection. Many healthcare workers succumbed to coronavirus, representing 8.3% of Italy cases by 19th March 2020. Working under pressure and burn out are likely to be experienced by cancer care providers during this pandemic.51
Mental health and psychological stress, especially vicarious traumatization in caregivers, can be caused by the COVID-19 pandemic. In a recent study from China that enrolled front-line nurses to evaluate vicarious traumatization scores via a mobile app-based questionnaire showed significant prevalence.61–63
Recommendations
Currently, there are no harmonized guidelines on the management of cancer patients with COVID-19. Institution-based guidelines are followed, and this varies from center to center. This has posed significant challenges, especially for integrative cancer treatments.31,51
American Society of Clinical Oncology (ASCO) recently released a COVID-19 related frequently asked questions document addressing the recommendations on screening of cancer patients, in-patients and outpatients visits, collection of laboratory samples, home drug infusion options and engaging in telehealth.64
Multiple registries of COVID-19 in cancer patients have been launched in order to characterize the severity and clinical outcomes of cancer patients. A new registry of such is TERAVOLT (Thoracic cancERs international coVid 19 cOLlaboraTion) that collect lung cancer tailored risk-assessment data across multiple international centers.65
In ten-point recommendations for oncology practices on the safety of cancer patients and their caregivers, Pelin et al, suggest prescreening, telemedicine, switch therapy, among others during this pandemic.66
The World Health Organization67 has given several general recommendations, which include; adopting isolate, test, treat and trace policy, hand washing after visiting every patient, screening, and isolation of suspected COVID-19 cancer patients in different wards. Additionally, care providers are advised to limit the use of aerosol-generating procedures like intubations in cancer patients.51
The Center for Disease Control (CDC) recommends high-risk individuals like cancer patients to stay home and should avoid cruise ship and nonessential air travel. However, they should be provided access to several weeks of medication.68
During the Middle East Respiratory Syndrome Coronavirus (MERS-CoV), leaders in oncology care instituted a plan to manage the crisis in cancer patients. The main components of this plan include; leadership and communication, staff management, infection control, patient management, and recovery plan. Such a plan presents a template for the COVID-19 epidemic and should be adopted for the benefit of immunosuppressed groups like cancer patients.69
A recently published experience from China on the countermeasures against COVID-19 in cancer recommends postponing elective surgery or adjuvant chemotherapy for stable patients and those with advanced cancer in endemic areas. Strict and thorough surveillance should be considered if cancer patients are infected with SARS-CoV-2, especially elderly patients and those with other comorbidities.15,31
The Italian authors recommend the importance of prioritizing urgent cancer treatment, especially in the setting of colorectal surgery. Minimally invasive procedures should be preferred and done only on highly selected patients.
Radiotherapy, as an essential treatment modality, can be used as an alternative to surgery where feasible, especially by the use of hypofractionated regimens, as this will minimize the number of visits.70 Delaying radiotherapy poses detrimental effects, especially in patients who need palliative radiotherapy. Similarly, according to the Early Breast Cancer Trialists’ Collaborative Group, radiotherapy delays obviate survival benefits associated with the adjuvant treatments of breast cancer.71
European/American Society for Radiation Oncology (ESTRO-ASTRO) issued a consensus statement on lung cancer radiotherapy during the COVID-19 pandemic. This practice recommendation addresses six different lung cancer cases and the prioritization of hypofractionation in the setting of risk mitigation and reduced resources.72
In a special issue about their experience from the COVID-19 epicenter in the United States, Masumi et al recommend the continuation of therapy in patients with curative intent. Hematologic malignancy like acute leukemias needs greater urgency; hence should be handled as such. And finally, cellular immunotherapies and hematopoietic stem cell transplantation are invaluable curative interventions for most patients with aggressive disease; therefore should not be delayed if possible.48
Patients on immunotherapy with underlying lung diseases like interstitial pneumopathy are considered a high-risk group for immunotherapy related pneumonitis. As mentioned above, there are controversies about COVID-19 and anticancer treatment with ICI. However, patients should not be denied the crucial benefits of ICI treatment, especially in highly responsive diseases.73
A panel made up of 15 experts in the UK has recently published updated recommendations during the ongoing COVID-19 pandemic in the radiotherapy setting recommending hypofractionation for bowel cancer to minimize visits while maintaining the efficacy of treatment. The panel also recommends delaying any therapy in elderly patients and patients with poor performance status or those who are unfit for chemotherapy.60,74
