Back to Journals » Clinical Interventions in Aging » Volume 13
The influence of carbon dioxide field flooding in mitral valve operations with cardiopulmonary bypass on S100ß level in blood plasma in the aging brain
Authors Listewnik M , Kotfis K , Ślozowski P , Mokrzycki K , Brykczyński M
Received 21 June 2018
Accepted for publication 27 July 2018
Published 25 September 2018 Volume 2018:13 Pages 1837—1845
DOI https://doi.org/10.2147/CIA.S177356
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Mariusz Listewnik,1 Katarzyna Kotfis,2 Paweł Ślozowski,1 Krzysztof Mokrzycki,1 Mirosław Brykczyński1
1Department of Cardiac Surgery, Pomeranian Medical University, Szczecin, Poland; 2Department of Anesthesiology, Intensive Therapy and Acute Intoxications, Pomeranian Medical University, Szczecin, Poland
Introduction: The risk of air microembolism during cardiopulmonary bypass (CPB) is high and influences the postoperative outcome, especially in elderly patients. The use of carbon dioxide (CO2) atmosphere during cardiac surgery may reduce the risk of cerebral air microembolism. The aim of our study was to assess the influence of CO2 field flooding on microembolism-induced brain damage assessed by the level of S100ß protein, regarded as a marker of brain damage.
Materials and methods: A group of 100 patients undergoing planned mitral valve operation through median sternotomy using standard CPB was recruited for the study. Echocardiography was performed prior to and after the CPB. CO2 insufflation at 6 L/minute was conducted in the study group. Blood samples for S100ß protein analysis were collected after induction of anesthesia, 2 hours after aorta de-clamping, and 24 hours after operation.
Results: The S100ß level in blood plasma did not differ significantly between the study and the control group (0.13±0.08 µg/L, 1.12±0.59 µg/L, and 0.26±0.23 µg/L and 0.18±0.19 µg/L, 1.31±0.62 µg/L, and 0.23±0.12 µg/L, P=0.7, 0.14, and 0.78). The mean increase of the S100ß concentration was 13% lower in the group with CO2 protection than in the control group (0.988 µg/L vs 1.125 µg/L), although statistically insignificant. Tricuspid valve annuloplasties (TVAs) had significant impact on the increase in S100ß concentration in the treatment group after 24 hours (TVA [-] 0.21±0.09 vs TVA [+] 0.42±0.42, P=0.05). In patients >60 years, there were significant differences in the S100ß level 2 and 24 hours after the procedure (1.59±0.682 µg/L vs 1.223±0.571 µg/L, P=0.048, and 0.363±0.318 µg/L vs 0.229±0.105 µg/L, P=0.036) as compared with younger patients.
Conclusion: The increase in S100ß concentration was lower in the group with CO2 protection than in the control group. Age and an addition of TVA significantly influenced the level of S100ß concentration in the tests performed 2 hours after aortic clamp release.
Keywords: mitral valve surgery, S100ß, air microembolism, cardiopulmonary bypass, carbon dioxide insufflation
Introduction
Multiple co-existing mechanisms of neurological injury after cardiac operations have been described, especially in elderly patients with impaired vasomotor function or severe cerebrovascular disease. These include microembolization and macroembolization of the atheromatous plaque, air or clot from the aorta or from the cardiopulmonary bypass (CPB) circuit, and hypoperfusion of the brain when on CPB, not to forget hypoperfusion associated with the systemic inflammatory response.1 Risk and prevention of air embolism associated with the CPB appears to be a real problem known almost from the beginning of cardiac surgery.2,3
To evacuate air from the heart after open heart surgery, standard surgical maneuvers are used including filling and venting of the left ventricle, heart and lungs’ ballottement, mechanical ventilation, placing the patient in Trendelenburg position, and needle aspiration. Despite these efforts, a significant amount of air remains. Flooding the surgical field with carbon dioxide (CO2) reduces the incidence of intracardiac air by approximately 85% due to the difference in CO2 density (1.5 times that of air) and solubility (360 times more soluble than air), which leads to the preferential filling of the dependent areas of surgical field with CO2 and a reduction in size of microemboli.4,5 Transesophageal echocardiography (TEE) shows that, when CO2 is used, the intracardiac air disappears rapidly (within minutes).4 Obviously, the use of CO2 atmosphere may cause an increase in the partial pressure of CO2 and a decrease in the pH and base excess (BE).
