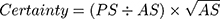Back to Journals » Journal of Inflammation Research » Volume 14
The Influence of an Occult Infection on the Outcome of Autologous Bone Grafting During Surgical Bone Reconstruction: A Large Single-Center Case-Control Study
Authors Tanner MC , Heller RA, Grimm A, Zimmermann S, Pilz M, Jurytko L, Miska M, Helbig L, Schmidmaier G , Haubruck P
Received 14 December 2020
Accepted for publication 10 February 2021
Published 22 March 2021 Volume 2021:14 Pages 995—1005
DOI https://doi.org/10.2147/JIR.S297329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Michael C Tanner,1 Raban Arved Heller,1– 3 Andreas Grimm,1 Stefan Zimmermann,4 Maximilian Pilz,5 Louisa Jurytko,1 Matthias Miska,1 Lars Helbig,1 Gerhard Schmidmaier,1 Patrick Haubruck1,6
1HTRG – Heidelberg Trauma Research Group, Center for Orthopedics, Trauma Surgery and Spinal Cord Injury, Trauma and Reconstructive Surgery, Heidelberg University Hospital, Heidelberg, D-69118, Germany; 2Institute for Experimental Endocrinology, Charité - Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt Universität Zu Berlin, Berlin Institute of Health, Berlin, D-13353, Germany; 3Department of General Practice and Health Services Research, Heidelberg University Hospital, Heidelberg, D-69120, Germany; 4Division Bacteriology, Department of Infectious Diseases, Heidelberg University Hospital, Heidelberg, D-69120, Germany; 5Institute of Medical Biometry and Informatics, Heidelberg University Hospital, Heidelberg, D-69120, Germany; 6Raymond Purves Bone and Joint Research Laboratory, Institute of Bone and Joint Research, Kolling Institute, Royal North Shore Hospital, University of Sydney, St. Leonards, New South Wales, A-2068, Australia
Correspondence: Patrick Haubruck
Raymond Purves Bone and Joint Research Laboratory, Institute of Bone and Joint Research, Kolling Institute, Royal North Shore Hospital, University of Sydney, St Leonards, NSW, A-2068, Australia
Tel +61 (0) 437320789
Email [email protected]
Background: Occult infections (OI) lack typical inflammatory signs, making them challenging to diagnose. Uncertainty remains regarding OI’s influence on the outcome of autologous bone grafting (ABG), and evidence-based recommendations regarding an appropriate course of action are missing. Thus, we sought to determine the incidence of an OI in patients receiving ABG, evaluate whether it influences the outcome of ABG and whether associated risk factors have a further negative influence.
Methods: This study was designed as a large size single-center case-control study investigating patients treated between 01/01/2010 and 31/12/2016 with a minimum follow-up of 12 months. Patients ≥ 18 years presenting with a recalcitrant non-union of the lower limb receiving surgical bone reconstruction, including bone grafting, were included. A total of 625 patients were recruited, and 509 patients included in the current study. All patients received surgical non-union therapy based on the “diamond concept” including bone reconstruction using ABG. Additionally, multiple tissue samples were harvested and microbiologically analyzed. Tissue samples were microbiologically evaluated regarding an OI. Bone healing was analyzed using clinical and radiological parameters, patient characteristics and comorbidities investigated and ultimately results correlated.
Results: Forty-six out of 509 cases with OI resulted in an incidence of 9.04%. Overall consolidation time was increased by 15.08 weeks and radiological outcome slightly impaired (79.38% vs 71.42%), differences were at a non-significant extent. Diabetes mellitus had a significant negative influence on consolidation time (p=0.0313), while age (p=0.0339), smoking status (p=0.0337), diabetes mellitus (p=0.0400) and increased BMI (p=0.0315) showed a significant negative influence on the outcome of bone grafting.
Conclusion: Surgeons treating recalcitrant non-unions should be aware that an OI is common. If an OI is diagnosed subsequent to ABG the majority of patients does not need immediate revision surgery. However, special attention needs to be paid to high-risk patients.
