Back to Journals » Clinical Ophthalmology » Volume 15
The Influence of Blue-Filtering Intraocular Lenses Implant on Exudative Age-Related Macular Degeneration: A Case–Control Study
Authors Hamel T, Rheault J, Simonyan D, Bourgault S, Rochette PJ
Received 5 January 2021
Accepted for publication 30 March 2021
Published 1 June 2021 Volume 2021:15 Pages 2287—2292
DOI https://doi.org/10.2147/OPTH.S300461
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Thierry Hamel,1,2 Justine Rheault,1,2 David Simonyan,3 Serge Bourgault,1,2,* Patrick J Rochette1,2,4,*
1Département d’ophtalmologie et d’ORL-CCF, Faculté de médecine, Université Laval, Québec, QC, Canada; 2Centre Universitaire d’Ophtalmologie (CUO), Hôpital du Saint-Sacrement, CHU de Québec – Université Laval, Québec, QC, Canada; 3Clinical and Evaluative Research Platform, CHU de Québec-Université Laval Research Centre, Québec, QC, Canada; 4Centre de Recherche du CHU de Québec – Université Laval, Axe Médecine Régénératrice, Hôpital du Saint-Sacrement, Québec, QC, Canada
*These authors contributed equally to this work
Correspondence: Patrick J Rochette
Centre de recherche du CHU de Québec – Université Laval, Axe Médecine Régénératrice, Hôpital du Saint-Sacrement, Bureau H2-10, 1050 Chemin Sainte-Foy, Québec, QC, G1S 4L8, Canada
Tel +1 418 682-7568
Email [email protected]
Purpose: To determine whether the use of a blue light-filtering intraocular lens (IOL) prevents the onset of wet age-related macular degeneration (AMD). More precisely, we examined the proportion of blue light-filtering IOL in a wet AMD patients’ sample and compared it with a general North American pseudophakic population sample.
Design: Retrospective case–control study.
Methods: Case patients were diagnosed and treated for wet AMD and had prior IOL implantation at least 3 years before the diagnosis of wet AMD. Control patients were randomly selected among patients who had cataract surgery at our institution. They were exempt of AMD and paired for the year of surgery, sex and age at cataract surgery. A total of 196 patients were included in each study group.
Results: Among patients with wet AMD, 62.8% had a blue light-filtering IOL compared with 63.3% among control patients (p = 0.92). Mean time between implantation and injection of anti-VEGF in AMD patients was 6.62 years (95% confidence interval (CI): 6.04– 7.19) in non-blue light-filtering IOL group and 5.76 years (95% CI: 5.41– 6.11) in blue light-filtering IOL group (p = 0.0120).
Conclusion: No correlations could be established between the presence of a blue light filter in the IOL and the occurrence of wet AMD. AMD patients without blue light-filtering IOL were injected significantly later than patients with an IOL filtering blue light, which contradict the potential clinical benefit of the blue light filter.
Keywords: blue-light filter, intraocular lens, cataract surgery, age-related macular degeneration, anti-VEGF injection
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe vision loss in developed countries. Risk factors for AMD include aging, smoking and genetic factors.1,2 Clinically, the degenerating macula is characterized by the accumulation of drusen and pigmentary changes resulting from lipoproteinous material accumulation.3 In later stages, we may observe geographic atrophy in the dry stage and/or choroidal neovascular membrane (CNVM) in exudative (or wet) AMD, both associated with loss of visual function. Vascular endothelial growth factor inhibitors (anti-VEGF) are commonly used to treat CNVM.