Massive air embolism occurring due to a medical error of the perfusionist or the surgeon is extremely rare, but microembolism during open heart surgery despite using de-airing procedures is a common situation. Results of the former can be observed as stroke or death of the patient immediately during or after the operation. The latter is difficult to observe because the lesions, if they occur, are very small and may appear days, weeks, or even months later, for example, postoperative delirium, changes in personality, neurocognitive dysfunction, and intensification of dementia. However, it is possible that these changes are connected not with the operation itself but only with the status of the patient, their age, sex, severity of the disease, or genetic factors.6
The use of CO2 atmosphere during cardiac surgery has become standard practice in many cardiac surgery departments. CO2 is a natural gas, 25 times more soluble in blood than air. This means that bubbles of CO2 dissolve in blood more quickly than air bubbles.7–9 It can be hypothesized that the shorter the time during which the gaseous microemboli dissolve and the fewer their number, the smaller the postoperative brain injury. So far, psychological tests and ultrasound examination were used to assess the size and impact of microemboli on the brain. However, there is very little direct evidence to demonstrate any real benefit of this practice. In order to determine the impact of the microemboli release on the brain, we used S100ß protein considered to be a specific marker of brain damage. Recently, this method was used by other authors in the evaluation of neurological changes after coronary artery by-pass grafting (CABG).10,11
The S100 proteins are a family of small, dimeric multi-genic calcium-binding proteins comprising various combinations of A1 and B subunits, with a molecular weight of 10–12 kDa and a biological half-life of approximately 2 hours, first isolated from central nervous tissue in 1965. In the biologically active form, called S-100A1-A1, this protein is predominantly found in the heart, kidney, and striated muscles, as S-100A1-B in glial cells, melanocytes, adipocytes, chondrocytes, and epidermal Langerhans cells and S-100BB in astrocytes and Schwann cells.12,13
The neuronal destruction and destabilization of the blood–brain barrier are accompanied by a release of S100ß protein into the blood. The S100ß is measurable within minutes after event and can be detected for an extended period in the blood. The S100ß is removed from the serum by the renal clearance pathway, with a half-life of 20–25 minutes. S100ß protein level is a sensitive and specific marker for brain injury after stroke, head trauma, and brain damage caused by circulatory arrest or cardiac surgery with CPB.14–17 The role of serial measurement of serum S100ß protein has already been described. The protein has been proven to be a good marker of brain damage during CPB.18
Aim
The aim of our study was to measure the level of S100ß in blood plasma to assess the influence of CO2 field flooding on microembolism-induced brain damage.
Materials and methods
To enable this research, we obtained a grant from the Ministry of Science and Higher Education in Poland (NN403289536). An approval was obtained from the ethics committee of the Pomeranian Medical University in Szczecin, Poland (BN-001/6/08). The study was performed according to the Declaration of Helsinki. All patients received detailed written information about the background, aim, and methods of the study, and only those who signed an informed consent form were included in the study.
Study group
The study group consisted of adult patients scheduled for a planned mitral valve operation through median sternotomy with the use of CPB. We screened all patients between March 2008 and February 2011 and included 100 consecutive patients. The inclusion criteria were as follows: 1) age above 18 years, 2) mitral valve operation, 3) ability to understand Polish language, 4) ability to sign an informed consent, 5) no previous dementia, and 6) no history of psychiatric disorder. There were 357 patients undergoing mitral annuloplasty or replacement. The exclusion criteria were as follows: 1) patient refusal (68 cases), 2) reoperation (29 cases), 3) history of stroke (23 cases), 4) active endocarditis (16 cases), 5) concomitant aortic valve or aortic arch procedure (90 cases), and 6) mini-invasive mitral valve operation through right thoracotomy (30 cases). A concomitant procedure of tricuspid valve annuloplasty (TVA), or CABG performed as required, was not an exclusion criterion. Patients qualified for the operation of the mitral valve through median sternotomy were randomized into the following two groups: treatment group and control group.
