Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
The Incidence of Adverse Drug Reaction Among Adult Patients on Antiretroviral Therapy in Ethiopia: Frailty Model
Authors Menza M
Received 14 January 2022
Accepted for publication 4 April 2022
Published 13 April 2022 Volume 2022:14 Pages 155—165
DOI https://doi.org/10.2147/HIV.S358351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Mesfin Menza
School of Public Health, College of Medicine and Health Sciences, Wachemo University, Hossana, Ethiopia
Correspondence: Mesfin Menza, Email [email protected]
Introduction: Adverse drug reactions are a major global public health concern and an important cause of hospitalization, discontinuation of the drug, morbidity and mortality. Even though the prevalence in Ethiopia was declining at a moderate rate, still, far too many people are suffering and dying unnecessarily due to adverse drug reactions.
Objective: The aim of this study was to determine the incidence of adverse drug reaction and its predictors among adult patients on antiretroviral therapy.
Methods: A retrospective follow-up study was conducted at Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital, Southern Ethiopia. Data were extracted from patients’ medical records. The Weibull model with gamma frailty distribution was fitted. Statistical significance was employed at a 5% level of significance and adjusted hazard ratio with 95% confidence interval was used.
Results: Out of the total 376 participants followed for 1988 person years of observations, 96 developed adverse reaction with the incidence rate of 4.820/100 per (95%CI: 4.102– 5.317). The univariate frailty was statistically significant (theta=0.306, 95%CI: 0.102– 0.521). Baseline CD4 count (AHR: 0.997, 95%CI: 0996– 0.998), fair adherence (AHR: 2.358, 95%CI: 1.133– 4.904), poor adherence (AHR: 3.069, 95%CI: 1.730– 5.445), HIV/TB coinfection (AHR: 2.069, 95%CI: 1.115– 3.843), WHO stage II (3.128, 95%CI: 1.414– 6.916), WHO stage III (AHR: 2.709, 95%CI: 1.048– 7.025) and WHO stage IV (1.516, 10.352) were associated with the incidence adverse reaction.
Conclusion: Most of the ADR cases occurred within two years after initiation of ART. Advanced clinical stage, TB coinfection, CD4 count, and poor adherence were predictors of ADRs. Continuous counseling for clients in advanced clinical stage and patients with TB coinfection need to get close follow-up to prevent the associated ADRs by the concerned parties.
Keywords: antiretroviral therapy, adverse reaction, HIV/AIDS, frailty model
Background
The human immunodeficiency virus (HIV) targets the immune system and weakens the body’s defense mechanism against many infections and some types of cancer that people with healthy immune systems can fight off. Acquired immunodeficiency syndrome (AIDS), the most advanced stage of HIV infection is defined by the development of certain cancers, infections or other severe long-term clinical manifestations.1 Globally 37.7 million people were living with HIV in 2020; among those 36 million were adults.2 In Africa almost 26 million people living with HIV, which accounts for 70% of all AIDS-related deaths in the world. East and Southern Africa remain the regions in the world most seriously affected by the pandemic.1,2 In 2020, HIV prevalence in Ethiopia was 0.9%. Ethiopia is one of the countries hardest hit by HIV/AIDS, with a prevalence of 1.1% among people of all ages and 59% of those infected receiving highly active antiretroviral therapy (HAART).3
Even though HIV is not curable, it can be controlled and treated through antiretroviral therapy (ART). ART can keep patients healthy for many years by reducing the amount of virus (viral load) in the blood and body fluids. It is recommended for all people diagnosed with HIV, as it not only helps slow its progression, but also reduces the chances of transmitting the virus to other people.4 However these drugs are associated with adverse effects. Adverse drug reactions (ADRs) are harmful and unintended reactions to medicines given at standard doses through a proper route of administration for the purpose of prophylaxis, diagnosis, or treatment.5 Hepatotoxicity was the most prevalent ADR, accounting for 29.4% of hospital admissions, followed by acute renal injury (22.7%), skin reactions (6.7%), hypokalemia (5.7%), and gastrointestinal hemorrhage or gastritis (5.7%).6,7