Keywords: infection, bone healing, bone regeneration, non-union, bone infection
Plain Language Summary
Long-bone fractures that do not show timely healing remain challenging to treat. Augmentation with bone material harvested from the patient has been shown to be highly effective in treating these non-unions. However, local infections are widely believed to be a risk factor to the success of this treatment. Occult infections lack classical signs and are commonly diagnosed after bone grafting with the use of intraoperative tissue cultures unsettling surgeons. At the moment it is unclear if these occult infections affect the outcome of treatment and how common they are. The current study was designed as a large single-center case-control study including a total of 509 patients. Our data shows that despite thorough preoperative screening, occult infections occurred in 9.04%, which increased the healing duration slightly (20.4 vs 16.92 months) and impaired the outcome to a minor extent (71.42% vs.79.38%). In addition, diabetes mellitus was identified to prolong healing while diabetes mellitus, a higher BMI/age, smoking were identified as risk factors that contribute to an inferior outcome. In conclusion the current study was able to provide robust data showing that while occult infections are frequent the majority of patients don't need immediate revision surgery. However, surgeons should pay special attention to high-risk patients.
Introduction
Failed fracture healing resulting in non-union (NU) remains a frequent and challenging clinical problem. A recent large-scale cohort study suggested an overall risk of 1.9% NUs per fracture, ranging as high as 9% in certain high-risk groups.1 Considering the overall fracture-incidence (4017 per 100.000 people/year in the US in 2010),2 affected patients are numerous. NUs, often incur substantial defects and impaired biology,3 not only severely impacting patients’ quality of life, but also generating high socioeconomic costs.4–6
Despite advances, treating NUs remains challenging. In 2007, core factors for successful bone regeneration were summarized in the “diamond concept (DC)”;7 an important foundation for all NU therapies. Autologous bone grafting (ABG) remains the standard for bone-defect-augmentation8 and is a cornerstone in NU therapy,5,9 fulfilling all core factors of the DC. However, quantitative limitations remain, and alternatives have inferior biological properties.10 Thus, ABG should only be considered once risk factors negatively impacting its outcome, such as infections, have been minimized. Infection is detrimental towards both bone healing11 and bone regeneration4 and thus, if an infection is likely, transplantation of ABG should be avoided at all costs.4 Identifying occult infections (OI) remains challenging due to a lack of clinical or systemic signs11–13 making microbiological testing of tissue samples the indispensable gold standard.11,12 Unfortunately, microbiological analysis requires days4 and typically reveals an OI only after completed transplantation of ABG, unsettling treating surgeons, as uncertainty remains regarding the influence of an OI on the outcome of bone grafting.
In this study, we sought to determine the incidence of an OI in patients receiving ABG, despite thorough preoperative screening. Secondly, we evaluated whether OI influences the outcome of bone grafting compared to non-infected controls. Thirdly, we looked for associated risk factors negatively influencing therapeutic outcome.
Materials and Methods
Study Design
This study was designed as a retrospective case-control study based on a large clinical database. The institutional ethics committee of the University of Heidelberg approved the study and review of both patient data and files before commencement of the study (S-262/2017). The need for individual patient consent was waived by the ethics committee as the initial review of the patient data was carried out by the treating surgeons involved in the individual cases and all data entered into the database and used for further analysis was fully anonymized by them. Thus, patient data confidentiality was maintained at all times and the study was performed in compliance with the Declaration of Helsinki and the STROBE guideline.
All patient-related data between 01/01/2010 and 31/12/2016 was reviewed and results of microbial testing were analyzed, stratified based on bacterial species and correlated with the clinical outcome.
Inclusion and Exclusion Criteria
All patients ≥18 years presenting with a NU of the lower limb and receiving surgical treatment between 01/01/2010 and 31/12/2016 in our institution with a minimum of both clinical and radiological follow-up duration of 12 months were included. Patients requiring corticosteroid medication or chemotherapy, and patients without intraoperative microbial testing were excluded.
