Back to Journals » Infection and Drug Resistance » Volume 15
The Influence of Two Priming Doses of Different Anti-COVID-19 Vaccines on the Production of Anti-SARS-CoV-2 Antibodies After the Administration of the Pfizer/BioNTech Booster
Authors Wolszczak Biedrzycka B , Bieńkowska A, Smolińska-Fijołek E, Biedrzycki G, Dorf J
Received 23 September 2022
Accepted for publication 26 November 2022
Published 29 December 2022 Volume 2022:15 Pages 7811—7821
DOI https://doi.org/10.2147/IDR.S390351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Blanka Wolszczak Biedrzycka,1,2 Anna Bieńkowska,1,2 Elwira Smolińska-Fijołek,3 Grzegorz Biedrzycki,4 Justyna Dorf5
1Department of Psychology and Sociology of Health and Public Health, University of Warmia and Mazury in Olsztyn, Olsztyn, 10-082, Poland; 2The Oncology Center of the Region of Warmia and Mazury in Olsztyn, Hospital of the Ministry of the Interior and Administration, Olsztyn, 10-228, Poland; 3Department of Physiology, Medical University in Gdańsk, Gdańsk, 80-211, Poland; 4Hospital Dispensary, Regional Specialist Hospital in Olsztyn, Olsztyn, 10-561, Poland; 5Department of Clinical Laboratory Diagnostics, Medical University of Białystok, Bialystok, 15-269, Poland
Correspondence: Blanka Wolszczak Biedrzycka, Tel +48-505-970-699, Email [email protected]
Introduction: A global vaccination program was implemented in late 2020 to end the pandemic caused by the SARS-CoV-2 virus. However, the immune response elicited by the vaccines proved to be insufficient due to the rapid emergence of new viral mutations. Therefore, the factors influencing cellular and humoral immune responses after the administration of different vaccines against SARS-CoV2 need to be identified.
Materials: In the present study, anti-SARS-CoV-2 antibody titers were analyzed 20 to 50 days after the administration of a third (booster) dose of the BNT162b2 vaccine in 192 residents of the city of Olsztyn (Poland) primed with two AstraZeneca or Pfizer/BioNTech vaccines.
Methods: Antibody titers were determined in venous blood serum in the ECLIA test using the Cobas e411 Roche analyzer.
Results: The study revealed that persons who received three doses of the Pfizer/BioNTech vaccine had significantly higher antibody titers than those who received two doses of AstraZeneca and a booster dose of Pfizer/BioNTech.
Keywords: vaccination, COVID-19, BNT162b2, ChAdOx1, anti-SARS-CoV-2
Introduction
According to the latest available data (late July 2022), the severe acute respiratory syndrome coronavirus (SARS-CoV-2) caused more than 572 million confirmed cases of COVID-19 and caused more than 6.39 million deaths around the world.1 The virus is known to be acquired from zoonotic source and mainly spreads airborne through direct and contact transmission.2 Since the beginning of the pandemic, SARS-CoV-2 has undergone numerous genetic and antigen mutations. The most prevalent and best researched mutations include the Alpha (B. 1. 1. 7/20I/N501Y. V1), Beta (B. 1. 351/20H/N501Y. V2), Gamma (B. 1. 1. 28. P1/P. 1/20J/N501Y. V3), Delta (B. 1. 617. 2/21A), and Omicron (B. 1. 1. 529/21K/BA. 1) variants. Several vaccines have been developed to protect against COVID-19 and decrease the risk of severe illness and death.3 The vaccines manufactured by Oxford/AstraZeneca (ChAdOx1) and Pfizer–BioNTech (BNT162b2) were most popular in Europe. In early studies evaluating the efficacy of these vaccine brands, the BNT162b2 mRNA vaccine demonstrated >90% efficacy, whereas the AstraZeneca AZD1222 vaccine containing the adenovirus vector demonstrated 60–70% efficacy against symptomatic infections.4–7
 |
Figure 2 Heat map of correlation of total anti-SARS-CoV2 antibodies in patients after Pfizer vaccination with chosen clinical parameters. |
 |
Figure 3 Heat map of correlation of total anti-SARS-CoV2 antibodies after AstraZeneca vaccination with chosen clinical parameters. |
Clinical study reports have shown that both vaccines were characterized by high safety and high immunogenicity. However, very few studies have directly compared the efficacy of these vaccines. According to many authors, the effectiveness of immunization is determined not only by vaccine type but also by the patient’s age and gender.8 A number of studies performed on the elderly population, which is at highest risk of serious complications from COVID-19, revealed that vaccine-induced immunity is insufficient to confer full protection against infections caused by SARS-CoV-2.4,9 Studies show that older people and women comply more closely with the pandemic guidelines.10 The protective effect of certain medicinal products (including statins) should be considered in high-risk patients.11
Numerous studies have confirmed that the protection conferred by two doses of the vaccine against COVID-19 (symptomatic and asymptomatic infections) wanes over time. Despite the above, two vaccine doses still effectively protect against severe COVID-19 infections and death all over the world.8,9,12 In Poland, the booster dose campaign began in September 2021 to maintain a high level of protection in the population at the greatest risk of severe complications from COVID-19 (elderly persons, persons with comorbidities, health care employees), and the booster dose became available to the general population in November 2021.13 Only Pfizer/BioNTech and Moderna vaccines were authorized for use as boosters. A booster dose of the AstraZeneca vaccine was not available.
