Back to Journals » The Application of Clinical Genetics » Volume 13
The Influence of IL-1B Gene Polymorphisms on H. pylori Infection and Triple Treatment Response Among Jordanian Population
Authors Shakhatreh MAK, Khabour OF , Alzoubi KH , BaniHani MN , Abu-Siniyeh A , Bashir NA, Sabi SH, Mahafdah M
Received 13 March 2020
Accepted for publication 10 June 2020
Published 2 July 2020 Volume 2020:13 Pages 139—145
DOI https://doi.org/10.2147/TACG.S253778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Muhamad Ali K Shakhatreh,1 Omar F Khabour,1 Karem H Alzoubi,2 Mohammed N BaniHani,3 Ahmed Abu-Siniyeh,4 Nabil A Bashir,5 Salsabeel H Sabi,2 Mahmoud Mahafdah3
1Department of Medical Laboratory Sciences, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Clinical Pharmacy, Jordan University of Science and Technology, Irbid, Jordan; 3Department of General Surgery and Urology, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Taif University, Taif, Kingdom of Saudi Arabia; 5Department of Physiology and Biochemistry, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Muhamad Ali K Shakhatreh Email [email protected]
Background: Helicobacter pylori (H. pylori) is considered the main cause of gastritis, peptic ulcer and gastric carcinoma in the human populations. H. pylori infection influences the secretion level of several proinflammatory cytokines including IL-1β, which encoded by the IL-1B gene.
Objective: The current study aimed to investigate whether IL-1B gene polymorphisms are associated with H. pylori infection among the Jordanian population and responses to triple therapy.
Subjects and Methods: The gastroscopic examination was performed on 412 subjects for H. pylori infection diagnosis, 257 subjects were found to be infected by H. Pylori (positive cases), whereas 155 subjects were uninfected (negative controls). The IL-1B gene T-31C and C3954T polymorphisms were genotyped by PCR-RFLP.
Results: It was found that the T-31C polymorphism has a significant association with H. pylori infection (P< 0.05), and the TT genotype frequency was significantly higher in infected subjects (50.2%) compared to controls (38.7%). On the other hand, no significant association was detected between C3954T SNPs and H. pylori infection among the Jordanian population. In addition, none of the examined polymorphisms were found to influence the responses to triple therapy.
Conclusion: The IL-1B gene T-31C SNP might be associated with an enhanced risk of H. pylori infection among the Jordanian population.
Keywords: IL1B gene, IL-1β, single nucleotide polymorphism, Helicobacter pylori, interleukins
Introduction
One of the main risk factors in human gastrointestinal diseases is Helicobacter pylori (H. pylori), which is gram-negative microaerophiles human pathogen bacteria. Different gastrointestinal diseases such as chronic gastritis, peptic ulcer, and gastric carcinoma have been linked to H. pylori infection.1,2 A strong association was found between H. pylori and the host immune system during progress of gastrointestinal diseases,3 where H. pylori induces the production of many proinflammatory cytokines, such as interleukin 1 beta (IL- 1β), interleukin 10 (IL-10) and tumor necrosis factor-alpha.4
It has been found that certain polymorphisms have an impact on the secretion levels of these cytokines, such as IL-1β, which is encoded by IL-1B gene on the long arm of chromosome 2 at band q13.5 IL-1β is produced by activated macrophages as a proprotein that is proteolytically processed to its active form by caspase 1.6 IL-1β plays an important role as a mediator of inflammatory response and has been shown to be involved in cell proliferation, differentiation, and apoptosis.7 It was also shown to be a vital pro-inflammatory cytokine in the pathogenesis of H. pylori associated gastrointestinal diseases.8 Two key single nucleotide polymorphisms (SNPs) were detected in the IL-1B gene (IL-1B T-31C and IL-1B C3954T), where they were shown to reduce the IL-1B gene expression levels.9 Consequently, reduced IL-1β levels decrease gastric acid secretion,10 which provides a proper environment for H. pylori to provoke an infection in the human stomach. The correlation between IL-1B promoter polymorphisms and H. pylori infection has been studied previously in populations such as Turkish, Brazillian, and Italian.11–13 For example, in meta-analysis studies, a strong synergistic interaction between IL-1β polymorphisms and H. pylori infection in the development of gastric cancer was detected.14–18 In addition, an association between IL-1β polymorphisms and H. pylori infection was reported in Asian, European, and Latin American populations.19–24 Polymorphisms in IL-1B have also been shown to influence the responses to H. pylori triple therapy in different populations including American,25–27 Japanese,28–30 and Korean.31 However, no association between IL-1B and response to triple therapy was detected among the Chinese population.32 Thus, the association between IL-1B and H. pylori infection/response to triple treatment could be population-specific and affected by the genetic background of the individual. Therefore, in the current study, the impact of IL-1β on H. pylori infection and its eradication by triple therapy were examined in the Jordanian population.