Preoperative Screening Program
Patients were examined in our dedicated NU-clinic. Special focus lay on detecting any evidence of previous or ongoing infection, both via blood samples (eg, CRP & WBC) and physical examination (soft tissue, mechanical stability and function). Furthermore, X-rays and CT-scans of the affected long bones were obtained to evaluate the extension of the NU and implanted materials. Previous studies in our department showed that contrast-enhanced ultrasound sonography (CEUS) could detect OI,12 thus CEUS examination was conducted. Results were evaluated by an interdisciplinary board.
Surgical Treatment
Depending on the type of NU (atrophic=impaired bone biology/hypertrophic=intact bone biology, impaired biomechanical stability) as well as the overall risk for OI, a one- or two-step procedure based on the DC14 was performed. A one-step procedure (debridement, filling of defect with ABG and BMP, revision of osteosynthesis) was conducted in NU with a small defect and no history of infection or suspicion thereof. The two-step procedure is known as “induced membrane-” or Masquelet-therapy.15 This procedure has been developed for the treatment of both infected NUs and NUs with a bone defect >5cm3,16 and since then has been widely accepted due to its clinical efficacy.16–21 Details regarding the peri- and postoperative antibiotic treatment have been published elsewhere.4
Microbial Testing
During surgical treatment, multiple tissue samples (≥5) were harvested and analyzed according to the standard of care of our microbiological department.4,22 All agar plates were at least incubated for 5 days, fluids provided in blood culture bottles even for 14 days to ensure detection of slow growing organisms, eg, cutibacteria or specific yeast. All evidence of bacteria was reviewed by an experienced microbiologist and stratified based on their origin and pathogenicity. Evidence of bacteria was classified as infection if obligate pathogen bacteria were evident or if more than 2 independent samples were positive for the same facultative pathogen bacteria.4 In order to assess the probability of a deep tissue infection compared to a risk of sample contamination from bacteria that are evident on the skin of a patient we developed a simple scoring system termed “certainty score” (number of positive samples (PS), absolute number of samples (AS)):
Here, a higher number indicates a higher probability of deep tissue infection.
Evaluation of Outcome
Subsequent to treatment, clinical and radiological follow-up was scheduled after 6 weeks as well as 3, 6 and 12 months and thereafter annually. During each follow-up appointment, two blinded experienced trauma surgeons independently evaluated bone fusion based on the latest available x-rays and CT-scans. Consolidation was defined as cortical bridging of at least three out of four cortices visible in x-rays and/or CT-scans. Healing time was defined as the duration between the final surgical treatment and the date on which the NU was rated as consolidated. In addition, two experienced trauma surgeons independently performed clinical evaluation of weight-bearing, walking distance free of pain and clinical signs of mechanical stability during the regular follow-up examinations.
Statistics
Statistical analysis was performed with the open software R, version 4.0.2,23 applying the packages “tidyr”,24 “dplyr”,25 “pROC”,26 and “ggplot2”27 for receiver operator characteristics (ROC) analysis corresponding to our previous studies.28,29 The Shapiro–Wilk test was used for assessing the normal distribution of values. Boschloos’ test was used to assess statistically significant differences in 2-level variables such as the patients’ sex, smoking, diabetes and the treatment.30 To assess differences in categorical variables with more than 2 levels the Fisher’s exact test was utilized (Tables 1 and 2). For variations of the variable age, the non-parametric Kruskal–Wallis H-test for independent samples was used. To predict both the time to as well as the event of a consolidation multiple regression modelling was utilized. The model selection was performed via 10-fold cross-validation of a stepwise RMSE (Root Mean Square Error) comparison and backward selection using the packages “MASS”31 and “leaps”.32 The selection process was intended to choose an optimized combination from 3 to 7 out of 7 variables of interest. The primary measure for predictive performance of any logistic regression model was the area under the curve (AUC) of the ROC-curve. Differences between ROC curves were assessed by the DeLong’s test for two correlated ROC curves.33 The level of significance (α) was set at 0.05. All p values reported are to be interpreted descriptively as they were not adjusted for multiple testing. Flowchart and figures were edited and/or created using BioRender.com.
 |
Table 1 Patients’ Characteristics of the Entire Collective |
 |
Table 2 Patients Characteristics of the Study Group (SG) |
Results
Incidence of OI
To evaluate the incidence of OI while minimizing potential selection bias, we analyzed all cases treated in our level-1 trauma center regardless of the follow-up duration. A total of 625 patients suffering from NU were surgically treated at our institution and anatomically the overwhelming majority was located in the lower extremity (509 cases (81.4%)). Only 116 cases (18.6%) were located in the upper extremity. All 509 cases received ABG transplantation as part of NU treatment and 46 cases were tested positive for an OI in the procedure in which application of ABG was performed. Thus, despite our thorough preoperative screening program, an incidence of 9.04% of OI after NU treatment of the lower limb remains.