Blue light (400–500 nm) exposure is a suspected aggravating factor for AMD development.4–7 A systematic review of the epidemiological literature on the subject showed a significant link between light exposure and the higher risk of developing AMD.8 With the aging lens, transmission of blue light reaching the retina is reduced.9 This blue light natural filtration of the lens is abolished when cataract surgery using non-blue light-filtering intraocular lens (IOL) is performed. In 1991, blue light-filtering IOL were introduced but since then, a limited number of studies have attempted to determine whether the use of blue light-filtering IOL protects against the progression of AMD. In consequence, non-blue light-filtering models are still widely used in cataract surgeries.10 A recent systematic review of the published data on blue light exposure and AMD concluded that using blue-filtering IOL could not be supported by published evidence.11
Ophthalmologists are lacking solid data to justify the choice of using a blue light-filtering IOL in order to mitigate the development of CNVM, which is the leading cause of blindness among AMD patients. The purpose of our study was to assess the protective effect of implanting blue light-filtering IOL on neovascular AMD development. We have thus evaluated if a lower proportion of blue light-filtering IOL could be found in a cohort of patients with wet AMD in comparison to a general North American pseudophakic population sample. Moreover, in an attempt to determine whether the presence of a blue filter could delay the onset of wet AMD, we have compared the time between cataract surgery and the first injection of anti-VEGF in patients with and without blue light-filtering IOL.
Materials and Methods
This study has received approval by the Centre de Recherche du CHU de Québec – Université Laval institutional ethic committee for the protection of human subjects and was conducted in accordance with the Declaration of Helsinki. Individual patient consent was waived by the institutional ethic committee. To be waived, we had to comply with specific conditions, which we did, i.e. all data must be anonymized and confidential, each subject has to be identified by a number and all files should be destroyed at the end of the study. We conducted a retrospective case–control study on patients with wet AMD who had prior cataract extraction in a tertiary care ophthalmic center (Centre Universitaire d’Ophtalmologie, Hôpital du St-Sacrement). They were randomly selected by a computer algorithm, from a chronological list of patients whose medical file included a billing code for a first injection of anti-VEGF during the study period starting in January 2007 and ending in December 2017. Injection was performed by one of six ophthalmologists with expertise in retinal diseases at our hospital or at the Centre Oculaire de Québec (a private institute treating patients with national health insurance). Selected patients had their medical file carefully reviewed and were excluded when the anti-VEGF injection was performed for other retinal diseases (e.g. diabetic macular edema, proliferative retinopathy or retinal vein occlusion) or when a patient was exempt of a cataract surgery (or left with aphakia). A patient was included in the study if a cataract surgery with implantation of IOL had been done at least 3 years prior to wet AMD diagnosis. The second eye of a same case patient could be included as a different entry for our secondary outcome only, i.e. time between cataract surgery and the first anti-VEGF injection. The 3-year minimum period was deemed appropriate in order to allow a certain environmental exposure to blue light. This period was determined empirically since no comparable study had been published at the beginning of our study. Control patients were randomly selected from a list of patients who had implantation of an IOL between 2004 and 2013. Patients were excluded if a diagnosis of dry or wet AMD was established in their medical record. They were then matched according to sex, age at cataract surgery and year of surgery. Systemic comorbidities (high blood pressure, use of aspirin (ASA), hyperlipidemia and history of smoking) were collected in patients’ medical records.
IOL models implanted were classified according to the presence or absence of a chromophore filtering blue light. Blue light-filtering IOL qualified when transmission of light between 400 nm and 450 nm was reduced by at least 50% according to the spectral transmittance data provided by the manufacturer.12,13 Blue light-filtering IOL included in the study were manufactured by Alcon (SN60WF, SN60AT). IOL without blue light filter were manufactured by Johnson & Johnson (AR40E, AR40E, ZCB00, ZA9003, PCB00), Bausch & Lomb (AKREOS ADAPT-AO, LI61Se, LI61A0, MI60P, MX60P) and Alcon (SA60AT, MZ60BD, MA60BM, LX10BD). All IOL models included in the study blocked ultraviolet wavelengths below 380 nm. Sample size was determined to be at least of 356 patients in order to detect a 10% difference in the type of IOL between cases and controls with an 80% power. Multivariate analysis was performed to consider the factors that could influence the relationship between the type of IOL implanted and the occurrence of wet AMD.