The entire world is still experiencing the COVID-19 pandemic, which is why the efficacy of anti-SARS-CoV-2 vaccines should be monitored in patients with a history of COVID-19 as well as in individuals who received different vaccines. Anti-SARS-CoV-2 antibody titers are a reliable indicator of vaccine efficacy.14 In this study, anti-SARS-CoV-2 antibody titers were evaluated after the administration of the BNT162b2 booster dose in individuals who had been primed with different vaccines and differed in age, gender, BMI, comorbidities, and history of COVID-19.
Materials and Methods
Study Population
The study involved 192 residents of the city of Olsztyn (Poland) aged 23–77, of both sexes. The inclusion criterion was vaccination with two doses of BNT162B2 or ChAdOx1-S in the second quarter of 2021, followed by a booster dose of BNT162b2 between 15 December 2021 and 15 January 2022. The exclusion criterion was vaccination at least one dose outside the stated period. The studied population was divided into two groups: persons who were primed with two doses of BNT162B2 and persons who were primed with two doses of ChAdOx1-S.
All participants provided the following information in the survey questionnaire: the date of second and the third vaccine dose, side effects after vaccination, and comorbidities. Then, the two groups were divided into subgroups based on gender (male, female), age (≤50, >50 years), BMI (≤24.9, >24.9 kg/m2), comorbidities (absent, present), and side effects after vaccination (side effects, no side effects). All participants gave their written consent to participate in the study and have blood samples taken.
Blood samples for analyses of antibody titers were collected between 1 and 4 February 2022, ie, 20 to 50 days after the administration of the BNT162B2 booster. Blood was collected into Vacutainer tubes for serum separation by centrifugation (red stopper). Blood was centrifuged for 10 minutes at 2000 × g at room temperature. The serum was separated and stored at −2°C to −8°C until analysis.
Method of Determining Antibody Levels
The Elecsys anti-SARS-CoV-2 S test (Roche S-RBD tAb) was used to assess total anti-SARS-CoV-2 S antibody levels. This test is based on the electrochemiluminescence immunoassay (ECLIA) which is used for in vitro quantitative determination of total antibodies (IgG/IgA/IgM) against the SARS-CoV-2 S-RBD protein in the human serum. The assay was conducted with the use of the fully automated Roche Cobas E411 analyzer (Roche Diagnostics). The ECLIA test is a three-step procedure that uses a recombinant protein representing the receptor binding domain (RBD) of the S antigen in a double-antigen assay format and favors the detection of high-affinity antibodies against SARS-CoV-2. Serum samples are incubated with a mix of biotinylated and ruthenylated RBD antigens to produce double antigen immune-complexes. Streptavidin-coated microparticles are then added, and DAGS complexes bind to the solid phase. The reagent mixture is transferred to the measuring cell where the microparticles are magnetically captured. Electrochemiluminescence is initiated by applying voltage, and it is measured with a photomultiplier. Signal yield increases with the antibody titer. The test has a measuring range of 0.40–250 U/mL (up to 25,000 U/mL in a 1:100 dilution), and concentrations below 0.80 U/mL are regarded as negative, whereas concentrations of ≥0.80 U/mL are regarded as positive.15
Statistical Analysis
Data were processed statistically in GraphPad Prism 8.4.3 for Windows (GraphPad Software, La Jolla, USA). The distribution of results was analyzed by the Shapiro–Wilk test. Student’s t-test was used to determine the normality of distribution, and the Mann–Whitney U-test was used to compare data without normal distribution. The results were presented as median values (minimum – maximum). Statistical significance was established at p < 0.05. Spearman correlation coefficient was used to evaluate the relationships between total anti-SARS-CoV-2 S antibodies levels and clinical-demographic parameters describing the study group.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Warmian-Masurian Medical Chamber in Olsztyn (protocol code 36/2021/VIII; 8 November 2021).