The Influence of an OI on the Outcome of ABG Transplantation
After applying our strict inclusion and exclusion criteria, a total of 307 cases had to be excluded due to an insufficient follow-up duration. Interestingly, 303 (98.7%) of the excluded cases were patients without an OI, and only 4 (1.3%) were cases in which an OI was detected (exclusion and allocation process is shown in Figure 1). After applying the exclusion criteria, 160 patients were assigned to the control group (CG, no OI), and 42 patients were assigned to the study group (SG, OI). Patient demographics are shown in Table 1. While groups were comparable regarding age, the number of smokers, patients suffering from diabetes, ASA and anatomical localization, significant differences were observed in the gender distribution (p=0.004) as well as serum levels of CRP and leukocyte count (p=0.050 and p=0.007, respectively).
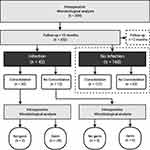 |
Figure 1 The patient allocation and inclusion process into individual analyses is visualized. Abbreviation: OP, operative procedure. |
Average consolidation time was longer in the SG compared to the CG (1.70 years vs 1.41 years, respectively) although differences were at a non-significant extent (p=0.345). In addition, the final evaluation of the outcome revealed an impaired consolidation rate in the SG compared to the CG to a non-significant extent (127/160 (79.38%) in the CG vs 30/42 (71.42%) in the SG, p=0.299). Consolidation times and outcome for both groups are visualized in Figure 2A.
Identifying Both Individual and Clusters of Risk Factors Predisposing for Treatment Failure
Table 2 shows the patient demographics of the SG stratified regarding the outcome of therapy (responders to therapy (R) and non-responders (NR)). No differences were detected in the distribution of sex, BMI, anatomical localization, fracture type and leukocyte count. NR showed a higher number of smokers (NR: 50.0% vs R: 23.3%, p=0.104), a significantly higher number of patients suffering from diabetes (NR: 33.3% vs R: 3.3%, p=0.011), a higher ASA score (ASA score >2 in NR: 91.67% vs R: 60.00%, p=0.075), significantly higher average CRP levels (NR: 18.98 mg/l vs R: 8.16 mg/l, p=0.036) and were significantly older (NR: 53.06 years old vs R: 43.26 years old, p=0.014). No significant differences in pathogen distributions were found between NR and R (p=0.067), while the overall majority of bacteria in both groups was Staphylococcus epidermidis (NR: 61.1% vs R: 75.0%), more Candida parapsilosis, Enterococcus faecalis and Staphylococcus aureus were found in NR, and more Enterobacterales and other gram-positive bacteria found in R. Individual outcomes for each individual bacterium and grouped pathogens are shown in Figure 3A and B.
Samples with a lower bacteria count needed further enrichment to specify the individual pathogen, which is known to be associated with sample contamination. In order to test our developed “certainty score” we assigned all cases needing enrichment into a group (E) and compared the pathogen distribution and the “certainty score” with samples that needed no enrichment (NE) (Supplementary Table 1). This analysis showed that 93.3% of bacteria in the E group were Staphylococcus epidermidis compared to 60% in NE and the “certainty score” was significantly higher in the NE (NE: 1.06 vs E: 0.78, p=0.041). In contrast, the “certainty score” showed no differences regarding NR and R (p=0.909).
