Back to Journals » International Journal of General Medicine » Volume 15
The Influence of Obstetric Factors on the Occurrence of Pelvic Floor Dysfunction in Women in the Early Postpartum Period
Received 26 December 2021
Accepted for publication 9 March 2022
Published 25 March 2022 Volume 2022:15 Pages 3353—3361
DOI https://doi.org/10.2147/IJGM.S355913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fan Yang,1 Hongyu Liao2
1Department of Surgery, Huazhong University of Science and Technology Hospital, Wuhan, Hubei, 430074, People’s Republic of China; 2Department of Obstetrics and Gynecology, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan, Hubei, 430074, People’s Republic of China
Correspondence: Hongyu Liao, Department of Obstetrics and Gynecology, Hubei Provincial Hospital of Traditional Chinese Medicine, No. 856, Luoyu Road, Wuhan, Hubei, 430074, People’s Republic of China, Tel +86-18086669896, Email [email protected]
Background: This study aimed to analyze the effect of obstetric factors on the development of pelvic floor dysfunction (PFD) in women in the early postpartum period.
Methods: Clinical data of 300 women who were reviewed in our outpatient clinic from July 2016 to December 2019 in the postpartum period were retrospectively analyzed. The occurrence of pelvic organ prolapse (POP) and stress urinary incontinence (SUI) was assessed using the Pelvic Organ Prolapse Quantification System and International Consultation on Incontinence Questionnaire Short Form. Factors affecting the occurrence of PFD in women in the early postpartum period were analyzed using univariate and multifactorial logistic regression models.
Results: A total of 46 cases of POP (15.33%) and 82 of SUI (27.33%) occurred in 300 women at 6– 8 weeks after birth. Unconditional logistic regression confirmed that age ≥ 35 years, vaginal delivery, BMI before delivery ≥ 25 kg/m2, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia were risk factors influencing the occurrence of POP (OR > 1, P < 0.05), whereas age ≥ 35 years, vaginal delivery, perineal tear, protracted or prolonged second stage of labor, fetal macrosomia, and SUI during pregnancy were risk factors influencing the occurrence of SUI (OR> 1, P< 0.05).
Conclusion: Obstetric factors such as age, mode of delivery, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia may increase the risk of developing PFD in women in the early postpartum period; hence, these risk factors should be correctly identified and promptly addressed to prevent the development of PFD.
Keywords: pelvic floor dysfunction, pelvic organ prolapse, stress urinary incontinence, obstetric factors
Introduction
Pelvic floor dysfunction (PFD) refers to a weakening of the supporting tissue of the fascia, neuromuscles, and ligaments of the pelvic floor, which result in relaxation of the supporting tissues of the pelvic floor and dysfunction of the pelvic organs, such as the genital tract, lower urinary tract, and lower gastrointestinal tract, inducing a series of disorders, including pelvic organ prolapse (POP), stress urinary incontinence (SUI), sexual dysfunction, and fecal incontinence (FI).1–3 In terms of physiological structure, the female pelvic floor is composed of multiple layers of fascia and muscles, both of which maintain several physiological functions such as defecation and urination.4 When the supporting tissues such as pelvic floor and fascia become weak, and the pelvic floor and connective tissue become less supportive, frequent urination, abdominal distension and constipation may occur; in severe cases, disorders such as POP, SUI and sexual dysfunction may occur. If not treated and intervened reasonably, the aggravation of the condition may induce several adverse outcomes, which can seriously affect the quality of life of patients.5 Therefore, early diagnosis and prevention of PFD is of great clinical significance.