The incidence of ADRs varies from region to region, with India reporting 32.45%, Malaysia 42.2%, South Africa 44.5%, Nigeria 72%, and Ethiopia reporting 22.1% to 85.7%.8–11 The rate of adverse drug reactions is affected by a number of factors, but the most important ones were a decrease in CD4 cell count, late WHO clinical stages, not receiving OIP, a TDF-NVP-containing regimen, being bedridden at the time of treatment initiation, and concurrent treatment administration.8–11 There is significant evidence that treatment success is associated with adherence to ART. ADR is the major barrier to antiretroviral therapy adherence for patients living with the virus.12 In European countries 30.4%, Botswana 9%, Ethiopia 7.7% of patients discontinue ART due to ADR.13–15 The occurrence of ADRs associated with the use of ART is common and leads to non-adherence to ART, poor and inconsistent adherence to ART then lead to drug resistance and even death.16
The cost of a preventive adverse drug reaction (including direct and indirect costs) is enormous. ADR-related expenditure, including hospitalization, surgery, and lost productivity, in certain countries outweighs the cost of drugs.17 The expenditure of preventable ADRs (per hospitalization) varied from €2851 to €9015 in an in-patient environment, with an excess length of stay ranging from 4.2 to 13.0 days. In outpatient trials, however, expenses attributable to preventable ADRs ranged from €174 to €8515 (mean hospital cost per admission) with a duration of stay ranging from 7.0 to 9.3 days.18
ADRs are one of the leading causes of hospital admissions, morbidity and mortality in the world. The median (with interquartile range; IQR) prevalence of ADR-related hospitalization in developed and developing countries was 6.3% (3.3–11.0) and 5.5% (1.1–16.9), respectively.6 Evidence from previous studies showed that the rates of readmission due to ADRs varied from 3% to 64% (median: 21%, IQR: 14–23%). Readmissions were deemed preventable in 5% to 87% of cases (median: 69%, IQR: 19–84%).19
Even though the prevalence in Ethiopia was declining at a moderating rate from 2.4% in 2001 to 0.9% in 2020, still, far too many people are suffering and dying unnecessarily due to both HIV/AIDS and adverse drug reactions.20 Few studies in the region have shown that ARV drug toxicity is a common occurrence and major reason for ART regimen change, modification and non-adherence.21,22 In resource limited setting including Ethiopia, where treatment options are limited; information about the development of adverse reaction to ART is vital for monitoring the risks. However, there was limited information on the subject area in Ethiopia. The aim of this study is to assess the incidence and predictors of ADRs in adult patients on ART at Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital, Southern Ethiopia using frailty model which reduces bias in parameter estimates by capturing variability between individuals. It will also be profitable for the health care planners to develop successful evidence based targeted preventive strategies.
Materials and Methods
Study Setting, Study Design and Period
A retrospective follow-up study was conducted in Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital which is found in Hosanna Town, Hadiya Zone, Southern Ethiopia from March 1, 2017 to April 30, 2021. It is located 235 km from Addis Ababa, the capital city of Ethiopia and 194 km from Hawassa, the capital city of SNNPR. The Hospital provides general and specialty health care services along with teaching and research activities. It also renders comprehensive HIV/AIDS-related services including, voluntary counseling and testing (VCT), provider initiated testing and counseling (PITC), prevention of mother to child transmission (PMTCT) and ART program. ART clinic is one of the former clinics of the hospital where care and follow-ups are given for patients with HIV/AIDS.
Populations
All adult patients above the age of 15 years on ART at the ART clinic of Nigist Elleni Mohamed Memorial Hospital were the source population. Study populations were all adult patients above the age of 15 years on ART at the ART clinic of Nigist Elleni Mohamed Memorial Hospital between March 1, 2017 and April 30, 2021. All adult patients above the age of 15 years on ART who initiated first-line ART from March 1, 2017 to April 30, 2021 at the ART clinic of Nigist Elleni Mohamed Memorial Hospital were included in the study.