Regression modelling was set up to analyze the predictive potential of clinical characteristics regarding (A) the time to consolidation and (B) the presence or absence of consolidation in the future. The linear regression Model A was composed by the patients' ASA, age, and the presence of diabetes. Here, a significantly negative influence on consolidation time was only seen for diabetes mellitus (p=0.0313). The favored logistic regression model (B) included the variables age, smoking, diabetes and the patients’ BMI. While all risk factors had a negative influence on the outcome of therapy, diabetes mellitus and subsequently, smoking had the highest impact (Supplementary Table 2)
The DeLong’s test indicated that model B outperformed any of the constituting variables in a univariate model, such as the patients' age (p=0.0236), BMI (p=0.0108), the presence of diabetes (p=0.0044), and the smoking status (p<0.0001). The ROC curve of the final logistic regression model is shown in Figure 2B and indicates an area under the curve (AUC) of 90.3%. Based on the Youden’s Index, an optimal cutoff was estimated with a sensitivity of 90.0% and a specificity of 91.7%.
Discussion
In the current study, we sought to determine the incidence of OI in context with bone grafting and to further analyze its impact on outcome and influencing risk factors.
The Incidence of OI Subsequent to Transplantation of ABG
Bone grafting is a commonly used surgical procedure, but evidence regarding the rate of infection subsequent to ABG remains limited. In addition, most studies analyzed new-onset postoperative infections which can be either caused by an OI or iatrogenic infections due to microbiological contamination of the grafting or implant material.34 Existing evidence indicates an overall infection risk of 3.05%35 and a new-onset postoperative infection rate around 12.4%.36 In the current study, samples were harvested immediately prior to bone grafting, thereby minimizing the iatrogenic contribution. Thus, our study gives first evidence regarding an incidence of 9.04% of an OI during NU treatment despite our dedicated preoperative screening program. Surgeons should be aware that despite a negative history of infection and diagnostics, a substantial number of patients suffer from an OI. Ultimately, our results emphasize the continuing need for microbiological testing of multiple (≥5) samples harvested during each surgery. Furthermore, our data indicate that when in doubt, surgeons should treat patients as if they have an infected NU rather than relying on the results of preoperative screening.
Does an OI Have a Detrimental Effect on the Outcome of Therapy?
If an infection is present, therapies intend to eradicate the infection before achieving bone regeneration and finally osseous union.3,5,7,15,16,37 A recent review from 2015 stated that no robust evidence regarding the outcome of the treatment of infected long-bone NU is available,38 thus highlighting the need for further large-scale studies of high-quality. The current study is the largest single-center study to date investigating the influence of OI on the outcome of bone reconstruction, and our data showed that union was achieved in patients without an OI in 79.38% and in patients with an OI in 71.4%. Our union rate was at the lower end of the previously published range, which may be due to our strict definition of osseous union, as well as strict inclusion criteria. Reported consolidation times of surgically treated NUs range between 4 and 16 months.39,40 In our study consolidation was achieved in patients without an OI after an average of 1.7 years compared to 1.41 years in patients of the study group. Compared to available evidence, consolidation time in our study was considerably longer. This may have been caused by our definition of osseous consolidation: while continuing osseous consolidation seen in X-rays during follow-up was evaluated as ongoing healing, NUs were only defined as consolidated once 75% of circumference was healed. Interestingly, consolidation of occult infected NUs took only slightly longer (15.08 weeks) and was only slightly impaired (7.98%) when compared to uninfected NUs and differences were at a non-significant extent. This rather mild influence might be explained by the standardized antibiotic treatment in our institution. Helbig et al showed that 90% of bacteria detected in NUs were susceptible to our antibiotic regime resulting in a high percentage of eradication.4
Evidence of an OI unsettles surgeons and patients alike. The results from the current study indicate that despite the detection of an OI, outcome and consolidation time are only slightly impaired. Thus, surgeons should inform patients and encourage a meticulous follow-up in order to detect non-responders early.