Subjects and Methods
Subjects
The study participants were adult patients who underwent a gastroscopic examination at King Abdullah University Hospital (KAUH) for suspected H. pylori infection. Inclusion criteria were patients diagnosed with non-ulcer dyspepsia in the gastroscopy examination with normal finding/mild gastritis. Exclusion criteria were individuals who had gastric surgery or were under medications of anticoagulants, antibiotics, and proton pump inhibitors.33,34 This study procedure was conducted in accordance with the Declaration of Helsinki. The IRB of Jordan University of Science and Technology approved the study (approval ID number: 16/6/14/3141). Written informed consent was obtained from all participants according to the IRB regulations. Of the invited subjects, 412 agreed to participate from which 257 subjects were found to be H. pylori positive (cases). The rest 155 patients were found to be H. pylori negative and were considered as control group.35 Demographics of the participants and medical information were obtained using a structured form and medical files.
Diagnosis of H. pylori Infection
To diagnose H. pylori infection, biopsy samples from the gastric antrum were obtained from all participants by an expert physician. Biopsy samples were directly sent to the pathological examination to diagnose H. pylori infection.
In brief, samples were stained using Harris’ hematoxylin-eosin and Giemsa staining as previously described.36 Stained sections were evaluated for the presence of H. pylori infection by two experts under the oil immersion objective (1,000×). Positive diagnosis of H. pylori was reported for cases that showed typical H. pylori morphology (comma/S-shaped bacilli of 2 to 4 μm long and 0.5 to 1 μm thick) attached to the surface of the cells or free in the mucous layer, and forming small colonies for the least. Otherwise, the diagnosis was considered negative.37 To validate the result, a positive control tissue and a negative control tissue were included with every run.
Therapeutic Regimens
Patients who participated in the study were revisited after taking their standard H. pylori eradication therapy as per hospital procedures. The used triple eradication therapy consisted of clarithromycin 500 mg twice daily, amoxicillin 1g twice daily, and PPIs twice daily, for 14 days. Responsiveness to therapy was defined as improvement in clinical symptoms and reduction in IgG serum levels 3 months after treatment, which are considered as alternative less invasive methods for detection of responsiveness of treatment compared to endoscopy.38
Isolation of Genomic DNA
DNA was isolated from whole blood (collected in EDTA tubes) using a Promega kit (Madison, USA) according to the manufacturer’s instructions.39,40 The quality and quantity of isolated DNA were evaluated using a Bio-Rad SmartSpect_3000 instrument (Hertfordshire, UK). Samples were stored at - 20° C until used for the genotyping of IL-1B SNPs.