An epidemiological survey has found that the incidence of PFD-related diseases is approximately 2–40% among adult women, and it has been increasing over the years, posing a threat to public health.6 The occurrence factors of PFD are complex, which are closely related to the mode of delivery, pregnancy and obesity, chronic injury, and the impact of uneven hormone distribution on the nutrient supply to pelvic floor tissues,7 of which delivery and pregnancy are considered the primary causes; hyperextension produced during delivery and pregnancy can weaken fascial and muscle fiber elasticity and induce tearing of the muscle fibers, causing anterior and posterior vaginal wall prolapse and destruction of the pelvic floor supporting structures.8 Meanwhile, changes in pelvic floor function during pregnancy are related to changes in hormones in the body. Zheng et al9 showed that high levels of serum relaxin during pregnancy can act on pelvic floor collagen tissue, which may be one of the pathological mechanisms of PFD during pregnancy and postpartum, and it still takes a certain period for the hormone levels in the body to return to the normal level state after maternal delivery. Therefore, timely identification of risk factors for the occurrence of pelvic floor dysfunction is of great clinical significance for preventing and treating disease and promoting maternal rehabilitation. In this study, the clinical data of 300 women who were reviewed in our hospital (tertiary A-level hospital) at 6–8 weeks after delivery from July 2016 to December 2019 were screened, of which 46 cases of POP occurred, with an incidence rate of 15.33%, and 82 cases of SUI, and the following study was conducted to further analyze the factors influencing the occurrence of postpartum POP and SUI.
Materials and Methods
Clinical Data
Retrospective analysis of clinical data of 300 women who were all first pregnancies, aged 22–42 years (mean age: 28.84±3.15) at 6–8 weeks after delivery, with a mean gestational age of 39.32±1.04 weeks and BMI of 18–29 kg/m2 before delivery (mean: 24.36±2.35 kg/m2), who were admitted to our outpatient clinic from July 2016 to December 2019, was performed. This study was approved by the medical ethics committee of our hospital.
Inclusion Criteria
The inclusion criteria were as follows: ethnic Han; primipara; singleton pregnancy, full-term delivery; review at 6–8 weeks after delivery; no previous history of chronic diseases such as hypertension and diabetes; no previous history of large pelvic mass, pelvic surgery, abdominal surgery, urinary tract disease, renal disease, chronic cough, or chronic constipation; and complete clinical data. Exclusion criteria were as follows: genital tract inflammation, acute pelvic inflammation; multiple pregnancy; abnormal urinalysis results; presence of congenital organ developmental abnormalities, such as urethral malformation, abnormal urethra and bladder development, and urinary fistula; SUI caused by nervous system disease complicated with placental abruption, placenta previa, heart disease, etc.; and patients with hypertension or diabetes.
Criteria for Determination
(1) POP. POP refers to a type of weak pelvic floor supporting tissue caused by various reasons, which results in the descending displacement of pelvic organs and abnormal positioning and functioning of organs. Patients were assessed using the POP-Q,10 with POP-Q ≥ grade I considered to be abnormal. (2) SUI. SUI refers to the involuntary leakage of urine from the ureteral orifice during increased abdominal pressure, such as sneezing, coughing, laughing, or exercise. The degree of SUI was assessed by (ICIQ-SF)11 involving four aspects, that is amount of leakage (0, 2, 4, 6 scores), frequency of leakage (0–5 scores), timing of leakage (not involved in scoring), and impact on daily life (0–10 scores). On a scale of 0–21, a total ICIQ-SF score of ≤7 was determined as mild, 8–13 as moderate, and 14–21 as severe.
Study Methods
Questionnaires were designed to cover age, mode of delivery, BMI before delivery, performance of forceps-assisted delivery, perineal tear (a tear of the skin, mucous membrane, or deep tissue of the soft tissue between the anus and the external genital of a woman during delivery), persistent occipital transverse position, active stagnation or prolongation of the second stage of labor, fetal macrosomia (birth weight of the fetus ≥ 4000 g), and SUI during pregnancy. Trained investigators assisted enrollees in completing the valeted questionnaires, which were collected immediately after completion.
Statistical Analysis
Statistical Product and Service Solutions (SPSS) 22.0 software (IBM, Armonk, NY, USA) was used for data processing and statistical analysis, and the count data are expressed as percentages and were compared using the χ2 test. Logistic regression analysis was used to analyze the significant variables in the univariate analysis, multivariate logistic regression was used to analyze risk factors, and the test level was set at α=0.05. P < 0.05 was considered to indicate statistical significance.