Statistical Analysis
Quantitative variables are described as mean and standard deviation, and qualitative variables as frequencies and percentages. Parametric (F-test or t-test) or non-parametric (Kruskal–Wallis, Wilcoxon rank sum) tests were used to compare continuous data by groups after normality verification; chi-squared or Pearson exact tests were used for categorical data comparisons. In case of multiple comparisons, Tukey–Kramer adjustment was applied. To test the impact of blue-filtering IOL on AMD, univariate and multivariate generalized estimating equation (GEE) logistic regression modeling were performed. To obtain adjusted odds ratio, multivariate GEE logistic regression modeling was fitted with the following adjustment factors: high blood pressure, ASA, hyperlipidemia and history of smoking. To test the impact of blue-filtering on the mean time between cataract surgery and the first injection in the case group, we used uni- and multivariate GEE linear regression modeling adjusted for sex, high blood pressure, ASA, hyperlipidemia and history of smoking. This GEE model considered the correlation induced by including both eyes of the same patients. All GEE models were fitted with independent structure of working correlation matrix having minimal quasi-likelihood criterion. Statistical analyses were performed using SAS Statistical Software v.9.4 (SAS Institute, Cary, NC, USA) with a two-sided significance level set at p < 0.05.
Results
A total of 1790 patients had been treated with anti-VEGF from 2007 to 2017. Of these 1663 were reviewed in order to identify 196 case patients meeting the inclusion criteria. They were matched with 196 control patients. Among case patients, 46 patients had bilateral injections for wet AMD for a total of 242 eyes. Of these 46 patients, 18 patients had blue light-filtering IOL in both eyes, 27 patients had non-blue light-filtering IOL in both eyes and 1 patient had non-blue light-filtering IOL in his first eye and then blue light-filtering IOL in his second eye. Overall, the mean follow-up time was 6.08 years.
Demographics and baseline characteristics of the study population are depicted in Table 1. The incidence of high blood pressure is higher in wet AMD group (146/196; 74.5%) than in control group (130/196; 66.3%), but this is not statistically significant (p = 0.08). The incidence of aspirin usage is higher in wet AMD group (109/196; 55.6%) than in control group (91/196; 46.4%), but the data do not reach statistical significance (p = 0.07). We observed a significantly higher proportion of hyperlipidemia (125/196; 63.8%) and smoking history (88/196; 44.9%) in wet AMD group when compared with control (hyperlipidemia 102/196 (52.0%) and smoking history 58/196 (29.6%)) (p = 0.02 and p < 0.0001, respectively).
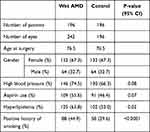 |
Table 1 Baseline Characteristics |
Proportion of Blue Light-Filtering IOL in AMD Patients Compared with Non-AMD Individuals
We found a highly similar proportion of blue light-filtering IOL in both the case and control groups. Indeed, blue light-filtering IOL was found in 123 patients in wet AMD group (123/196; 62.8%) and 124 patients in control group (124/196; 63.3%) (p = 0.92). Multivariate GEE logistic regression adjusted for high blood pressure, ASA, hyperlipidemia and history of smoking indicated no significant influence of blue light-filtering IOL on wet AMD: odds ratio (blue light-filtering vs non-blue light-filtering) = 0.88 (95% confidence interval (CI): 0.54–1.41, p = 0.5831).
Time Between Cataract Surgery and First Anti-VEGF Injection with Blue Light-Filtering IOL Compared with Non-Blue Light-Filtering IOL
The mean time between cataract surgery and the first injection in eyes with blue-filtering IOL (n = 150) was 5.76 years (95% CI: 5.41–6.11) compared with 6.62 years (95% CI: 6.04–7.19) in eyes without blue light filtration (n = 92). This difference is statistically significant (p = 0.0120). Multivariate GEE linear regression adjusted for sex, high blood pressure, ASA, hyperlipidemia and history of smoking indicated that time between cataract surgery and the first injection in patients with blue filtering IOL is still significantly lower with blue filtering IOL (5.59 years, 95% CI: 5.09–6.09) compared with eyes without blue light filtration (6.43 years, 95% CI: 5.79–7.06) (p = 0.0098).