Genotyping of IL-1B Polymorphisms
The IL-1B T-31C and C3954T polymorphisms were genotyped by polymerase chain reaction-restriction fragment length polymorphisms (PCR-RFLP) as previously described.41–43 The PCR reaction was done using Eppendorf thermocycler (Hamburg, Germany). Amplification was performed in 0.2 µL PCR tubes containing 50 ng of DNA, 1 µ of each primer and ready to use PCR master mix obtained from Promega (Madison, USA). Table 1 shows primer used, PCR conditions and restriction conditions. Digestion of the PCR products of the IL-1B T-31C and C3954T polymorphisms was mediated by the AluI and Taq I restriction enzymes (New England Biolabs, Beverly, CA, USA) respectively. The restriction reaction contained 10 µg of the PCR, 10 units of the restriction enzyme and appropriate amounts of deionized H2O and the smart cut buffer (supplied with the kit). The reaction mixture was incubated in the water bath at 37°C for 16 hours. The reaction was then heat inactivated by incubation at 80°C for 20 minutes. Electrophoresis conditions were 1% TBE buffer, 2% agarose, 80 volts for 45 minutes.
 |
Table 1 PCR and Restriction Conditions of IL-1B Gene Polymorphisms |
Statistical Analysis
Analysis of the association between IL-1B polymorphisms and H. pylori infection/response to triple treatment was achieved using SNPstat statistical software. G. Power version 3.0.10 was used for power analysis (Franz Faul, Universität Kiel, Kiel, Germany). Distribution of examined SNPs according to Hardy–Weinberg equilibrium was also examined using SNPstat. A P value of less than 0.05 was used to infer statistical significance.
Results
Table 2 shows the demographics of the study subjects. The mean age of the control group was 43 years and for the cases was 41 years. Male participants represented 51.3% of the control group and 49% of the cases group. No differences in medical history and other demographics were detected between the two groups (Table 2).
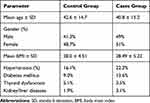 |
Table 2 Demographics of Participants |
The association between SNPs of the IL1B gene (C3954T and T-31C) and H. pylori infection is shown in Table 3. A significant association between T-31C and H. pylori infection was detected (P<0.05). The frequency of the TT genotype was significantly higher in cases (50.2%) compared to controls (38.7%). Thus, homozygotes TT state was associated with higher H. pylori infection risk compared to other genotypes. In addition, the T allele was enriched in cases (66%) compared to controls (60%). With respect to C3954T SNP, no significant association was detected with H. pylori infection (P>0.05, Table 3).
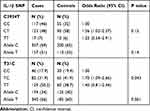 |
Table 3 Association Between IL1B SNPs and H. pylori Infection |
Haplotype analysis of C3954T and T-31C SNPs is shown in Table 4. Among all examined haplotypes, the TC haplotype was present in 2.58% of cases compared to 8.62% of controls (P<0.005). Thus, TC haplotype seems to lower the risk of H. pylori infection. No significant association between the rest of the haplotype and H. pylori infection was observed (P>0.05) (Table 4).
 |
Table 4 Analysis of IL-1B Haplotypes of the Examined Polymorphisms |
Among the 257 patients, 41 patients showed resistance to triple therapy (resistance rate of 16%). The IL-1B C3954T and T-31C SNPs were tested for their association with triple therapy response (Table 5). The results showed no association between examined SNPs and response to triple therapy (P>0.05). In addition, none of the haplotypes were associated with response to triple therapy (data not shown). Thus, IL-1B SNPs might not be associated with response to triple therapy treatment of H. pylori infection among Jordanians.
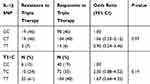 |
Table 5 IL-1B SNPs Association with Triple Therapy Response |
Discussion
The aim of the study is to assess whether there is an association between IL-1B gene polymorphisms and H. pylori infection in the Jordanian population. Current data revealed a significant association between IL-1B - T-31C genetic polymorphism and H. pylori infection. The TT genotype was higher in H. pylori positive subjects compared to the control ones.