Defining Risk Factors That Influence the Outcome of NU Therapy in Patients with an OI
Thirdly, we sought to determine whether risk factors prolonging or impairing consolidation of NUs could be identified. Our results indicated that consolidation time is significantly prolonged by diabetes mellitus, while higher age, smoking, higher BMI and diabetes mellitus have a significantly negative effect on the outcome of therapy. Recent studies have shown that age itself does not impair healing of NUs when compared to a younger control group,16 however, it is known that elderly patients are more susceptible for infectious diseases due to physiological changes on a cellular and molecular basis.41 Therefore, the negative influence of age on the outcome of NU therapy might be reserved to NUs with an (occult) infection. While the detrimental effects of smoking on bone healing are well known, obesity has only recently been associated with an impaired immune response and thus higher susceptibility to infections.42 Diabetes mellitus is established as an important risk factor for bacterial infections due to higher oxidative stress in the tissue of affected patients.43 The results of the current study are supported by the findings of a large-scale population-based study that identified higher age and diabetes mellitus as risk factors regarding the outcome of bone grafting.35 In summary, our data indicate that surgeons should be aware of these distinct risk factors that directly influence the outcome of NU therapy. Furthermore, measures should be taken to mitigate the influence of risk factors such as smoking and diabetes mellitus by encouraging smoking cessation and reduction of HbA1c prior to NU treatment.
Limitations
This study has several limitations. Despite treating numerous patients at our institution, strict inclusion and exclusion criteria, as well as only a small number of patients showing OI, led to a reduced number of patients being included in the current study. Both national and international patients are treated in our institution. Thus, off-site follow-up care is frequently reducing the follow-up in our hospital. Nevertheless, the current study is the largest single center studies to date investigating occult infection in context with the outcome of ABG. Therefore, our results give surgeons and clinicians reliable and robust information on how to treat such cases effectively. While Candida species in the current were analyzed a full fungal culture is missing. Fungal osteomyelitis is considered a rare disease and patients at risk are mostly severely immunocompromised,44 however, readers should be aware that the current study cannot address the influence and incidence of other fungal species. In addition, mycobacterium tuberculosis was not included in the current study. Reasons are the low incidence of tuberculosis in Germany as well as the low rate of skeletal manifestations,45,46 resulting in a likelihood of less than one per million for mycobacterial osteomyelitis at the beginning of the observation period 2010. Although we believe that these limitations do not influence the importance and validity of our findings caregivers should be aware of these missing pathogens when reading and interpreting the results of the study.
Conclusion
Surgeons treating recalcitrant non-unions should be aware that the incidence of an occult infection remains at 9.04% despite a negative history of infection and thorough diagnostics. If an occult infection is diagnosed subsequent to ABG transplantation, our data indicate that the majority of patients does not need immediate revision surgery. However, certain risk factors exist that prolong and impair the outcome of therapy. Thus, special attention needs to be paid towards these high-risk patients.
Abbreviations
NU, Non-union; DC, Diamond concept; ABG, Autologous bone graft; OI, Occult infection; CRP, C-reactive protein; WBC, White blood cell; CEUS, Contrast-enhanced ultrasound sonography; BMP, Bone morphogenic protein; ROC, Receiver operator characteristics; SG, Study group; CG, Control group; R, Responder; NR, Non-responder; E, Enrichment; NE, No Enrichment.
Data Sharing Statement
The raw data used to support the findings of this study is highly sensitive patient data and cannot be shared due to confidentiality. Fully anonymized data is potentially available from the corresponding author upon reasonable request.
Funding
No funding was received for this study.
Disclosure
Mr Raban Arved Heller reports grants from Oskar-Helene-Heim foundation, Berlin, during the conduct of the study. The authors declare no other conflicts of interest.