Technical procedure description
In all cases, a routine intraoperative TEE was performed prior to and after the CPB. Standard CPB technique with membrane oxygenator, nonpulsatile flow, two venous cannulas, and mean arterial pressure control was used. Patients were normothermic with continuous blood heating at 36.6°C. All patients underwent general anesthesia with endotracheal intubation according to a local protocol, with the use of etomidate and fentanyl at induction, muscle relaxation with pancuronium, and maintenance of anesthesia with sevoflurane and fentanyl. The standard cold crystalloid intermittent cardioplegic solution type St Thomas II was used. The de-airing procedures after restarting the heart were conducted with vent suction from the left atrium and ascending aorta under the control of TEE. Effort was undertaken to check for any residual air during the de-airing procedures, and the de-airing time was precisely planned to ensure cessation of any additional air bubbles from the pulmonary veins. All operations were performed by the same surgeon. Chest tubes were removed on postoperative day 1 or 2.
CO2 insufflation devices
For CO2 insufflation, a standard Redon drain was used consisting of a flexible PVC tube with an inner diameter of 2.5 mm and perforated at 10.0 cm toward the end part. Distal lumen was occluded with a typical Luer stopper. The perforated part of the drain tube was fixed with two sutures to the left side of the pericardium just below the left branch of the retractor and covered with gauze. The flow of CO2 was measured with a standard flowmeter for medical CO2. The flow of CO2 at 6 L/minute started 1 minute before opening the left atrium and ended after its closure. An increase in the partial pressure of CO2, consequent decrease in the pH and BE, was corrected by the perfusionist with a minimal increase in the volume of air added to the gas exchanger.
S100ß protein measurements
To analyze the changes in S100ß protein level, blood was collected at three points during the operation. The first 10 mL of blood sample (P1) was taken just after induction, intubation, and installation of the central venous line. The next sample (P2) was taken in the postoperative unit 2 hours after de-clamping of the aorta. The third sample (P3) was collected 24 hours after operation. Blood was stored at 4°C, allowed to clot, and after centrifugation (10 minutes, 3,000 rpm) collected in 4 mL test tube Cryo (SARSTEDT AG & Co. KG, Nümbrecht, Germany). Samples of serum were stored at −30°C for later analysis. Protein S100ß was analyzed using the monoclonal two-site sandwich immunoluminometric assay Elecsys 100B, Cobas (Hoffmann-La Roche Ltd., Basel, Switzerland). The S-100 assay measures the ß-subunit of protein S-100 as defined by monoclonal antibodies, and the detection limit of the kit is 0.02 μg/L. The range of S100ß serum concentrations in 95% of healthy subjects is below 0.12 μg/L.
Statistical analysis
The descriptive statistics were expressed as mean ± SD. For noncontinuous variables, the chi-squared test, Yates’ chi-squared test, and Fisher’s exact test were used, when necessary. For continuous variables with a normal distribution in the Kolmogorov–Smirnov test, the two-sample t-test was used. For the continuous variables without normal distribution, the Mann–Whitney U test for independent data samples and the Wilcoxon signed-rank test for correlated data samples were used.
Patients in the treatment and control groups were paired according to age (≤60 years and >60 years) and sex. The first patient of each pair was chosen randomly using the Random Number Generator of Excel 97 (Microsoft Office; Microsoft Corporation, Redmond, WA, USA). The second patient from the opposite group was matched automatically. The results were considered significant when P<0.05. The statistical analysis was performed using the licensed Statistica 12.0 software (Nr JPZP602C295824AR-V; StatSoft, Inc., Tulsa, OK, USA).