The study findings are consistent with previous studies that reported a role for IL-1B-C31T SNP in the development of H. pylori-related diseases, particularly gastroduodenal diseases.12,44 Similarly, IL-1B T-31C has a significant association with H. pylori infection among Uzbeks population.45 In a case–control study among the Chinese population that was conducted on 392 patients, an association between IL-1B T-31C and gastric cancer was reported, which further augmented by H. pylori.46 In the Brazilian population, a strong association was found between IL-1B −31TT and H. pylori infection.47 Thus, IL-1B T-31C seems to be associated with H. pylori infection in several populations worldwide. The current study also reported lack of association between C3954T SNP and H. pylori infection in the Jordanian population. Similar findings were reported among Brazilian population.12,47 The mechanism by which IL-1B T-31C polymorphism might modulate H. pylori infection needs to be investigated. This might involve changes in IL-1B protein level in the gastric epithelium and the subsequent modulation of gastric acid secretion.12,48-50
The clinical significance of IL-1B T-31C polymorphism in the modulation of infection risk has been examined in several studies. For example, the TT genotype at IL-1B-31 has been showed to increase the risk of chronic HBV infection.51
Our haplotype analysis of T-31C and C3954T SNPs showed that the frequency of TC haplotype was lower in cases than in controls, which indicate that TC haplotype might reduce the risk of H. pylori infection among Jordanian population. More studies are required to confirm the present findings.
Among all patients, only a small number (16%) showed resistance to H. pylori triple therapy, and current results did not show any association between response to triple therapy, and IL-1B SNPs/haplotypes. The role of IL-1B polymorphisms in H. pylori eradication has been studied in different populations and came up with controversial findings. For example, in Japanese population, IL-1B polymorphisms were found to influence triple therapy of H. pylori infection.28 However, no effect for IL-1B polymorphisms was detected on the eradication of H. pylori in the Chinese population.32 Thus, H. pylori eradication might be influenced by different factors, such as lifestyle behavior, bacterial resistance to antibiotics, treatment adherence, and bacterial virulence factors. This might explain the reason for the inconsistency observed in the different studies.10,52
In conclusion, current results indicated that the IL-1B T-31C polymorphism was significantly correlated to H. pylori infection among Jordanian, while no association was detected between H. pylori infection and IL-1B C3954T polymorphism. Examining other cytokine polymorphisms such as IL-8 SNPs that previously has been reported to associate with H. pylori infection53,54 is strongly recommended in future studies.
Acknowledgments
This project was supported by Scientific Research Funds (SRF), Ministry of Higher Education and Scientific Research, Amman, Jordan, Project number (MPH/2/19/2016). In addition, the authors thank Jordan University of Science and Technology for its support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. de Korwin J-D, Ianiro G, Gibiino G, Gasbarrini A. Helicobacter pylori infection and extragastric diseases in 2017. Helicobacter. 2017;22:e12411. doi:10.1111/hel.12411
2. Venerito M, Vasapolli R, Rokkas T, Malfertheiner P. Gastric cancer: epidemiology, prevention, and therapy. Helicobacter. 2018;23:e12518. doi:10.1111/hel.12518
3. Kumar S, Dhiman M. Inflammasome activation and regulation during Helicobacter pylori pathogenesis. Microb Pathog. 2018;125:468–474. doi:10.1016/j.micpath.2018.10.012
4. de Brito BB, da Silva FA, de Melo FF. Role of polymorphisms in genes that encode cytokines and Helicobacter pylori virulence factors in gastric carcinogenesis. World J Clin Oncol. 2018;9(5):83–89. doi:10.5306/wjco.v9.i5.83
5. Akdis M, Aab A, Altunbulakli C, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2016;138(4):984–1010. doi:10.1016/j.jaci.2016.06.033