Canadian guidelines on prioritizing systemic therapies for genitourinary malignancies during this pandemic recommend the use of radium 223, especially in patients with bone-only lesions and androgen-receptor -axis targeted therapies in metastatic prostate cancer while also carefully weighing the risk of taxanes related neutropenias. They also recommend the use of virtual care of cancer patients at this time to minimize visits.75
The National Institute for Health and Care Excellence (NICE) and the European society of medical oncology (ESMO) issued tiered approach guidelines in delivering cancer care during the COVID-19 pandemic. It’s designed on three levels: high, medium, and low priority using the criteria of the Cancer Care Ontario and Magnitude of Clinical Benefit Scale (MCBS), as described in Table 2. 76
 |
Table 2 The Cancer Care Ontario, Huntsman Cancer Institute and Magnitude of Clinical Benefit Scale (MCBS) |
Patients with a terminal disease or with comorbid health who contract COVID-19 and require mechanical ventilation could have a dismal prognosis. A retrospective study from Wuhan reported the survival of only one patient among the 32 COVID-19 patients who were severely ill and required mechanical ventilation.77 Therefore, oncologists need to have advance care planning and discussions with cancer patients and their families on such possible outcomes.78
Similarly, the National Comprehensive Cancer Network (NCCN),48 underscores the importance of supportive care discussions with cancer patients should they become infected with COVID-19. With scarce resources, Oncologists should prioritize what treatments are most likely to be successful, symptom-relieving, and which patients are likely to benefit from such treatments. Finally, Oncologists should always be compassionate and do no harm–primum non nocere.
Proposed Organization Model from Our Institution
Fortunately, there have been no cases of COVID-19 in our oncology department, but that is not without contingency measures put in place. First, all medical staff is trained on the latest information on COVID-19 and how to carry out case-finding in their respective departments. Secondly, a COVID-19 ad hoc expert committee comprising of infectious disease experts, hematologists and oncologists, pharmacists and radiologists has been set up and institutional guidelines put in place. Thirdly, to minimize cross-infection, staff should not enter other departments other than their own without permission. Fourthly, the zoning of the institution into four special categories was used to screen for incoming patients. Zone 1 (screening and surveillance zone) is for patients who need surveillance as directed by the committee of experts in-order to rule out potential infection. Each patient must be isolated in a single room.
Zone 2 (quarantine zone) is for suspected cases, and therefore each patient must be quarantined in a single room.
Zone 3 (confirmed quarantine zone) is a treatment zone for patients with confirmed COVID-19.
Zone 4 (oncology ward) is used as the treatment zone for cancer patients who do not have COVID-19. The full protocol is shown in Figure 1
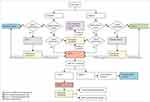 |
Figure 1 COVID-19 screening algorithm for the oncology department. *Positive: sputum and nasal or throat swab. †Negative: Two negative tests 24 hours apart. |
Even though cases in china have exponentially dropped and the curve flattened, continuous screening of cancer patients for COVID-19 before admission or before therapy is still strictly followed in our institution.
Conclusion
COVID-19 has changed the world order and, more so, the order of cancer care. Cancer patients and cancer survivors are at high risk for contracting and dying from COVID-19. The incidence data of COVID-19 in the cancer patient population is still emerging and could be higher than it is reported. The symptomology of COVID-19 in cancer patients is atypical and masked; hence misdiagnosis is possible, so Oncologists should have a high index of suspicion.
Early screening, diagnosis, isolation, treatment, and education of cancer patients are of utmost importance. Social distancing, handwashing should be observed by cancer patients. Similarly, hospital visits should be avoided, and clinicians should engage in virtual care.
Patients’ age, tumor type, underlying comorbidities, stage of the disease, and treatment type all affect the risk and outcomes of contracting SARS-CoV-2 in cancer patients. However, recent evidence shows that mortality in cancer patients from COVID-19 is mainly driven by old age and comorbidities and less likely by the use of cytotoxic drugs.
Impacts of this pandemic in cancer care include; Increased risk of mortality, missed early cancer diagnosis opportunities, cancelation, or interruptions of life-saving therapies, distraction effects and diagnostic overshadowing. Also, important clinical trials, research, and academic programs and have all been severely affected.
As more data emerges, the landscape of COVID-19 will be understood, but as of now, the guidelines issued by the relevant cancer organizations should be strictly followed to protect cancer patients. Preventative measures remain the only effective arsenal against this invisible pathogen.