Results
Baseline Data
Among the 300 patients, 225 were aged <35 years. There were 19 women with a history of abortion (including 14 who had abortion once, three who had abortions twice, and two who had abortions more than twice). Regarding BMI before pregnancy, 67 had BMI < 25 kg/m2 and 233 had BMI ≥25 kg/m2. Regarding the mode of delivery, 139 had vaginal delivery and 161 underwent cesarean delivery. In terms of BMI before delivery, 175 had BMI < 25 kg/m2 and 125 had BMI < 25 kg/m2. There were 106 cases of forceps-assisted delivery, 101 cases of perineal tear (including 35 cases of degree I, 40 cases of degree II, 23 cases of degree III, and three cases of degree IV tear), 62 cases of persistent transverse occipital position, 36 cases of protracted or prolonged active phase, 44 cases of protracted or prolonged second stage of labor, 49 cases of fetal macrosomia, and 62 cases of SUI during pregnancy.
Occurrence of PFD
There were 46 cases of POP (15.33%) in 300 women 6–8 weeks after giving birth, including 28 cases of grade I (9.33%), 13 cases of grade II (4.33%), 4 cases of grade III (1.33%), and 1 case of grade IV POP (0.33%). The incidence of SUI was 27.22% in 82 cases, including 20 cases of mild (6.67%), 51 cases of moderate (17.00%), and 11 cases of severe SUI (3.67%) (Figure 1).
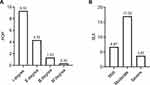 |
Figure 1 The specific proportion of PFD distribution. (A) POP; (B) SUI. |
Analysis of Factors Influencing the Occurrence of Postpartum POP
Univariate Analysis
There were significant differences in age, mode of delivery, pre-delivery BMI, perineal tear, protracted or prolonged second stage of labor, and proportion of fetal macrosomia in the POP group compared with those in the non-POP group (P< 0.05) (Table 1).
 |
Table 1 Univariate Analysis of Factors Influencing the Occurrence of POP (n) |
Multifactorial Analysis
After assigning the occurrence of POP as the dependent variable and the factors showing significant differences in the univariate analysis as independent variables (Table 2), unconditional multinomial logistic regression analyses were performed, which revealed that age ≥ 35 years, vaginal delivery, BMI before delivery ≥ 25 kg/m2, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia were potential risk factors for the occurrence of POP (OR> 1, P< 0.05) (Table 3).
 |
Table 2 Assignment Table of Factors Influencing the Occurrence of POP in the Postpartum Period |
 |
Table 3 Multifactorial Analysis of Factors Influencing the Occurrence of POP in the Postpartum Period |
Analysis of Factors Influencing the Occurrence of Postnatal SUI
Univariate Analysis
Differences in age, mode of delivery, perineal tear, protracted or prolonged second stage of labor, fetal macrosomia, and percentage of SUI during pregnancy were significant between the SUI and non-SUI groups (P< 0.05) (Table 4).
 |
Table 4 Univariate Analysis of Factors Influencing the Occurrence of SUI in the Postpartum Period (n) |
Multifactorial Analysis
After assigning the occurrence of SUI as the dependent variable and the factors with significant differences in the univariate analysis as independent variables (Table 5), unconditional multinomial logistic regression analyses were performed, which revealed age ≥ 35 years, vaginal delivery, perineal tear, protracted or prolonged second stage of labor, fetal macrosomia, and SUI during pregnancy as possible risk factors for the occurrence of SUI (OR> 1, P< 0.05) (Table 6).