6. Speaker KJ, Fleshner M. Interleukin-1 beta: a potential link between stress and the development of visceral obesity. BMC Physiol. 2012;12(1):8. doi:10.1186/1472-6793-12-8
7. Maedler K, Dharmadhikari G, Schumann DM, Størling J. Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin Biol Ther. 2009;9(9):1177–1188. doi:10.1517/14712590903136688
8. El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48(6):743–747. doi:10.1136/gut.48.6.743
9. Ren H-Y, Wen L-S, Geng Y-H, et al. Association between IL-1B gene polymorphisms and Helicobacter pylori infection: a meta-analysis. Microb Pathog. 2019;137:103769. doi:10.1016/j.micpath.2019.103769
10. Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp. 2009;57(1):45–56. doi:10.1007/s00005-009-0007-z
11. Akcil G, Dogan I, Cengiz M, et al. The role of interleukin-1 gene polymorphisms and Helicobacter pylori in gastroesophageal reflux disease. Turkish J Gastroenterol. 2015;25(1):81–85. doi:10.5152/tjg.2014.6512
12. Ramis IB, Vianna JS, Halicki PCB, et al. Relationship of interleukin-1B gene promoter region polymorphism with Helicobacter pylori infection and gastritis. J Infect Dev Ctries. 2015;9(10):1108–1116. doi:10.3855/jidc.6123
13. Figura N. Helicobacter pylori infection and gastric carcinoma: not all the strains and patients are alike. World J Gastrointest Oncol. 2016;8(1):40. doi:10.4251/wjgo.v8.i1.40
14. Zeng Z-R. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut. 2003;52(12):1684–1689. doi:10.1136/gut.52.12.1684
15. Yamada S, Matsuhisa T, Makonkawkeyoon L, Chaidatch S, Kato S, Matsukura N. Helicobacter pylori infection in combination with the serum pepsinogen I/II ratio and interleukin-1β-511 polymorphisms are independent risk factors for gastric cancer in Thais. J Gastroenterol. 2007;41(12):1169–1177. doi:10.1007/s00535-006-1951-6
16. Park M-J, Hyun M-H, Yang J-P, Yoon J-M, Park S. Effects of the interleukin-1β-511 C/T gene polymorphism on the risk of gastric cancer in the context of the relationship between race and H. pylori infection: a meta-analysis of 20,000 subjects. Mol Biol Rep. 2015;42(1):119–134. doi:10.1007/s11033-014-3748-7
17. Chen B, Luo M, Zhou X, Lv Y, Su G. Correlation between interleukin-1β-511 C/T polymorphism and gastric cancer in Chinese populations: a meta-analysis. Med Sci Monit. 2016;22:1742–1750. doi:10.12659/MSM.895771
18. Ying H-Y, Yu B-W, Yang Z, et al. Interleukin-1B 31 C>T polymorphism combined with Helicobacter pylori-modified gastric cancer susceptibility: evidence from 37 studies. J Cell Mol Med. 2016;20(3):526–536. doi:10.1111/jcmm.12737
19. Santos JC, Ladeira MSP, Pedrazzoli J, Ribeiro ML. Relationship of IL-1 and TNF-α polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Brazilian J Med Biol Res. 2012;45(9):811–817. doi:10.1590/S0100-879X2012007500099
20. Tahara T, Shibata T, Yamashita H, et al. Synergistic effect of IL-1β and TNF-α polymorphisms on the H. pylori-related gastric pre-malignant condition. Hepatogastroenterology. 2012;59(120):2416–2420. doi:10.5754/hge10605
21. Kim JJ, Kim N, Hwang S, et al. Relationship of interleukin-1β levels and gastroesophageal reflux disease in Korea. J Gastroenterol Hepatol. 2013;28(1):90–98. doi:10.1111/j.1440-1746.2012.07274.x
22. Zhao Y. Association between TNF-α and IL-1β genotypes vs Helicobacter pylori infection in Indonesia. World J Gastroenterol. 2013;19(46):8758. doi:10.3748/wjg.v19.i46.8758
23. Sun X, Xu Y, Zhang F, Jing T, Han J, Zhang J. Association between the IL1B −31C > T polymorphism and Helicobacter pylori infection in Asian and Latin American population: a meta-analysis. Microb Pathog. 2015;86:45–52. doi:10.1016/j.micpath.2015.07.010
24. Wang Y-M, Li Z-X, Tang F-B, et al. Association of genetic polymorphisms of interleukins with gastric cancer and precancerous gastric lesions in a high-risk Chinese population. Tumor Biol. 2016;37(2):2233–2242. doi:10.1007/s13277-015-4022-x
25. Hwang I-R, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1β production in Helicobacter pylori infection. Gastroenterology. 2002;123(6):1793–1803. doi:10.1053/gast.2002.37043