Abbreviations
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; MERS-CoV, Middle East respiratory syndrome coronavirus; JAMA, Journal of American Medical Association; CLL, chronic lymphocytic leukemia; ASCO, American Society of Clinical Oncology; TERAVOLT, Thoracic cancERs international coVid 19 cOLlaboraTion; ESTRO/ASTRO, European/American Society for Radiation Oncology; NICE, National Institute for Health and Care Excellence; MCBS, Magnitude of Clinical Benefit Scale; NCCN, National Comprehensive Cancer Network; ICI, immune checkpoint inhibitor; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protein serine 2.
Disclosure
The authors received no funding for this work and report no financial or non-financial conflicts of interest.
References
1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
2. WHO. Novel Coronavirus (2019-nCoV) SITUATION REPORT - 1 21 JANUARY 2020. SITUATION REPORT - 1 21 JANUARY 2020. Jan,2020.
3. XU MC YAR-H. SARS: epidemiology. Respirology. 2003;8:S9–S14. doi:10.1046/j.1440-1843.2003.00518.x
4. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi:10.1016/j.jare.2020.03.005
5. Xie M, Chen Q. Insight into 2019 novel coronavirus - an updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi:10.1016/j.ijid.2020.03.071
6. Commission CNH. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment (7th edition). China National Health Commission. 2020.
7. Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg. 2020;76:71–76. doi:10.1016/j.ijsu.2020.02.034
8. W.H.O. Coronavirua disease (COVID-19), Situation report-202 . 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200809-covid-19-sitrep-202.pdf?sfvrsn=2c7459f6_2. Accessed September 08, 2020.
9. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418–423. doi:10.1002/jmv.25681
10. Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi:10.1016/S0140-6736(20)30154-9
11. W.H.O. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available from: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19). Accessed September, 2020.
12. Abdul-Rahman Jazieh M, Alenazi TH, Alhejazi A, Safi FA, Olayan AA. Outcome of oncology patients infected with corona virus. Am Society Clin Oncol. 2020;6:471–475. doi:10.1200/GO.20.00064
13. Lee LYW, Cazier JB, Starkey T, Turnbull CD, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi:10.1016/S0140-6736(20)31173-9
14. Oh WK. COVID-19 infection in cancer patients: early observations and unanswered questions. Ann Oncol. 2020;31:838–839. doi:10.1016/j.annonc.2020.03.297
15. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi:10.1016/S1470-2045(20)30096-6
16. Wang Z, Wang J, He J. Active and effective measures for the care of patients with cancer during the COVID-19 spread in China. JAMA Oncol. 2020;6:631. doi:10.1001/jamaoncol.2020.1198
17. Cortiula F, Pettke A, Bartoletti M, Puglisi F, Helleday T. Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31:553–555. doi:10.1016/j.annonc.2020.03.286
18. Vanderpuye V, Elhassan MMA, Simonds H. Preparedness for COVID-19 in the oncology community in Africa. Lancet Oncol. 2020;21(5):621–622. doi:10.1016/S1470-2045(20)30220-5
19. Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:4. doi:10.1016/S1470-2045(20)30149-2
20. Lewis MA. Between Scylla and Charybdis — oncologic decision making in the time of Covid-19. N Engl J Med. 2020;382:2285–2287. doi:10.1056/NEJMp2006588
21. Tang X, Wu C, Li X, et al. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev. 2020;7:1012–1023. doi:10.1093/nsr/nwaa036
22. Amir EFJ, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19- a systematic review and meta-analysis. Archives Acad Emergency Med. 2020;8(1):e35.
23. Desideri I, Pilleron S, Battisti NML, et al. Caring for older patients with cancer during the COVID-19 pandemic: a young international Society of Geriatric Oncology (SIOG) global perspective. J Geriatr Oncol. 2020. doi:10.1016/j.jgo.2020.05.001
24. Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108. doi:10.1001/jamaoncol.2020.0980
25. RS SK Z, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. 41;1:19–28.
26. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894–901. doi:10.1016/j.annonc.2020.03.296
27. Prevention CCfDCa. The epidemiological characteristics of an outbreak of 2019 Novel Coronavirus Diseases (COVID-19) — china, 2020. China CDC Weekly. 2020;2(8).
28. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. doi:10.1001/jama.2020.4683
29. Dai M, Liu D, Liu M. et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov;2020.
30. Cai Y, Hao Z, Gao Y, et al. Coronavirus disease 2019 in the perioperative period of lung resection: a brief report from a single thoracic surgery department in Wuhan, People’s Republic of China. J Thorac Oncol. 2020;15(6):1065–1072. doi:10.1016/j.jtho.2020.04.003
31. Geliang Yang M, Zhang H, Yang Y. Challenges and countermeasures of integrative cancer therapy in the epidemic of COVID-19. Integr Cancer Ther. 2020;19(3):1–2.