Results
Characteristics of the Study Group
The study involved 192 people who received three doses of the anti-SARS-CoV-2 vaccine. The evaluated population was divided into two groups: persons who received three doses of the Pfizer vaccine (59%) and persons who received two doses of the AstraZeneca vaccine and a Pfizer booster dose (41%). In both groups, most participants were women (84%, 57%), persons older than 50 years (66%, 51%), and persons with history of COVID-19 (60%, 57%). Detailed characteristics of both study groups are presented in Table 1.
 |
Table 1 Characteristics of Study Group |
Comparison of Total Anti-SARS-CoV-2 Antibodies Level Depending on the Sex, Age, BMI, Coexisting Diseases, Post-Vaccination Symptoms and History of COVID-19 in Group of People Who Were Primed with Two Doses of BNT162B2 or ChAdOx1-S
In both groups, total anti-SARS-CoV-2 antibody levels were higher in patients who received Pfizer than AstraZeneca vaccines. Total anti-SARS-CoV-2 antibody titers were higher in women who received Pfizer vaccines than in women who received AstraZeneca vaccines (p=0.0111). In patients both younger (p=0.0189) and older than 50 years, anti-SARS-CoV-2 antibody titers were higher (p=0.0495) after Pfizer than AstraZeneca vaccination. Total anti-SARS-CoV-2 antibody levels were significantly higher in patients with normal BMI after Pfizer than AstraZeneca vaccination (p=0.0375). Patients with side effects from vaccination (p=0.0434) and a history of COVID-19 (p=0.0400) had much higher total anti-SARS-CoV-2 antibody titers after receiving the Pfizer vaccine than the AstraZeneca vaccine. In patients with comorbidities, total anti-SARS-CoV-2 antibody levels were significantly after Pfizer vaccination than AstraZeneca vaccination (p=0.0059). All results are shown in Table 2 and Figure 1.
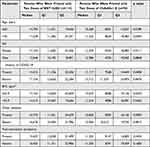 |
Table 2 Comparisions of Results Anti-SARS-CoV-2S Antibodies Concentration in Both Group of Residents with p value |
Correlation of Total Anti-SARS-CoV2 Antibodies in Patients After Pfizer Vaccination with Chosen Clinical Parameters
A positive correlation was also observed between total anti-SARS-CoV2 antibody levels and the patients’ age (p=0.012) Figure 2.
Correlation of Total Anti-SARS-CoV2 Antibodies After AstraZeneca Vaccination with Chosen Clinical Parameters
A negative correlation was noted between total anti-SARS-CoV2 antibody levels and a history of COVID-19 (p = 0.031) Figure 3.
Multiple Regression Analysis
In order to find a relationship between level of anti-SARS-CoV-2S antibodies to the patient’s age, gender and the presence of coexistence diseases we developed multiple linear regression models. Multiple regression analysis showed that the kind of vaccine was associated with the higher level of antibodies (p < 0.001). Moreover, we did not observe association between age (p = 0.5405), gender (p=0.4286) and coexistence diseases (p = 0.9588) to the concentration of antibodies (Table 3).