26. Sugimoto M, Furuta T, Shirai N, et al. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007;8(16):2701–2717. doi:10.1517/14656566.8.16.2701
27. Sugimoto M, Furuta T, Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J Gastroenterol Hepatol. 2009;24(11):1725–1732. doi:10.1111/j.1440-1746.2009.06047.x
28. Take S, Mizuno M, Ishiki K, et al. Interleukin-1beta genetic polymorphism influences the effect of cytochrome P 2C19 genotype on the cure rate of 1-week triple therapy for Helicobacter pylori infection. Am J Gastroenterol. 2003;98(11):2403–2408. doi:10.1111/j.1572-0241.2003.07707.x
29. Furuta T. Polymorphism of interleukin-1? Affects the eradication rates of by triple therapy. Clin Gastroenterol Hepatol. 2004;2(1):22–30. doi:10.1016/S1542-3565(03)00288-X
30. Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, ISHIZAKI T. Influences of proinflammatory and anti-inflammatory cytokine polymorphisms on eradication rates of clarithromycin-sensitive strains of Helicobacter pylori by triple therapy. Clin Pharmacol Ther. 2006;80(1):41–50. doi:10.1016/j.clpt.2006.03.007
31. Kim N, Cho S-I, Yim J-Y, et al. The effects of genetic polymorphisms of IL-1 and TNF-A on Helicobacter pylori-induced gastroduodenal diseases in Korea. Helicobacter. 2006;11(2):105–112. doi:10.1111/j.1523-5378.2006.00384.x
32. Zhang L, Mei Q, Li QS, Hu YM, Xu JM. The effect of cytochrome P2C19 and interleukin-1 polymorphisms on H. pylori eradication rate of 1-week triple therapy with omeprazole or rabeprazole, amoxycillin and clarithromycin in Chinese people. J Clin Pharm Ther. 2010;35(6):713–722. doi:10.1111/j.1365-2710.2009.01140.x
33. Saito Y. First-line eradication for Helicobacter pylori -positive gastritis by esomeprazole-based triple therapy is influenced by CYP2C19 genotype. World J Gastroenterol. 2015;21(48):13548. doi:10.3748/wjg.v21.i48.13548
34. Phiphatpatthamaamphan K, Vilaichone R, Siramolpiwat S, et al. Effect of IL-1 polymorphisms, CYP2C19 genotype and antibiotic resistance on Helicobacter pylori eradication comparing between 10-day sequential therapy and 14-day standard triple therapy with four-times-daily-dosing of amoxicillin in thailand: a prospective randomized study. Asian Pacific J Cancer Prev. 2016;17(4):1903–1907. doi:10.7314/APJCP.2016.17.4.1903
35. BaniHani MN, Khabour OF, Alzoubi KH, et al. The association between ABCB1 C1236T/C3435T SNPs and H. pylori infection among Jordanians. Genes. 2020;11(1):63. doi:10.3390/genes11010063
36. Kuo C-H, Lu C-Y, Shih H-Y, et al. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J Gastroenterol. 2014;20(43):16029. doi:10.3748/wjg.v20.i43.16029
37. Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015;3(1):10. doi:10.3978/j.issn.2305-5839.2014.11.03
38. Atherton JC. Non-endoscopic tests in the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11(Suppl 1):11–20. doi:10.1046/j.1365-2036.11.s1.3.x
39. Alzoubi KH, Khabour OF, Hassan RE, Qarqaz F, Al-azzam S, Mhaidat N. The effect of genetic polymorphisms of RARA gene on the adverse effects profile of isotretinoin-treated acne patients. Int J Clin Pharmacol Ther. 2013;51(08):631–640. doi:10.5414/CP201874
40. Khabour OF, ABU-RUMEH L, AL-JARRAH M, JAMOUS M, ALHASHIMI F. Association of adiponectin protein and ADIPOQ gene variants with lumbar disc degeneration. Exp Ther Med. 2014;8(4):1340–1344. doi:10.3892/etm.2014.1909
41. Hefler LA, Tempfer CB, Unfried G, et al. A polymorphism of the interleukin-1β gene and idiopathic recurrent miscarriage. Fertil Steril. 2001;76(2):377–379. doi:10.1016/s0015-0282(01)01914-8
42. Gehmert S, Velapatiño B, Herrera P, et al. Interleukin-1 beta single-nucleotide polymorphism’s C allele is associated with elevated risk of gastric cancer in Helicobacter pylori-infected Peruvians. Am J Trop Med Hyg. 2009;81(5):804–810. doi:10.4269/ajtmh.2009.08-0494
43. Haji-Aghamohammadi AA, Bastani A, Miroliaee A, Oveisi S, Safarnezhad S. Comparison of levofloxacin versus clarithromycin efficacy in the eradication of Helicobacter pylori infection. Casp J Intern Med. 2016;7(4):267–271.