Results
There were 49 patients in the treatment (T) group (23 women and 26 men, aged 41–77 years [mean 61.7±9.35]). In the control (C) group, there were 51 patients (22 women and 29 men, aged 45–79 years [mean 64.2±8.5]). The demographic and preoperative data are presented in Table 1. There were 60 mitral annuloplasty procedures using the Carpentier-Edwards Classic Annuloplasty Ring (Edwards Lifesciences, Irvine, CA, USA) or St Jude Medical™ Rigid Saddle Ring (St Jude Medical, St Paul, MN, USA) and 40 mitral valve replacements – 19 with a standard bi-leaflet mechanical valve (ATS Medical, Minneapolis, MN, USA) and 21 with the Hancock II Tissue Valve (Medtronic, Minneapolis, MN, USA). Additionally, some other procedures were performed: 60 CABGs, 32 TVA, 29 radiofrequency monopolar ablations (MRFA), and three atrial septal defect (ASD) closures (Table 2). The postoperative data and complications are summarized in Table 3.
  | Table 1 Demographic data |
The results of S100ß level in blood plasma samples taken from patients in the treatment and control groups before the operation, 2 hours after de-clamping of the aorta, and 24 hours after the operation did not differ significantly between each other (Table 4). An analysis according to age in whole study group showed that in patients aged >60 years, the serum level of S100ß 2 hours and 24 hours after the surgery was significantly higher compared to younger patients (1.358±0.634 μg/L vs 1.022±0.535 μg/L, P=0.008, and 0.279±0.218 vs 0.193±0.0908, P=0.005, respectively; Table 5). Similar results were observed in the study group (1.393±0.607 μg/L vs 0.847±0.45 μg/L, P<0.001, and 0.322±0.303 μg/L vs 0.191±0.084 μg/L, P=0.016), but in the control group, there were no significant age-dependent differences (Table 6). Comparing younger patients in the treatment and control groups, 2 hours after the surgery, there were significant differences in S100ß level (0.847±0.45 μg/L vs 1.264±0.561 μg/L, P=0.015), but not at the beginning and after 24 hours. In the older patients in the treatment and control groups, the differences were not significant (Table 7).
  | Table 4 The S100ß levels at predefined study times |
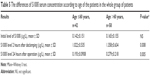  | Table 5 The differences of S100ß serum concentration according to age of the patients in the whole group of patients |
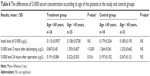  | Table 6 The differences of S100ß serum concentration according to age of the patients in the study and control groups |
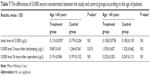  | Table 7 The differences of S100ß serum concentration between the study and control groups according to the age of patients |
It has also been observed that the additional procedure of TVA had an impact upon the increase in S100ß concentration in the whole patient cohort significant after 2 hours (P=0.049) and not significant after 24 hours from the operation (Table 8). The increase in S100ß concentration in the treatment group after 24 hours was statistically significant – TVA (−) 0.21±0.09 vs TVA (+) 0.42±0.42, P=0.05 (Table 8). It has been shown that the serum S100ß concentration depends on age and a concomitant performance of TVA. In the older group with TVA, results were significantly higher than in the younger group (1.59±0.682 μg/L vs 1.055±0.561 μg/L, P=0.043, and 0.363±0.318 μg/L vs 0.189±0.074 μg/L, P<0.001), both 2 hours and 24 hours after the surgery (Table 9). In the older group, there were significant differences in the S100ß level 2 hours and 24 hours after the procedure (1.59±0.682 μg/L vs 1.223±0.571 μg/L, P=0.048, and 0.363±0.318 μg/L vs 0.229±0.105 μg/L, P=0.036), when comparing the results of patients with and without TVA (Table 10). The only direct evidence that CO2 flooding could be effective was found when comparing the S100B results between younger patients in the study and control groups without TVA. In the second measure, 2 hours after surgery, we also found significant differences (0.845±0.465 μg/L vs 0.845±0.465 μg/L, P=0.028).
Discussion
In our study, the mean increase in the S100ß concentration was 13% lower in the group with CO2 protection than in the control group (0.988 μg/L vs 1.125 μg/L), but the differences were statistically insignificant. We observed significant differences in S100ß concentrations depending on the age of the patients. In the group of patients older than 60 years, the S100ß concentrations were significantly higher both 2 hours and 24 hours after the surgery. Among patients aged ≤60 years, significantly lower values of S100ß concentrations were found between the treatment and control groups 2 hours after the surgery and in the subgroup without additional procedures, namely, mitral valve annuloplasty. These results suggest the potential impact of CO2 flooding on S100ß concentrations but unfortunately not found in other analyses.