32. van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era. Nat Med. 2020;26(5):665–671. doi:10.1038/s41591-020-0874-8
33. Aakash Desai M. COVID-19 and cancer: lessons from a pooled meta-analysis. Am Society Clin Oncol. 2020.
34. Jin X-H, Zheng KI, Pan K-H, Xie Y-P, Zheng M-H. COVID-19 in a patient with chronic lymphocytic leukaemia. Lancet Haematol. 2020;7(4):e351–e352. doi:10.1016/S2352-3026(20)30074-0
35. Xuhan Zhang KS, Tong F, Fei M, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Advances. 2020.
36. Di Noia V, D’Aveni A, Squadroni M, Beretta GD, Ceresoli GL. Immune checkpoint inhibitors in SARS-CoV-2 infected cancer patients: the spark that ignites the fire? Lung Cancer. 2020;145:208–210. doi:10.1016/j.lungcan.2020.05.006
37. Cai C, Ahmed OA, Shen H, Zeng S. Which cancer type has the highest risk of COVID-19 infection? J Infect. 2020. doi:10.1016/j.jinf.2020.05.028
38. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi:10.1158/2159-8290.CD-20-0516
39. Luigi Palmieri XA, Bella A, Bellino S, et al. Characteristics of COVID-19 patients dying in Italy report based on available data on March 20th, 2020. COVID-19 Surveillance Group. 2020.
40. Banna G, Curioni-Fontecedro A, Friedlaender A, Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5:2.
41. Bersanelli M, Giannarelli D, Castrignano P, Fornarini G, Panni S, Mazzoni F. INfluenza vaccine indication during therapy with immune checkpoint inhibitors: a transversal challenge. The INVIDIa study. Immunotherapy. 2018;10(14):1229–1239. doi:10.2217/imt-2018-0080
42. M GD B, Castrignanò P, et al. Influenza vaccine indication during therapy with immune checkpoint inhibitors: a transversal challenge. The INVIDIa study. Immunotherapy. 2018;14(10):1229–1239.
43. Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10(8):1121–1128. doi:10.1158/2159-8290.CD-20-0596
44. Montopoli M, SZ R, Rugge VM, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS- CoV-2: a population-based study (n=4532). Ann Oncol. 2020;31:1040–1045. doi:10.1016/j.annonc.2020.04.479
45. Vrdoljak E, Sullivan R, Lawler M. Cancer and coronavirus disease 2019; how do we manage cancer optimally through a public health crisis? Eur J Cancer. 2020;132:98–99. doi:10.1016/j.ejca.2020.04.001
46. Singhal TA. Review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281–286. doi:10.1007/s12098-020-03263-6
47. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:14. doi:10.1001/jama.2020.2565
48. Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. 2020;1–4.
49. Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med. 2020;8:3. doi:10.1016/S2213-2600(20)30066-7
50. Zhan M, Qin Y, Xue X, Zhu S. Death from Covid-19 of 23 health care workers in China. N Engl J Med. 2020;382:2267–2268. doi:10.1056/NEJMc2005696
51. Shankar A, Saini D, Roy S, et al. Cancer care delivery challenges amidst coronavirus disease - 19 (COVID-19) outbreak: specific precautions for cancer patients and cancer care providers to prevent spread. Asian Pac J Cancer Prev. 2020;21(3):569–573. doi:10.31557/APJCP.2020.21.3.569
52. COVID-19: Recommendations for Management of Elective Surgical Procedures. Available from: https://www.facs.org/-/media/files/covid19/recommendations_for_management_of_elective_surgical_procedures.ashx. Accessed September 08, 2020.
53. Network CA. COVID-19 pandemic impact on cancer patients and survivors survey findings summary. Am Cancer Society. 2020.
54. The Lancet O. Safeguarding cancer care in a post-COVID-19 world. Lancet Oncol. 2020;21:5.
55. Amit Sud* BT, Jones ME, Broggio J, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21:1035–1044. doi:10.1016/S1470-2045(20)30392-2
56. Avinash G, OV D, Rob H, et al. Fewer cancer diagnosis during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21.
57. Gavillet M, Carr Klappert J, Spertini O, Blum S. Acute leukemia in the time of COVID-19. Leuk Res. 2020;92:106353. doi:10.1016/j.leukres.2020.106353
58. Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID −19) pandemic: an international collaborative group. Oncologist. 2020;25. doi:10.1634/theoncologist.2020-0213
59. Schrag D, Hershman DL, Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323:2005. doi:10.1001/jama.2020.6236
60. Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;21:629–630. doi:10.1016/S1470-2045(20)30217-5
61. Li Z, Ge J, Yang M, et al. Vicarious traumatization in the general public, members, and non-members of medical teams aiding in COVID-19 control. Brain Behav Immun. 2020.