References
1. Mills LA, Aitken SA, Simpson A. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017;88(4):434–439. doi:10.1080/17453674.2017.1321351
2. Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ
3. Giannoudis PV, Harwood PJ, Tosounidis T, Kanakaris NK. Restoration of long bone defects treated with the induced membrane technique: protocol and outcomes. Injury. 2016;47(Suppl 6):S53–S61. doi:10.1016/S0020-1383(16)30840-3
4. Helbig L, Bechberger M, Aldeeri R, et al. Initial peri- and postoperative antibiotic treatment of infected nonunions: results from 212 consecutive patients after mean follow-up of 34 months. Ther Clin Risk Manag. 2018;14:59–67. doi:10.2147/TCRM.S152008
5. Moghaddam A, Thaler B, Bruckner T, Tanner M, Schmidmaier G. Treatment of atrophic femoral non-unions according to the diamond concept: results of one- and two-step surgical procedure. J Orthop. 2017;14(1):123–133. doi:10.1016/j.jor.2016.10.003
6. Moghaddam A, Zietzschmann S, Bruckner T, Schmidmaier G. Treatment of atrophic tibia non-unions according to ‘diamond concept’: results of one- and two-step treatment. Injury. 2015;46(Suppl 4):S39–50. doi:10.1016/S0020-1383(15)30017-6
7. Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3–6. doi:10.1016/S0020-1383(08)70003-2
8. Hannouche D, Petite H, Sedel L. Current trends in the enhancement of fracture healing. J Bone Joint Surg Br. 2001;83(2):157–164. doi:10.1302/0301-620X.83B2.0830157
9. Schmidmaier G, Moghaddam A. Long bone nonunion. Z Orthop Unfall. 2015;153(6):659–674. (). doi:10.1055/s-0035-1558259
10. Haubruck P, Ober J, Heller R, Miska M, Schmidmaier G, Tanner MC. Complications and risk management in the use of the reaming-irrigator-aspirator (RIA) system: RIA is a safe and reliable method in harvesting autologous bone graft. PLoS One. 2018;13(4):e0196051. doi:10.1371/journal.pone.0196051
11. Mills L, Tsang J, Hopper G, Keenan G, Simpson AH. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Joint Res. 2016;5(10):512–519. doi:10.1302/2046-3758.510.BJR-2016-0138
12. Fischer C, Preuss EM, Tanner M, et al. Dynamic contrast-enhanced sonography and dynamic contrast-enhanced magnetic resonance imaging for preoperative diagnosis of infected nonunions. J Ultrasound Med. 2016;35(5):933–942. doi:10.7863/ultra.15.06107
13. Haubruck P, Heller R, Tanner MC, et al. A preliminary study of contrast-enhanced ultrasound (CEUS) and cytokine expression analysis (CEA) as early predictors for the outcome of tibial non-union therapy. Diagnostics (Basel). 2018;8(3). doi:10.3390/diagnostics8030055.
14. Giannoudis PV, Einhorn TA, Schmidmaier G, Marsh D. The diamond concept–open questions. Injury. 2008;39(Suppl 2):S5–8. doi:10.1016/S0020-1383(08)70010-X
15. Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41(1):27–37. doi:10.1016/j.ocl.2009.07.011
16. Tanner M, Vlachopoulos W, Findeisen S, et al. Does age influence the outcome of lower limb non-union treatment? A matched pair analysis. J Clin Med. 2019;8(9):9. doi:10.3390/jcm8091276
17. Calori GM, Giannoudis PV. Enhancement of fracture healing with the diamond concept: the role of the biological chamber. Injury. 2011;42(11):1191–1193. doi:10.1016/j.injury.2011.04.016
18. Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42(6):591–598. doi:10.1016/j.injury.2011.03.036
19. Gugala Z, Gogolewski S. Healing of critical-size segmental bone defects in the sheep tibiae using bioresorbable polylactide membranes. Injury. 2002;33:71–76. doi:10.1016/S0020-1383(02)00135-3
20. Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22(1):73–79. doi:10.1016/S0736-0266(03)00165-7
21. Raven TF, Moghaddam A, Ermisch C, et al. Use of Masquelet technique in treatment of septic and atrophic fracture nonunion. Injury. 2019;50:40–54. doi:10.1016/j.injury.2019.06.018
22. Dapunt U, Spranger O, Gantz S, et al. Are atrophic long-bone nonunions associated with low-grade infections? Ther Clin Risk Manag. 2015;11:1843–1852. doi:10.2147/TCRM.S91532
23. Team RDC, Liverani S, Richardson S. R: a language and environment for statistical computing. R Found Stat Comput. 2015;25(5):1. doi:10.1007/s11222-014-9471-3