 |
Table 5 Assignment Table of Factors Influencing the Occurrence of SUI in the Postpartum Period |
 |
Table 6 Multifactor Analysis Affecting the Occurrence of SUI |
Discussion
The female pelvic floor is composed of fascia and multiple layers of muscles that close the pelvic outlet. The pelvic floor tissue plays an important role in maintaining the normal position of pelvic organs, such as the rectum, bladder, and uterus. When PFL, fascia, and neuromuscular function are impaired, it can lead to the occurrence of PFD-related diseases such as POP and SUI.12
Shao found that obstetric factors such as age, mode of delivery, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia may increase the risk of early PFD in women,13 so these risk factors should be correctly identified and treated in a timely manner to prevent the occurrence of PFD. This study revealed that the incidence of POP and SUI at 6–8 weeks after birth was 15.33% and 27.33% in 300 women, respectively; that unconditional logistic regression analysis confirmed that age ≥ 35 years, vaginal delivery, BMI before delivery ≥ 25 kg/m2, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia may be risk factors affecting the occurrence of POP; and that age ≥ 35 years, vaginal delivery, perineal tear, protracted or prolonged second stage of labor, fetal macrosomia, and SUI during pregnancy may be risk factors affecting the occurrence of SUI, which is similar to the findings of Shao. The findings of this study suggested that regardless of whether a parturient has one or more of these conditions (age ≥ 35 years, SUI during pregnancy, vaginal delivery, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia), she should be informed of the high risk of pelvic floor dysfunction in time, and the pelvic floor rehabilitation training plan should be developed preventively to guide parturient rehabilitation. Possible explanations for the above findings may be described as follows: (1) Age. Progesterone and estrogen levels gradually decrease with age, which tend to affect the structure and function of the urinary tract. Increasing age can lead to atrophy of the urethral mucosa, increasing the incidence of POP and SUI.14 (2) Vaginal delivery and perineal tears. Previous evidence found that Oxford grading of the pelvic floor muscle strength in the vaginal delivery group 6 weeks after delivery was lower than that in the cesarean section group and that the PFIQ-7 questionnaire score and incidence of SUI and POP were higher than those in the cesarean section group.15 This suggested that women undergoing vaginal delivery had a higher incidence of SUI and POP, which had a greater impact on their pelvic floor function. This may be because during vaginal delivery, the sudden increase in abdominal pressure and lowering of the bladder neck cause impaired pressure transmission that can lead to damage to the vaginal wall and fascial supporting structures in the pelvic cavity, which can cause direct or indirect damage to the pelvic floor muscles and nerves, inducing SUI and POP in the postpartum period.16,17 Moreover, ischemia-reperfusion injury during vaginal delivery can lead to fracture and degeneration of elastic fibers and pelvic floor collagen fibers, resulting in damage to the pelvic floor nerves, muscles, and collagen, and cause morphological changes in the anorectal muscle.17 (3) BMI before delivery. Evidence has confirmed that obesity is one of the main high-risk factors for the occurrence of SUI and POP in women.18 As BMI increases during pregnancy, fetal weight also increases, which continues to increase the length of muscle fibers being stretched, aggravates the compression of the pelvic floor muscles, and increases the area of the pelvic diaphragm fissure. When the pressure exceeds the threshold, the cervical, bladder neck, and anorectal junction move down, leading to irreversible damage to the pelvic muscles and muscle fiber rupture, thereby increasing the risk of POP.19,20 (4) Stagnation or prolongation of the second stage of labor. Under pressure, the supporting tissues around the vagina change and fibroblasts undergo metabolic changes and corresponding cytoskeletal changes, often manifested as extreme distortion, expansion, stretching, and deformation. On the other hand, the abnormal second stage of labor can increase the abdominal pressure and pressure of the fetal head on the pelvic floor tissues, causing pelvic floor muscle fatigue and damage to the pelvic floor muscle fibers when the pressure is excessive.21 (5) Fetal macrosomia. Excess neonatal weight can have a direct, long-term gravitational effect on maternal soft tissues (eg, fascia, pelvic floor muscles, etc.), causing ischemia or even rupture of connective tissue, pelvic floor muscle fibers, and nerve tissue, resulting in secondary degeneration, necrosis, atrophy, and vascular lesions. Eventually, this leads to a decrease in the number of muscle fibers and loss of connective tissue support, causing damage to the pelvic floor tissues.22,23
In order to prevent the occurrence of postpartum PFD, this study proposes the following prevention strategies: (1) strengthen the guidance related to pelvic floor function exercise before and during pregnancy, improve women’s knowledge and awareness of pelvic muscles by uptake of enough nutrition while also preventing overnutrition, and develop an exercise program to control pregnancy weight and neonatal weight; (2) fully assess the uncontrollable factors, select the appropriate mode of delivery, closely observe the changes in labor, minimize the duration of the second stage of labor, identify the indications for perineal laterotomy and forceps-assisted delivery, and prevent damage to the muscles and fascia of the pelvic floor; (3) regularly perform postpartum pelvic floor function screening, as well as good contraceptive measures, and receive pelvic floor rehabilitation treatment training to improve women’s reproductive health. Lipschuetz et al24 reported that timely pelvic floor muscle recovery exercises within 10 months after delivery helped to reduce the risk of PFD.