Discussion
Estimating retinal blue light exposure in humans is challenging using epidemiological studies. Indeed, many confounding factors such as age, geographic locality and patient’s own recall on their exposure can interfere with the analysis. Studies have been inconsistent regarding the role of blue light on the risk of AMD progression.11,14–18 Giving the morbidity associated with wet AMD and the absence of a curative treatment, finding strategies to prevent progression of AMD is of great interest. Using blue light-filtering IOL is one. These implants are used in 25% of cataract surgery worldwide.15
A paucity of studies has shown a benefit of blue light-filtering IOL on mitigating AMD.17,18 In a retrospective study of 40 patients, Pipis et al. showed less geographic atrophy progression over 1 year in patients with blue light-filtering IOL compared with patients with no color filter.17 Nagai et al. conducted a prospective observational study on 174 patients which primary objective was to compare changes in fundus autofluorescence (FAF) 2 years after implantation of yellow-tinted or colorless IOL.18 They found no new or increased abnormal FAF in the yellow-tinted IOL group but 12 eyes (15.2%) in the colorless IOL group showed progressive abnormal FAF (p = 0.0016). Eyes with colorless IOL were more likely to show any form of AMD (p = 0.042) but a statistical conclusion could not be drawn regarding exudative AMD.
Our study confirms that patients with wet AMD were more likely to be elderly females and to present recognized risk factors including high blood pressure (p = 0.08), ASA (p = 0.07), hyperlipidemia (p = 0.02) and history of smoking (p < 0.0001). Those associations have previously been shown in major clinical studies, which validates our patient cohort.19–23 Our randomly generated control group was matched to our AMD group for certain demographics (age, gender and year of surgery) and constitutes a sample of a population living in the same geographic area. We can thus extrapolate that control patients have had a similar blue light exposure compared with wet AMD patients over the study period (mean = 6.08 years). The control group was exempt of any form of AMD, dry or wet. Conversely, we can assume that a wide proportion of our case patients did exhibit some features of dry AMD at the time of cataract surgery based on the AREDS study, which showed that only 1.3% and 2.0% of patients with category 2 (mild) AMD progressed to category 4 (advanced) AMD at 5 and 7 years respectively.24
We found a similar proportion of blue light-filtering IOL in wet AMD group (62.8%) when compared with control group (63.3%, p = 0.92). Considering that blue light has been found to be toxic to RPE cells in vitro and in animal models it was plausible to expect this correlation in humans. Indeed, we were expecting that patients without protection against the blue part of the visible light spectrum would be more likely to develop wet AMD. This association has not been observed in our retrospective case–control study. Moreover, patients with blue-filtering IOL who subsequently developed wet AMD were first treated almost one year prior (mean = 5.76 years) to patients without blue light filtering IOL (mean = 6.62 years). A recent in vitro study using A2E-loaded RPE cells showed a decrease in VEGF production with exposure to blue light (440 nm). The authors suggested that synthesis of VEGF is suppressed by blue light thus limiting damage to the RPE.25 Greater time between cataract surgery and anti-VEGF injection in our group without blue light-filtering IOL could potentially be explained by this theory supporting a protective effect of blue light exposure.
The results of our study must be taken with caution given that this was a retrospective observational study. The rationale behind surgeons’ IOL choices was not indicated in the patients’ chart. One can think that patients with high-risk dry AMD would have been more likely to receive a blue light-filtering IOL leading to a susceptibility bias potentially causing a preponderance of blue light-filtering IOL among the case patients. However, the proportion of this type of IOL was similar in patients treated for wet AMD and those without AMD. We can assume that the choice of the type of IOL was more based on surgeons’ personal preferences than on the presence of dry AMD. Also, data concerning the severity of dry AMD in our wet AMD group could not be extracted. Most charts lacked a standardized classification of dry AMD making it impossible to match cases and controls for baseline AMD. In addition, macular optical coherence tomography and fundus photography are not routinely performed in pre-operative evaluation for cataract surgery in our institution. Furthermore, the >3-year period between cataract surgery and anti-VEGF treatment might not be long enough to see a significant effect of blue light filtration. Indeed, the blue light exposure after cataract surgery might not be significant compared with the amount of blue light received during a lifetime. However, one could hypothesize that blue light filtering becomes more critical for the elderlies. Indeed, as we age, blue-light absorbing chromophores (the main one being lipofuscin) accumulate in the retina making them at greater risk of developing wet AMD after they lose the protection provided by their cataractous lenses. Also, other factors, notably smoking history, could play a more important role in the development of wet AMD. Our cases and controls were not matched for smoking history and a higher proportion of patients did smoke in our case group (44.9% vs 29.9%). However, multivariate GEE logistic regression adjusted for multiple factors (high blood pressure, ASA, hyperlipidemia and history of smoking) showed no influence of blue filtration on wet AMD.