We have also demonstrated that performing an additional TVA procedure has a significant impact on the increase in S100ß concentrations in postoperative studies. This might have been caused by increased suction from the operating field since the application and tying of sutures to the tricuspid ring are performed after aortic cross-clamp release and the blood, which flows from the coronary sinus, requires intensive suction for a period of 10–20 minutes. This difference in the results of both groups (with and without TVA) made us realize the mistake of not including a proportional distribution of patients with TVA in our randomization assumptions. Due to the fact that the control group contained significantly more patients with TVA (treatment group: 11/49 vs control group: 21/51, P=0.045), which is associated with an increase in S100ß concentration, it has to be concluded that the differences between the two groups were even smaller in reality.
The important problem of finding a way to effectively replace air with CO2 in the operating field has been addressed by several clinical trials that established the principles of operating field insufflation offering an appropriate concentration of CO2 in the heart chambers in order to make sure that residual microbubbles of gas are largely filled with CO2.19–21
Yet, whether the method is legitimate or not remains an open question. Microbubbles of air reach cerebral circulation during virtually all of cardiac surgery procedures. Although their number increases considerably when opening the chambers of the heart, they do not cause measurable symptoms and it is impossible to detect consequences of microembolism using typical neurological tests. Several studies demonstrated a correlation between small lesions in the brain detected on an magnetic resonance imaging (MRI) scan and neurological tests, but other studies have not confirmed these observations.22–29
Some authors were of the opinion that when comparing patients a few months after a cardiac procedure, neurological (behavioral) changes appear more often in patients after CABG than in patients after OPCAB, but these associations were denied by others.30,31 Martens et al13 demonstrated that the number of air bubbles released to carotid arteries after weaning from CPB does not have to correlate with the occurrence of cognitive dysfunction.
Haggag et al27 have shown, in a study performed on rats, that injection of air into the arterial system does not cause lesions typical for microembolism. Neville et al,28 in a study comparing valve procedures with CABG, also did not find differences in the frequency of postoperative neurological dysfunction between the two groups, despite detecting much more air in carotid arteries on ultrasonography during valve procedures. In contrast, Herrmann et al29 have shown that operations involving opening of the heart chambers result in a higher percentage of neurological dysfunctions and a higher increase in brain damage biomarkers in comparison to CABG. Martens et al30 examined the influence of delivering air and CO2 to the carotid arteries of pigs on the occurrence of lesions on an MRI scan and concluded that air caused extensive cerebral infarction while CO2 did not cause any lesions that would be detectable with MRI. However, it must be underlined that a large amount of air reaching cerebral arteries during a cardiac surgery may only be a result of technical error.
Our study is not without limitations. First, other concomitant procedures (TVP or CABG) may influence the CPB times and the levels of S100ß. Second, the study reports laboratory findings, without a clinical postoperative perspective. The postoperative dementia screening (Mini Mental State Examination [MMSE]) or a detailed neurocognitive examination during the follow-up period would provide an important insight into the effect of CO2 flooding on neurological outcome. Third, the number of patients included in the study is relatively small and further research might be required to eliminate sample size effect. Fourth, although elevated serum S100B levels after cardiac operations with CPB are sensitive and specific for ischemic stroke, its appearance in surgical patients without neurological injury and its potential release from extracranial sources (ie, adipocytes, chondrocytes, and melanocytes) make its use as a postoperative biomarker of neurological insult debatable.4,32–36 Moreover, the use of CO2 atmosphere has been debatable.37,38 Nevertheless, we believe that notwithstanding shortages in our randomization assumptions, our study still offers important insights that are relevant to surgical practice.
Conclusion
The mean increase in S100ß concentration was lower in the group with CO2 protection than in the control group. Both age and an addition of TVA significantly influenced the level of S100ß concentration in the tests performed 2 hours after aortic clamp release.