In this study, screening for pelvic floor diseases and analyzing related risk factors was found to be helpful for the early identification of high-risk groups, which is of great significance in preventing the occurrence of PFD in the early postpartum period. However, this study has some limitations. The study had a retrospective and cross-sectional design, and there may be some biases involved with data collection. Further, there was only one source of cases. Few possible factors were included, and the related contents of FI and occurrence of long-term postpartum PFD were not analyzed. In a future study, we will consider the above deficiencies as the focus of the research direction for further analysis. Besides, this study has another deficiency. Due to the retrospective analysis, the pelvic floor functions of all the parturients before delivery were not recorded, leading to the ignorance of the possible influence of prenatal POP on the results. Since only one case in this population developed stage IV POP, relevant medical history was not excluded, resulting in certain data bias. In the next step, we will collect cases from prenatal period and directly track the occurrence of POP from prenatal to postpartum period for data comparison. Furthermore, since it was a preliminary study with insufficient fund and a small number of cases, only two specific pelvic floor dysfunctions with high incidences were selected for comparison. In the next step, we will expand the number of cases and increase the types of related pelvic floor dysfunction to conduct a comprehensive study.
Conclusions
In conclusion, obstetric factors such as age, mode of delivery, perineal tear, protracted or prolonged second stage of labor, and fetal macrosomia may increase the risk of developing PFD; therefore, these risk factors should be correctly identified and promptly addressed to prevent the development of PFD. In the population with age ≥ 35 years, vaginal delivery, perineal tear, protracted or prolonged second stage of labor, fetal macrosomia, SUI during pregnancy with risk factors for PFD, preventive pelvic floor rehabilitation trainings should be carried out after delivery, including the commonly used methods of pelvic floor muscle rehabilitation exercises, low-frequency pulse electrical therapy, and biofeedback therapy, so as to promote the recovery of pelvic floor muscle function as soon as possible.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethical Statement
This study was approved by the ethics committee of Hubei Provincial Hospital of Traditional Chinese Medicine. Signed written informed consents were obtained from all participants before the study. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
We would like to express my gratitude to all those who helped me during the writing of this manuscript. We specially thank to all the peer reviewers and editors for their opinions and suggestions.
Author Contributions
FY and HL designed the study, FY collected the data, HL analyzed the data, FY and HL prepared the manuscript. All authors read and approved the final manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest relevant to this study.
References
1. Wallace SL, Miller LD, Mishra K. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019;31(6):485–493. doi:10.1097/GCO.0000000000000584
2. Kam HA, Yagel S, Eisenberg VH. Ultrasonography in pelvic floor dysfunction. Obstet Gynecol Clin North Am. 2019;46(4):715–732. doi:10.1016/j.ogc.2019.07.006
3. Louis-Charles K, Biggie K, Wolfinbarger A, Wilcox B, Kienstra CM. Pelvic floor dysfunction in the female athlete. Curr Sports Med Rep. 2019;18(2):49–52. doi:10.1249/JSR.0000000000000563
4. Diao X, Ding K, Ma X. Efficacy of normodyne-magnesium sulfate combination treatment on pregnancy-induced hypertension, and its effect on VEGF and Flt-1 levels. Trop J Pharm Res. 2021;20(10):2155–2161. doi:10.4314/tjpr.v20i10.20
5. Bo K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol. 2012;30(4):437–443. doi:10.1007/s00345-011-0779-8
6. Hatam N, Partovi Y, Najibi SR, Marzaleh MA, Najibi SM. Healthcare system functions in Iran and successful developing countries regarding access to universal health coverage: a comparative study. Iran Red Crescent Med J. 2021;23(7):e710.