 |
Table 3 Multiple Regression Analysis of the Level of Anti-SARS-CoV-2S Antibodies |
Discussion
The role of anti-SARS-CoV-2 antibody levels changed since the beginning of the COVID-19 pandemic. At present, antibody titers are not only regarded as indicators of humoral immunity but also used to diagnose infections and perform epidemiological evaluations.16 Antibody levels in the blood provide information about the immune response to stimulation with the pathogenic antigen both during infection and after vaccination. After vaccination or infection, antibody levels may wane over time, but the number of memory cells that rapidly induce the production of antibodies after repeated contact with the pathogen remains unchanged.17 Research into the immune response to the SARS-CoV-2 infection has demonstrated that antibodies targeting the spike (S) protein of SARS-CoV-2 play a key role in protection against the virus.18 In patients with a history of COVID-19, those antibodies persist in the blood for longer than antibodies targeting the nucleocapsid (N) protein of SARS-CoV-2. It should be noted that all vaccines authorized for use in Europe (Pfizer/BioNTech, Moderna, AstraZeneca) rely on the S protein of SARS-CoV-2.19
The Elecsys test quantifies antibodies targeting the S protein of SARS-CoV-2 which are able to neutralize the virus.15 Research has demonstrated that a positive result of the antibody test was correlated with a positive result of a microneutralization assay which assesses the immune response, ie, the extent to which antibodies are able to neutralize the virus.20,21 In the literature, post-vaccination antibody responses were compared after the administration of one dose and two doses of different vaccines against SARS-CoV-2 (AstraZeneca, Pfizer/BioNTech, Moderna). However, very few studies have analyzed differences in anti-SARS-CoV-2 antibody levels after the administration of three doses of different vaccines. A cohort study conducted by Munro et al produced highly important findings. The cited authors analyzed seven different vaccines that have been authorized for use as boosters after two priming doses of BNT162b2 or ChAdOx1. Each booster produced a satisfactory humoral immune response, and the noted differences were not significant.22 Most researchers evaluated changes in antibody levels after the administration of the same vaccine, and a similar analysis was conducted in our previous studies.23,24
Hillus et al compared immune responses after the administration of two doses of the AstraZeneca vaccine, two doses of the Pfizer/BioNTech vaccine, and a mixed AstraZeneca and Pfizer/BioNTech vaccine schedule. They found that heterologous immunization produced a stronger humoral immune response, expressed by higher antibody titers, than homologous immunization.25 Similar observations were made by Groß et al26 and Rose et al27 who reported higher humoral immune responses after mixed vaccine regimens than those noted in this study. However, the cited authors evaluated patients who received two, rather than three doses of the vaccine, as in the present study. Soytas et al demonstrated that a BNT162b2 booster after two priming doses of ChAdOx1 elicited a stronger humoral immune response than three doses of ChAdOx1.28 Keskin et al recommended BNT162b2 as a booster after two priming doses of CoronaVac.29
Atmar et al compared immune responses after mRNA-1273 (Moderna), Ad26.COV2.S (Johnson & Johnson–Janssen), and BNT162b2 (Pfizer/BioNTech) boosters administered in various combinations after two priming doses of the same vaccine brands. They found that heterologous boosters were more effective than homologous boosters.30 However, the cited authors did not evaluate the AstraZeneca vaccine which was compared with the Pfizer/BioNTech vaccine in the current study. Faustini et al found that immunity against SARS-CoV-2 is determined not only by the type of vaccine but also by viral mutations. The group administered BNT162b2 as a booster following two priming doses of the same vaccine had a stronger immune response against the Omicron variant that persons who received two doses of AstraZeneca, followed by a Pfizer/BioNTech booster. In turn, three doses of Pfizer/BioNTech were less effective against the Delta variant that two doses of AstraZeneca, followed by a Pfizer/BioNTech booster.31
The results of this study indicate that persons primed with AstraZeneca produced significantly fewer antibodies after a Pfizer/BioNTech booster than patients who received three Pfizer/BioNTech doses. Despite the fact that antibody levels were not assessed after two vaccine doses, our results seem to confirm the observations made by Aldridge et al32 who found that two priming doses of Pfizer/BioNTech produced significantly more anti-SARS-CoV-2 antibodies than two priming doses of AstraZeneca 21 days (antibody levels were more than ninefold higher) and 3 months (antibody levels were more than fivefold higher) after vaccination. Other researchers also reported that Pfizer/BioNTech elicited a stronger humoral immune response than AstraZeneca on the same days post vaccination.6,33–36
The side effects of anti-SARS-CoV-2 vaccines were also described in the literature. The most common side effects include pain at the injection site, fever, and fatigue. In the current study, side effects were significantly more prevalent in patients subjected to the heterologous rather than the homologous vaccine schedule. The relevant results were reported by Shaw.37 Interesting observations were made by Munro22 who compared the prevalence of side effects 7 days after the administration of different vaccines as boosters. Moderate or severe side effects were reported in patients who received the AstraZeneca booster after two doses of Pfizer/BioNTech, Moderna (m1273) or Curevac (CVn) boosters after two doses of AstraZeneca, and Pfizer/BioNTech after two doses of AstraZeneca. The last combination produced the same results to those noted in our study, and the results generated by the remaining schedules were similar to those reported by other researchers.30,38–40
The humoral immune response can also be affected by other factors, including age, gender, or history of COVID-19. In the present study, anti-SARS-CoV-2 antibody levels were higher in women than in men after the homologous regimen. Similar observations were made by other authors.41,42 Our previous research revealed that antibody titers after two and three vaccines were higher in patients with a history of COVID-19,23 and similar findings were reported by other authors.31,43 However, in the present study, anti-SARS-CoV-2 antibody levels were higher in patients without a history of COVID-19 in both groups. These findings could be attributed to the fact that many individuals have asymptomatic infections and are unaware of their infection status. Our previous study analyzed health care employees who are more likely to undergo a test when they develop symptoms or after they had contact with an infected person, even if they are asymptomatic.