A cohort study, including baseline standardized AREDS dry AMD classification combined with modern imaging, could be done to further support our conclusion. The number of patients needed would be much greater in such a study compared with our case–control design. Recently, Achiron et al. published a retrospective cohort study of 11,397 patients which did not show any evidence of reduction of the incidence of neovascular AMD with blue light-filtering IOL.26 A total of 164 cases of new neovascular AMD were identified. The vast majority of patients were lost to follow-up after the 1-month post-operative visit. As we did in our study for our control patients, it was assumed that a patient did not develop wet AMD if they had not received an intravitreal injection of anti-VEGF at their institution. This study did not use the standardized AREDS dry AMD classification and the minimal time interval between cataract surgery and the first anti-VEGF injection was considerably shorter than in our study: 1 versus 3 years. Unfortunately, the authors did not compare the mean time between cataract surgery and the first anti-VEGF injection for both IOL groups. We can observe from their Kaplan-Meier survival plot that patients with blue light-filtering IOL were injected sooner than patients without filters between 12- and 72-months follow-up; this would corroborate our findings.
Ideally, a randomized controlled trial (RCT) would be conducted but would most likely need thousands of patients and extensive follow-up to provide statistically significant data. A recent Cochrane review on blue light-filtering IOLs for macular protection included 51 RCTs but only one study evaluated the development of late-stage AMD at 3 years of follow-up; two studies, the development of any stage of AMD at one year of follow-up. Moreover, there were no events (any or late-stage AMD development) in either intervention group (with or without blue filter).11
Based on our results, we cannot recommend usage of blue light filtering IOL for the purpose of macular protection. A similar conclusion was drawn by a recent Cochrane review which also concluded that there was no difference in best corrected visual acuity and little to no evidence of superiority of blue light filtering IOL on contrast sensitivity, color discrimination, daytime alertness, reaction time or patient satisfaction.11 The general interest in blue light-filtering IOL is still debated, with recent evidence suggesting improved chromatic contrast and performance under glare conditions.27
Conclusions
In our retrospective case–control study, no correlations between the presence of a blue light filter in the IOL and the occurrence of wet AMD has been found. Moreover, patients without blue light- filtering IOL were first treated with anti-VEGF later than patients with an IOL filtering blue light. This is in contradiction with the potential clinical benefit of blue light-filtering IOL use in AMD patients. Further prospective studies are necessary to improve our understanding of blue light impact on AMD and to determine whether cataract surgery parameters could alleviate burden of the disease.
Highlights
- We found no correlations between blue light-filtering IOL and wet AMD.
- First anti-VEGF injection was delayed in patients without blue light-filtering IOL.
Disclosure
None of the authors has a financial or proprietary interest in any material or method mentioned.
References
1. Coleman HR, Chan -C-C, Ferris FL, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi:10.1016/S0140-6736(08)61759-6
2. Klein RJ, Zeiss C, Chew EY, et al. Complement Factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi:10.1126/science.1109557
3. Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. 1994. Retina. 2005;25(5 Suppl):130–142. doi:10.1097/00006982-200507001-00016
4. Beatty S, Boulton M, Henson D, Koh -H-H, Murray IJ. Macular pigment and age-related macular degeneration. Br J Ophthalmol. 1999;83(7):867–877. doi:10.1136/bjo.83.7.867
5. Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5(5):450–473.