44. Rad R. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53(8):1082–1089. doi:10.1136/gut.2003.029736
45. Abdiev S, Ahn KS, Khadjibaev A, et al. Helicobacter pylori infection and cytokine gene polymorphisms in Uzbeks. Nagoya J Med Sci. 2010;72(3–4):167–172.
46. He B-S, Pan Y-Q, Xu Y-F, Zhu C, Qu -L-L, Wang S-K. Polymorphisms in interleukin-1B (IL-1B) and interleukin 1 receptor antagonist (IL-1RN) genes associate with gastric cancer risk in the Chinese population. Dig Dis Sci. 2011;56(7):2017–2023. doi:10.1007/s10620-010-1557-y
47. Caleman Neto A, Rasmussen LT, de LRW, et al. Gene polymorphism of interleukin 1 and 8 in chronic gastritis patients infected with Helicobacter pylori. J Venom Anim Toxins Incl Trop Dis. 2014;20(1):17. doi:10.1186/1678-9199-20-17
48. Guo YS, Fujimura M, Lluis F, Tsong Y, Greeley GH, Thompson JC. Inhibitory action of peptide YY on gastric acid secretion. Am J Physiol. 1987;253(3 Pt 1):G298–302. doi:10.1152/ajpgi.1987.253.3.G298
49. Beales IL, Calam J. Interleukin 1β and tumour necrosis factor α inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42(2):227–234. doi:10.1136/gut.42.2.227
50. Chang Y-W, Jang J-Y, Kim N-H, et al. Interleukin-1B (IL-1B) polymorphisms and gastric mucosal levels of IL-1beta cytokine in Korean patients with gastric cancer. Int J Cancer. 2005;114(3):465–471. doi:10.1002/ijc.20724
51. Huebinger RM, Smith AD, Zhang Y, et al. Variations of the lung microbiome and immune response in mechanically ventilated surgical patients. PLoS One. 2018;13(10):e0205788. doi:10.1371/journal.pone.0205788
52. Kamada T, Haruma K, Komoto K, et al. Effect of smoking and histological gastritis severity on the rate of H. pylori eradication with omeprazole, amoxicillin, and clarithromycin. Helicobacter. 1999;4(3):204–210. doi:10.1046/j.1523-5378.1999.99299.x
53. Kang HY, Kim SG, Lee MK, Kim JS, Jung HC, Song IS. Effect of Helicobacter pylori eradication according to the IL-8-251 polymorphism in Koreans. J Korean Med Sci. 2012;27(10):1202–1207. doi:10.3346/jkms.2012.27.10.1202
54. Yin Y-W, Hu A-M, Sun -Q-Q, et al. Association between interleukin-8 gene −251 T/A polymorphism and the risk of peptic ulcer disease: a meta-analysis. Hum Immunol. 2013;74(1):125–130. doi:10.1016/j.humimm.2012.09.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