Sample Size and Sampling Procedure
The sample size was determined using Schoenfeld formula23 using STATA 14.1 based on the power approach under the following assumptions: Cox proportional hazard model, 95% confidence level, 80% power, 10% withdrawal probability. By taking a hazard ratio for three predictors significantly associated with ADRs in a study conducted in Bahir Dar,11 the sample size calculation is summarized in Table 1.
 |
Table 1 Minimum Sample Size Calculated for Predictors Significantly Associated with ADRs |
Accordingly, a final sample size of 376 was obtained. The study subjects were selected by simple random sampling technique using computer generated random numbers by R software version 3.6.1. Sampling frame was prepared by collecting the identification number of from the registration book.
Operational Definitions
Adverse drug reaction: a new case of unfavorable condition reported at least once on HIV-infected adult patients after enrolment on ART. The major ADRs included anemia, skin rash, pain (tingling) of extremities, vomiting, hepatotoxicity, and jaundice.
Censored: the event of interest may not be observed at the follow-up period, death before the occurrence of ADRs, and lost to follow-up before the occurrence of ADRs.
Event: the outcome of interest (the occurrence of ADRs), which occurs during the follow-up time.
Time to adverse reaction: the time interval between initiations of ART to occurrence of first episode of adverse reaction.
BMI: based on the WHO classification, normal weight is between 18.5 and 24.9 kg/m2, BMI less than 18.5 and greater than 25 are underweight and overweight, respectively.
Adherence: adherence was classified as good, fair, or poor, according to the percentage of drug dosage calculated from a monthly total dose of ART drugs. Therefore, good was reported if equal to or greater than 95% or ≤3 doses were missed per month, fair if 85–94% or 4–8 doses were missed per month, or poor if less than 85% or ≥9 doses were missed per month.
Comorbidities: diabetes mellitus, chronic kidney diseases, hypertension, asthma, heart disease.
Normal hemoglobin level: indicates free of anemia with an interval of ≥11.9 g/dL.
Data Collection Procedure and Quality Assurance
Secondary data were collected from patient intake form and follow-up, which are recorded routinely by the hospitals for follow-up, monitoring and evaluation of patients. Before actual data collection, a preliminary review was conducted among 5% of the sample size to evaluate the adequacy of the checklist. Variables such as residence and income level are not available in the record and not considered in this study. Data were collected using ODK which was prepared with relevant restrictions and necessary commands, by health officers and nurses working in the hospital. The completed checklist was checked for consistency and completeness before entry. Then data entry was cross-checked and we clarified the missing data.
Data Management and Statistical Analysis
After data collection by ODK version 1.25.2, each completed forms were checked for completeness, inconsistencies, coding error and missing values and were exported to STATA 14 and R statistical software version 3.6.1 for further analysis. Descriptive measures such as mean with standard deviation, median with interquartile range (IQR), percentages and frequencies were used to characterize the data. Besides, graphical methods and charts were used for descriptive data. The median follow-up time to ADRs was estimated using the life table and the log rank test was used to compare survival time between groups of categorical variables. Proportional hazard assumption was checked using the Kaplan–Meier survival plot graphically and Schoenfeld residual test statistically before fitting the survival model.
Different survival analysis models were fitted and compared to select best fitted model. The conventional Cox proportional hazard model may not fit the data well all the time, and may leads to incorrect inferences when all levels of relevant covariates have not been observed or included. The good fitted model was selected for multivariable analysis, by comparing parametric and semi-parametric model. Even though individuals are similar on the basis of the observed variables some individuals are frailer than others, since there are random variables that varies over the population frailty model was carried out to examine predictors of ADRs. The factors significantly associated with ADRs in the univariable analysis at P-values less than 0.25 were included in the multivariable survival model. To examine random variables that vary over the population univariate frailty model was carried out. Statistical significance was employed at level of significance of 5%, and adjusted hazard ratio with 95% confidence interval was used to present estimates of the strength of the association.