7. Chen YS, Hua KQ. Research on androgen and its receptors and pelvic floor dysfunction. Foreign Med Sci (Obstet Gynecol Fascicle). 2007;4:285–287.
8. Hilde G, Staer-Jensen J, Siafarikas F, Ellstrom EM, Bo K. Postpartum pelvic floor muscle training and urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2013;122(6):1231–1238. doi:10.1097/AOG.0000000000000012
9. Zheng Y, Li RM, Shuai HL, Wang XY, Luo X. The relationship between serum concentration of relaxin and the pelvic floor function. Chin J Clin Obstet Gynecol. 2011;12(2):96–99.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi:10.1016/S0002-9378(96)70243-0
11. Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322–330. doi:10.1002/nau.20041
12. Bo K, Hilde G, Tennfjord MK, Sperstad JB, Engh ME. Pelvic floor muscle function, pelvic floor dysfunction and diastasis recti abdominis: prospective cohort study. Neurourol Urodyn. 2017;36(3):716–721. doi:10.1002/nau.23005
13. Shao L. Influence of obstetric factors on pelvic floor dysfunction in early postpartum women. Zhejiang J Trauma Surg. 2021;26(4):692–694.
14. Kolberg TM, Hilde G, Staer-Jensen J, Siafarikas F, Engh ME, Bo K. Effect of postpartum pelvic floor muscle training on vaginal symptoms and sexual dysfunction-secondary analysis of a randomised trial. BJOG. 2016;123(4):634–642. doi:10.1111/1471-0528.13823
15. Ghorat F, Esfehani RJ, Sharifzadeh M, Tabarraei Y, Aghahosseini SS. Long term effect of vaginal delivery and cesarean section on female sexual function in primipara mothers. Electron Physician. 2017;9(3):3991–3996. doi:10.19082/3991
16. Venegas M, Carrasco B, Casas-Cordero R. Factors influencing long-term adherence to pelvic floor exercises in women with urinary incontinence. Neurourol Urodyn. 2018;37(3):1120–1127. doi:10.1002/nau.23432
17. Sacomori C, Berghmans B, de Bie R, Mesters IP, Cardoso FP. Predictors for adherence to a home-based pelvic floor muscle exercise program for treating female urinary incontinence in Brazil. Physiother Theory Pract. 2020;36(1):186–195. doi:10.1080/09593985.2018.1482583
18. Hwang JY, Kim BI, Song SH. Parity: a risk factor for decreased pelvic floor muscle strength and endurance in middle-aged women. Int Urogynecol J. 2019;30(6):933–938. doi:10.1007/s00192-019-03913-0
19. Murad-Regadas SM, Rodrigues LV, Furtado DC, et al. The influence of age on posterior pelvic floor dysfunction in women with obstructed defecation syndrome. Tech Coloproctol. 2012;16(3):227–232. doi:10.1007/s10151-012-0831-8
20. Hai-Nan X, Zhi-Jun X, Bao-Xiang L, et al. Investigation of correlation between diameters of pelvic inlet and outlet planes and female pelvic floor dysfunction. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):461–464. doi:10.1016/j.ejogrb.2011.07.034
21. Tennfjord MK, Engh ME, Bo K. The influence of early exercise postpartum on pelvic floor muscle function and prevalence of pelvic floor dysfunction 12 months postpartum. Phys Ther. 2020;100(9):1681–1689. doi:10.1093/ptj/pzaa084
22. Woodley SJ, Lawrenson P, Boyle R, et al. Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2020;5:D7471.
23. Knorst MR, Cavazzotto K, Henrique M, Resende TL. Physical therapy intervention in women with urinary incontinence associated with pelvic organ prolapse. Rev Bras Fisioter. 2012;16(2):102–107. doi:10.1590/S1413-35552012000200004
24. Lipschuetz M, Cohen SM, Liebergall-Wischnitzer M, et al. Degree of bother from pelvic floor dysfunction in women one year after first delivery. Eur J Obstet Gynecol Reprod Biol. 2015;191:90–94. doi:10.1016/j.ejogrb.2015.05.015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