A quantitative anti-SARS-CoV-2 antibody test is a highly helpful in planning the anti-COVID-19 vaccine schedule. In addition to patient interviews, the results of an antibody test provide valuable information about factors that can confer protection against SARS-CoV-2. In view of the rapid emergence of new viral mutations, any information that can help contain COVID-19 is invaluable.
Conclusions
In conclusion, this study demonstrated that a homologous vaccine schedule comprising three Pfizer/BioNTech doses elicits a stronger humoral immune response than a mixed schedule composed of two AstraZeneca doses and a booster dose of Pfizer/BioNTech.
Data Sharing Statement
The full data presented in this study are available on request from the corresponding author.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Warmian-Masurian Medical Chamber in Olsztyn (protocol code 36/2021/VIII; 8 November 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Acknowledgments
The authors would like to thank Roche Diagnostics for providing the analytical kits, and the Director of the Hospital of the Ministry of the Interior and Administration in Olsztyn and Manager of the Medical Diagnostics Laboratory of the Hospital of the Ministry of the Interior and Administration in Olsztyn for providing access to the Cobas e4111 analyzer.
Author Contributions
Conceptualization, B.W.-B. and A.B.; methodology, B.W.-B.; software, G.B.; validation, B.W.-B., A.B. and J.D.; formal analysis, B.W.-B.; investigation, A.B.; resources, B.W.-B.; data curation, G.B.; writing—original draft preparation, B.W.-B.; writing—review and editing, B.W.-B. and J.D.; visualization, B.W.-B.; supervision, J.D.; project administration, E.S.-F.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Roche Diagnostics, Hospital of the Ministry of the Interior and Administration in Olsztyn, University of Warmia and Mazury in Olsztyn.
Disclosure
The authors declare no conflict of interest.
References
1. COVID-19 data explorer - our world in data; 2022. Available from: https://ourworldindata.org/explorers/coronavirus-data-explorer.
2. Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–758. doi:10.1136/postgradmedj-2020-138234
3. Umakanthan S, Patil S, Subramaniam N, Sharma R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines. 2021;9(10):1064. doi:10.3390/vaccines9101064
4. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111.
5. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi:10.1056/NEJMoa2101544
6. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577
7. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389
8. Eyre DW, Lumley SF, Wei J, et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and oxford–AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. 2021;27(10):1516.e7–1516.e14. doi:10.1016/j.cmi.2021.05.041
9. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–1578. doi:10.1056/NEJMc2036242
10. Umakanthan S, Lawrence S. Predictors of COVID-19 vaccine hesitancy in Germany: a cross-sectional, population-based study. Postgrad Med J. 2022;98(1164):756–764. doi:10.1136/postgradmedj-2021-141365
11. Umakanthan S, Senthil S, John S, et al. The protective role of statins in COVID-19 patients: a retrospective observational study. Transl Med Commun. 2021;6. doi:10.1186/s41231-021-00102-4
12. Umakanthan S, Bukelo MM, Gajula SS. The Commonwealth Caribbean COVID-19: regions resilient pathway during pandemic. Front Public Health. 2022;10:1288. doi:10.3389/fpubh.2022.844333
13. Raport zakażeń koronawirusem (SARS-CoV-2) - Koronawirus: informacje i zalecenia - Portal Gov.pl; 2022. Available from: https://www.gov.pl/web/koronawirus/wykaz-zarazen-koronawirusem-sars-cov-2.
14. Lee N, Jeong S, Lee SK, et al. Quantitative analysis of anti-N and anti-S antibody titers of SARS-CoV-2 infection after the third dose of COVID-19 vaccination. Vaccines. 2022;10(7):1143. doi:10.3390/vaccines10071143
15. Elecsys® Anti-SARS-CoV-2; 2022. Available from: https://diagnostics.roche.com/gb/en/products/params/elecsys-anti-sars-cov-2.html.
16. Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2020;184:169–183.e17. doi:10.1016/j.cell.2020.11.029
17. Angyal A, Longet S, Moore SC, et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: a multicentre prospective cohort study. Lancet Microbe. 2021;3:e21–e31. doi:10.1016/S2666-5247(21)00275-5
18. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23:186–193. doi:10.1038/s41590-021-01122-w
19. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6(1):1–13. doi:10.1038/s41541-021-00369-6
20. Hansen CB, Jarlhelt I, Pérez-Alós L, et al. SARS-CoV-2 antibody responses are correlated to disease severity in COVID-19 convalescent individuals. J Immunol. 2021;206:109–117. doi:10.4049/jimmunol.2000898
21. Fujigaki H, Inaba M, Osawa M, et al. Comparative analysis of antigen-specific anti–SARS-CoV-2 antibody isotypes in COVID-19 patients. J Immunol. 2021;206:2393–2401. doi:10.4049/jimmunol.2001369
22. Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, Phase 2 trial. Lancet. 2021;398:2258–2276. doi:10.1016/S0140-6736(21)02717-3
23. Wolszczak-Biedrzycka B, Bié Nkowska A, Dorf J, Leitão JH. Assessment of post-vaccination antibody response eight months after the administration of BNT1622b2 vaccine to healthcare workers with particular emphasis on the impact of previous COVID-19 infection. Vaccines. 2021;9:1508. doi:10.3390/vaccines9121508
24. Wolszczak-Biedrzycka B, Bieńkowska A, Zaborowska JE, Smolińska-Fijołek E, Biedrzycki G, Dorf J. Anti-SARS-CoV-2S antibody levels in healthcare workers 10 months after the administration of two BNT162b2 vaccine doses in view of demographic characteristic and previous COVID-19 Infection. Vaccines. 2022;10(5):741. doi:10.3390/vaccines10050741
25. Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255–1265. doi:10.1016/S2213-2600(21)00357-X
26. Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022;75:103761. doi:10.1016/j.ebiom.2021.103761
27. Rose R, Neumann F, Grobe O, Lorentz T, Fickenscher H, Krumbholz A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022;20(1). doi:10.1186/s12916-021-02231-x
28. Soytas RB, Cengiz M, Islamoglu MS, et al. Antibody responses to COVID-19 vaccines in older adults. J Med Virol. 2021.
29. Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2022;94(1):39–41. doi:10.1002/jmv.27350
30. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–1057. doi:10.1056/NEJMoa2116414
31. Faustini SE, Shields AM, Banham G, et al. Cross reactivity of spike glycoprotein induced antibody against delta and omicron variants before and after third SARS-CoV-2 vaccine dose. medRxiv. 2022;2021:1.
32. Aldridge RW, Yavlinsky A, Nguyen V, et al. Waning of SARS-CoV-2 antibodies targeting the Spike protein in individuals post second dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and risk of breakthrough infections: analysis of the Virus Watch community cohort. medRxiv. 2021;2021. doi:10.1101/2021.11.05.21265968v1
33. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi:10.1016/S0140-6736(21)00432-3
34. Wei J, Stoesser N, Matthews PC, et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6(9):1140–1149. doi:10.1038/s41564-021-00947-3
35. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585–594. doi:10.1056/NEJMoa2108891
36. Ward H, Cooke G, Whitaker M, et al. REACT-2 round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv. 2021;2021. doi:10.1101/2021.02.26.21252512v1
37. Shaw RH, Stuart A, Greenland M, et al. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. doi:10.1016/S0140-6736(21)01115-6
38. Falsey A, Frenck R, Walsh E, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627–1629. doi:10.1056/NEJMc2113468
39. Flaxman A, Marchevsky N, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002). Lancet. 2021;398(10304):981–990. doi:10.1016/S0140-6736(21)01699-8
40. Li J, Hou L, Guo X, et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv. 2021. doi:10.1101/2021.09.03.21263062
41. Ward H, Whitaker M, Flower B, et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun. 2022;13(1):1–6. doi:10.1038/s41467-022-28527-x
42. Poland GA, Ovsyannikova IG, Kennedy RB. Personalized vaccinology: a review. Vaccine. 2018;36(36):5350–5357. doi:10.1016/j.vaccine.2017.07.062
43. Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397(10280):1178–1181. doi:10.1016/S0140-6736(21)00502-X
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