Acknowledgment
The authors of this study would like to thank Mrs Małgorzata Pawelec for her help during the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Seco M, Edelman JJ, Wilson MK, Bannon PG, Vallely MP. Serum biomarkers of neurologic injury in cardiac operations. Ann Thorac Surg. 2012;94(3):1026–1033. | ||
Nichols HT, Morse DP, Hirose T. Coronary and other air embolization occurring during open cardiac surgery; prevention by the use of gaseous carbon dioxide. Surgery. 1958;43(2):236–244. | ||
Oka Y, Inoue T, Hong Y, Sisto DA, Strom JA, Frater RW. Retained intracardiac air. Transesophageal echocardiography for definition of incidence and monitoring removal by improved techniques. J Thorac Cardiovasc Surg. 1986;91(3):329–338. | ||
Selnes OA, McKhann GM. Neurocognitive complications after coronary artery bypass surgery. Ann Neurol. 2005;57(5):615–621. | ||
Skidmore KL, Jones C, DeWet C. Flooding the surgical field with carbon dioxide during open heart surgery improves segmental wall motion. J Extra Corpor Technol. 2006;38(2):123–127. | ||
Webb WR, Harrison LH Jr, Helmcke FR, et al. Carbon dioxide field flooding minimizes residual intracardiac air after open heart operations. Ann Thorac Surg. 1997;64(5):1489–1491. | ||
Selman MW, McAlpine WA, Albregt H, Tatan R. An effective method of replacing air in the chest with CO2 during open-heart surgery. J Thorac Cardiovasc Surg. 1967;53(5):618–622. | ||
O’Connor BR, Kussman BD, Park KW. Severe hypercarbia during cardiopulmonary bypass: a complication of CO2 flooding of the surgical field. Anesth Analg. 1998;86(2):264–266. | ||
Lippmann M. Complications of CO2 flooding the surgical field in open heart surgery: an old technique revisited. Anesth Analg. 1998;87(4):978–979. | ||
Barnard J, Speake D. In open heart surgery is there a role for the use of carbon dioxide field flooding techniques to reduce the level of post-operative gaseous emboli? Interact Cardiovasc Thorac Surg. 2004;3(4):599–602. | ||
Giordano S, Biancari F. Does the use of carbon dioxide field flooding during heart valve surgery prevent postoperative cerebrovascular complications? Interact Cardiovasc Thorac Surg. 2009;9(2):323–326. | ||
Baudet E. Personal Contact. Bordeaoux, France; 1998. | ||
Martens S, Dietrich M, Doss M, Wimmer-Greinecker G, Moritz A. Optimal carbon dioxide application for organ protection in cardiac surgery. J Thorac Cardiovasc Surg. 2002;124(2):387–391. | ||
Svenarud P, Persson M, Van Der Linden J. Efficiency of a gas diffuser and influence of suction in carbon dioxide deairing of a cardiothoracic wound cavity model. J Thorac Cardiovasc Surg. 2003;125(5):1043–1049. | ||
Kalpokas MV, Nixon IK, Kluger R, Beilby DS, Silbert BS. Carbon dioxide field flooding versus mechanical de-airing during open-heart surgery: a prospective randomized controlled trial. Perfusion. 2003;18(5):291–294. | ||
Toner I, Peden CJ, Hamid SK, Newman S, Taylor KM, Smith PL. Magnetic resonance imaging and neuropsychological changes after coronary artery bypass graft surgery: preliminary findings. J Neurosurg Anesthesiol. 1994;6(3):163–169. | ||
Restrepo L, Wityk RJ, Grega MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting surgery. Stroke. 2002;33(12):2909–2915. | ||
Barber PA, Hach S, Tippett LJ, Ross L, Merry AF, Milsom P. Cerebral ischemic lesions on diffusion-weighted imaging are associated with neurocognitive decline after cardiac surgery. Stroke. 2008;39(5):1427–1433. | ||
Moody DM, Brown WR, Challa VR, Stump DA, Reboussin DM, Legault C. Brain microemboli associated with cardiopulmonary bypass: A histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59(5):1304–1307. | ||
Vanninen R, Aikiä M, Könönen M, et al. Subclinical cerebral complications after coronary artery bypass grafting: prospective analysis with magnetic resonance imaging, quantitative electroencephalography, and neuropsychological assessment. Arch Neurol. 1998;55(5):618–627. | ||