24. Wickham H, Wickham MH. Package ‘tidyr’. Easily Tidy Data with’spread’and’gather ()’Functions. 2017.
25. Wickham H, Francois R, Henry L, Müller K. dplyr: a grammar of data manipulation. R Package Version. 2015;3.
26. Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12(1):77. doi:10.1186/1471-2105-12-77
27. Wickham H. Ggplot2: Elegant Graphics for Data Analysis. R Package Version 2.1.0. New York: Springer-Verlag; 2009.
28. Moghaddam A, Heller R, Daniel V, et al. Exploratory study to suggest the possibility of MMP-8 and MMP-9 serum levels as early markers for remission after traumatic spinal cord injury. Spinal Cord. 2016.
29. Moghaddam A, Sperl A, Heller R, et al. Elevated serum insulin-like growth factor 1 levels in patients with neurological remission after traumatic spinal cord injury. PLoS One. 2016;11(7):e0159764. doi:10.1371/journal.pone.0159764
30. Boschloo RD. Raised conditional level of significance for the 2 × 2-table when testing the equality of two probabilities. Statistica Neerlandica. 1970;24(1):1–9. doi:10.1111/j.1467-9574.1970.tb00104.x
31. Ripley B, Venebles B, Bates D, Hornik K, Gebhardt A, Firth D. Package ‘MASS’. Support Functions and Datasets for Venables and Ripley’s MASS Web site. 2013.
32. Lumley T. Regression Subset Selection. Fortran code by Alan Miller Web site. 2013.
33. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
34. Takamoto M, Takechi M, Ohta K, et al. Risk of bacterial contamination of bone harvesting devices used for autogenous bone graft in implant surgery. Head Face Med. 2013;9(1):3. doi:10.1186/1746-160X-9-3
35. Lee FH, Shen PC, Jou IM, Li CY, Hsieh JL. A population-based 16-year study on the risk factors of surgical site infection in patients after bone grafting: a cross-sectional study in Taiwan. Medicine (Baltimore). 2015;94(47):e2034. doi:10.1097/MD.0000000000002034
36. Flierl MA, Smith WR, Mauffrey C, et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. J Orthop Surg Res. 2013;8:33. doi:10.1186/1749-799X-8-33
37. Moghaddam A, Ermisch C, Fischer C, Zietzschmann S, Schmidmaier G. Tibiadefekt- und Infektpseudarthrosen. Der Orthopäde. 2017;46(3):263–274. doi:10.1007/s00132-016-3305-2
38. Kanakaris NK, Tosounidis TH, Giannoudis PV. Surgical management of infected non-unions: an update. Injury. 2015;46(Suppl 5):S25–32. doi:10.1016/j.injury.2015.08.009
39. Giannoudis PV, Gudipati S, Harwood P, Kanakaris NK. Long bone non-unions treated with the diamond concept: a case series of 64 patients. Injury. 2015;46(Suppl 8):S48–54. doi:10.1016/S0020-1383(15)30055-3
40. Giannoudis PV, Kanakaris NK, Dimitriou R, Gill I, Kolimarala V, Montgomery RJ. The synergistic effect of autograft and BMP-7 in the treatment of atrophic nonunions. Clin Orthop Relat Res. 2009;467(12):3239–3248. doi:10.1007/s11999-009-0846-2
41. Gardner ID. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980;2(5):801–810. doi:10.1093/clinids/2.5.801
42. Dobner J, Kaser S. Body mass index and the risk of infection - from underweight to obesity. Clin Microbiol Infect. 2018;24(1):24–28. doi:10.1016/j.cmi.2017.02.013
43. Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144(2):171–185. doi:10.1111/imm.12394
44. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390–401. doi:10.5435/JAAOS-22-06-390
45. Corr PD. Musculoskeletal fungal infections. Semin Musculoskelet Radiol. 2011;15(5):506–510. doi:10.1055/s-0031-1293496
46. Fiebig L, Hauer B, Brodhun B, Altmann D, Haas W. Tuberculosis in Germany: a declining trend coming to an end? Eur Respir J. 2016;47(2):667–670. doi:10.1183/13993003.01410-2015
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.