6. Davies S, Elliott MH, Floor E, et al. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med. 2001;31(2):256–265. doi:10.1016/S0891-5849(01)00582-2
7. Sparrow JR, Miller AS, Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg. 2004;30(4):873–878. doi:10.1016/j.jcrs.2004.01.031
8. Sui GY, Liu GC, Liu GY, et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389–394. doi:10.1136/bjophthalmol-2012-302281
9. Van Norren D, Van De Kraats J. Spectral transmission of intraocular lenses expressed as a virtual age. Br J Ophthalmol. 2007;91(10):1374–1375. doi:10.1136/bjo.2007.117903
10. Mainster MA. The spectra, classification, and rationale of ultraviolet-protective intraocular lenses. Am J Ophthalmol. 1986;102(6):727–732. doi:10.1016/0002-9394(86)90400-9
11. Downie LE, Busija L, Keller PR. Blue-light filtering intraocular lenses (IOLs) for protecting macular health. Cochrane Database Syst Rev. 2018;5(5):CD011977. doi:10.1002/14651858.CD011977.pub2
12. Brockmann C, Schulz M, Laube T. Transmittance characteristics of ultraviolet and blue-light-filtering intraocular lenses. J Cataract Refract Surg. 2008;34(7):1161–1166. doi:10.1016/j.jcrs.2008.03.039
13. Mainster MA. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90(6):784–792. doi:10.1136/bjo.2005.086553
14. Symes RJ, Cuthbertson FM. Blue-blocking intraocular implants should be used routinely during phacoemulsification surgery-Yes. Eye. 2012;26:1397–1399. doi:10.1038/eye.2012.178
15. Lee RMH, Lam FC, Liu CSC. Blue-blocking intraocular implants should be used routinely during phacoemulsification surgery-No. Eye. 2012;26(11):1400–1401. doi:10.1038/eye.2012.177
16. Mainster MA, Turner PL. Blue-blocking IOLs vs. short-wavelength visible light: hypothesis-based vs. evidence-based medical practice. Ophthalmology. 2011;118(1):1–2. doi:10.1016/j.ophtha.2010.11.016
17. Pipis A, Touliou E, Pillunat LE, Augustin AJ. Effect of the blue filter intraocular lens on the progression of geographic atrophy. Eur J Ophthalmol. 2015;25(2):128–133. doi:10.5301/ejo.5000520
18. Nagai H, Hirano Y, Yasukawa T, et al. Prevention of increased abnormal fundus autofluorescence with blue light–filtering intraocular lenses. J Cataract Refract Surg. 2015;41(9):1855–1859. doi:10.1016/j.jcrs.2015.01.017
19. Sperduto RD, Hiller R. Systemic hypertension and age-related maculopathy in the framingham study. Arch Ophthalmol. 1986;104(2):216–219. doi:10.1001/archopht.1986.01050140070022
20. Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi:10.1001/archopht.122.4.564
21. Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye. 2005;19(9):935–944. doi:10.1038/sj.eye.6701978
22. Klein R, Klein BEK, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110(4):636–643. doi:10.1016/S0161-6420(03)00599-2
23. Wilson HL, Schwartz DM, Bhatt HRF, McCulloch CE, Duncan JL. Statin and aspirin therapy are associated with decreased rates of choroidal neovascularization among patients with age-related macular degeneration. Am J Ophthalmol. 2004;137(4):615–624. doi:10.1016/j.ajo.2003.10.025
24. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi:10.1001/archopht.119.10.1417
25. Marie M, Gondouin P, Pagan D, et al. Blue-violet light decreases VEGFa production in an in vitro model of AMD. PLoS One. 2019;14(10):e0223839. doi:10.1371/journal.pone.0223839
26. Achiron A, Elbaz U, Hecht I, et al. The effect of blue-light filtering intraocular lenses on the development and progression of neovascular age-related macular degeneration. Ophthalmology. 2021;128(3):410–416. doi:10.1016/j.ophtha.2020.07.039
27. Hammond BR, Sreenivasan V, Suryakumar R. The effects of blue light–filtering intraocular lenses on the protection and function of the visual system. Clin Ophthalmol. 2019;13:2427–2438. doi:10.2147/OPTH.S213280
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