62. Joob B, Wiwanitkit V. Traumatization in medical staff helping with COVID-19 control. Brain Behav Immun. 2020;87. doi:10.1016/j.bbi.2020.03.020
63. Bao Y, Sun Y, Meng S, Shi J, Lu L. 2019-nCoV epidemic: address mental health care to empower society. Lancet. 2020;395(10224):e37–e38. doi:10.1016/S0140-6736(20)30309-3
64. ASCO. ASCO special report: A Guide to cancer care delivery during the COVID-19 pandemic. Available from: https://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-Cancer-COVID19.pdf. Accessed September 08, 2020.
65. Marina Garassino LH, Peters S. TERAVOLT (Thoracic cancERs international coVid 19 cOLlaboraTion). Lancet Oncol. 2020 Jul; 21(7): 914–922.
66. Pelin Cinar M, Timothy Kubal MS, Freifeld A, et al. Safety at the time of the COVID-19 pandemic: how to keep our oncology patients and healthcare workers safe. JNCCN. 2020;18:5.
67. W.H.O. Novel Coronavirus (2019-nCoV) Situation Report. 11. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200131-sitrep-11-ncov.pdf?sfvrsn=de7c0f7_4. Accessed September 08, 2020.
68. CDC T. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings print page. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed September 08, 2020.
69. Abdul-Rahman Jazieh M, Hadab AA, Olayan AA, et al. Managing oncology services during a major coronavirus outbreak lessons from the saudi arabia experience. Am Society Clin Oncol. 2020;6:518–524.
70. Nagar H, Formenti SC. Cancer and COVID-19 - potentially deleterious effects of delaying radiotherapy. Nat Rev Clin Oncol. 2020;17:332–334. doi:10.1038/s41571-020-0375-1
71. Group EBCTC. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi:10.1016/S0140-6736(11)61629-2
72. Guckenberger M, Belka C, Bezjak A, et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: an ESTRO-ASTRO consensus statement. Radiotherapy Oncol. 2020;146:223–229. doi:10.1016/j.radonc.2020.04.001
73. Bersanelli M. Controversies about COVID-19 and anticancer. Immunotherapy. 2020;12:269–273. doi:10.2217/imt-2020-0067
74. Marijnen CAM, Rödel C, Bujko K. International expert consensus statement regarding radiotherapy treatment op‐ tions for rectal cancer during the COVID 19 pandemic. Radiotherapy Oncol. 2020;148:213–215. doi:10.1016/j.radonc.2020.03.039
75. Lalani AA, Chi KN, Heng DYC, et al. Prioritizing systemic therapies for genitourinary malignancies: canadian recommendations during the COVID-19 pandemic. Can Urol Assoc J. 2020;14(5):E154–E158. doi:10.5489/cuaj.6595
76. Ontario Health Cancer Care HCI. Pandemic planning clinical guideline for patients with cancer. Available from: https://www.accc-cancer.org/docs/documents/cancer-program-fundamentals/oh-cco-pandemic-planning-clinical-guideline_final_2020-03-10.pdf.
77. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
78. JR KE C, Stapleton RD. The importance of addressing advance care planning and decisions about do-not-resuscitate orders during novel coronavirus 2019 (COVID-19). JAMA. 2020. doi:10.1001/jama.2020.4894
79. Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi:10.1016/S1470-2045(20)30314-4
80. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904–913. doi:10.1016/S1470-2045(20)30310-7
81. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi:10.1136/bmj.m1091
82. Tomlins J, Hamilton F, Gunning S, Sheehy C, Moran E, MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J Infect. 2020;81:e59–e61. doi:10.1016/j.jinf.2020.04.020
83. Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–348. doi:10.1159/000507471
84. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China. Am J Gastroenterol. 2020;115(5):766–773. doi:10.14309/ajg.0000000000000620
85. Moiseev S, Avdeev S, Brovko M, Akulkina L, Fomin V. Cancer in intensive care unit patients with COVID-19. J Infection. 2020;81:e124–e125. doi:10.1016/j.jinf.2020.05.053
86. Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi:10.1164/rccm.202002-0445OC
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