Bendszus M, Reents W, Franke D, et al. Brain damage after coronary artery bypass grafting. Arch Neurol. 2002;59(7):1090–1095. | ||
Knipp SC, Matatko N, Wilhelm H, et al. Evaluation of brain injury after coronary artery bypass grafting. A prospective study using neuropsychological assessment and diffusion-weighted magnetic resonance imaging. Eur J Cardiothorac Surg. 2004;25(5):791–800. | ||
Cook DJ, Huston J 3rd, Trenerry MR, Brown RD Jr, Zehr KJ, Sundt TM 3rd. Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83(4):1389–1395. | ||
Motallebzadeh R, Kanagasabay R, Bland M, Kaski JC, Jahangiri M. S100 protein and its relation to cerebral microemboli in on-pump and off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;25(3):409–414. | ||
Bonacchi M, Prifti E, Maiani M, Bartolozzi F, di Eusanio M. Leacche M. Does off-pump coronary revascularization reduce the release of the cerebral markers, S-100beta and NSE? Heart Lung Circ. 2006;15(5):314–319. | ||
Martin KK, Wigginton JB, Babikian VL, Pochay VE, Crittenden MD, Rudolph JL. Intraoperative cerebral high-intensity transient signals and postoperative cognitive function: a systematic review. Am J Surg. 2009;197(1):55–63. | ||
Haggag KJ, Russell D, Walday P, Skiphamn A, Torvik A. Air-filled ultrasound contrast agents do not damage the cerebral microvasculature or brain tissue in rats. Invest Radiol. 1998;33(3):129–135. | ||
Neville MJ, Butterworth J, James RL, Hammon JW, Stump DA. Similar neurobehavioral outcome after valve or coronary artery operations despite differing carotid embolic counts. J Thorac Cardiovasc Surg. 2001;121(1):125–136. | ||
Herrmann M, Ebert AD, Tober D, Hann J, Huth C. A contrastive analysis of release patterns of biochemical markers of brain damage after coronary artery bypass grafting and valve replacement and their association with the neurobehavioral outcome after cardiac surgery. Eur J Cardiothorac Surg. 1999;16(5):513–518. | ||
Martens S, Theisen A, Balzer JO, et al. Improved cerebral protection through replacement of residual intracavital air by carbon dioxide: a porcine model using diffusion-weighted magnetic resonance imaging. J Thorac Cardiovasc Surg. 2004;127(1):51–56. | ||
Herrmann M, Ebert AD, Galazky I, Wunderlich MT, Kunz WS, Huth C. Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke. 2000;31(3):645–650. | ||
Kilminster S, Treasure T, McMillan T, Holt DW. Neuropsychological change and S-100 protein release in 130 unselected patients undergoing cardiac surgery. Stroke. 1999;30(9):1869–1874. | ||
Ueno T, Iguro Y, Yamamoto H, Sakata R, Kakihana Y, Nakamura K. Serial measurement of serum S-100B protein as a marker of cerebral damage after cardiac surgery. Ann Thorac Surg. 2003;75(6):1892–1897; discussion 1897–1898. | ||
Ramlawi B, Rudolph JL, Mieno S, et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg. 2006;244(4):593–601. | ||
Georgiadis D, Berger A, Kowatschev E, et al. Predictive value of S-100beta and neuron-specific enolase serum levels for adverse neurologic outcome after cardiac surgery. J Thorac Cardiovasc Surg. 2000;119(1):138–147. | ||
Jönsson H, Johnsson P, Alling C, Bäckström M, Bergh C, Blomquist S. S100beta after coronary artery surgery: release pattern, source of contamination, and relation to neuropsychological outcome. Ann Thorac Surg. 1999;68(6):2202–2208. | ||
Chaudhuri K, Storey E, Lee GA, et al. Carbon dioxide insufflation in open-chamber cardiac surgery: a double-blind, randomized clinical trial of neurocognitive effects. J Thorac Cardiovasc Surg. 2012;144(3):646–653.e1. | ||
Sun Y, Ji B, Zhu X, Zheng Z. Efficacy of carbon dioxide insufflation for cerebral and cardiac protection during open heart surgery: a systematic review and meta-analysis. Artif Organs. 2013;37(5):439–446. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