Result
Sociodemographic Characteristics of the Participants
A total of 376 patients who were on ART between 2017 and 2021 were collected. The mean baseline age (±SD) of study participants was 34.8 (±10) years. Half (50.27%) were females and nearly half of the participants (52.39%) were married. Among the study participants, 106 (28.19%) attended college and above followed by secondary 103 (27.39)(Table 2).
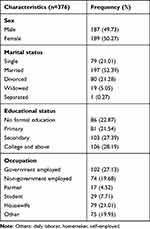 |
Table 2 Sociodemographic Characteristics of Patients on ART Follow-up at Nigist Elleni Mohamed Memorial Hospital, Hossana, Ethiopia, 2021 |
Baseline Clinical and Behavioral Characteristics
Among study participants, 329 (87.5%) of the study participants had a baseline CD4 count greater than 200 cells/mm3 and 196 (52.41%) were in the first WHO stage at the baseline. The predominant HAART regimen initially prescribed for the patients was a combination of tenofovir, lamivudine and efavirenz (TDF-3TC-EFV) 199 (53.07%) followed by zidovudine, lamivudine and nevirapine (AZT-3TC-NVP) 82 (21.87%). In this study, while 31.97% of the study participants had ever smoked tobacco products, nearly 38.00% had ever drunk alcoholic drinks. Among the study participants, 96 (25.53%) of the study subjects had developed ADRs (Table 3).
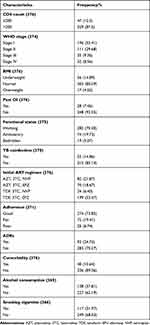 |
Table 3 Baseline Clinical and Behavioral Characteristics of Patients on ART on Follow-up at Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital, Hossana, Ethiopia, 2021 |
The Occurrence of ADRs
Out of 376 study participants followed retrospectively for the last five years; a total of 96 (25.5%) of them had developed adverse drug reactions in 1988 person years (PY) of observations. Of 96 events of ADRs, the most common were rash 38 (40.8%) followed by anemia 22 (23.6%) (Figure 1). The incidence rate was 4.820 per 100 person-year observation with 95%CI of (4.102–5.317).
 |
Figure 1 Type of ADRs of patients on ART at Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital, Hossana, Ethiopia, 2021. |
Survival Probability of Patients on ART
About 69 (71.9%) adverse drug reactions were occurred within the first two years of the initiation of ART. The cumulative probability of survival without developing ADRs at the end of two years and the end of the follow-up period was 0.66 and 0.022 years respectively. Due to the smaller proportion of the event in the cohort, the median survival time was not estimable (Table 4).
 |
Table 4 Life Table for Adverse Drug Reaction of Patients on ART at Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital, Hossana, Ethiopia, 2021 |
Predictors for the Incidence of ADRs
Before trying to fit the data with any of the models, the proportional hazard assumption was checked for each of the covariates using the Kaplan–Meier survival plot, and the results were not satisfactory to assume the lines were parallel for some covariates. However looking at the Kaplan–Meier survival plot is not enough to be certain of proportionality. Therefore, we checked the proportionality assumptions using Schoenfeld residual test. Schoenfeld residual test for all covariates was insignificant with (P>0.05) implied that the proportional hazards assumption has been fulfilled and also the global test was insignificant, which indicated that the PH assumptions holds (Figure 2).
Different techniques of survival analysis were tried to find a model that best fits the data. First, the proportional hazard model was fitted; then, the Weibull, exponential, log-logistic, Gompertz and log-normal parametric survival models were fitted. Finally, these parametric models with the gamma and inverse Gaussian univariate frailty models were fitted. Out of which the one that gave the best fit was selected based on AIC and BIC.
Accordingly, a model with a baseline hazard of Weibull distribution with a univariate frailty of gamma distribution was selected as the best fit to the data. Thus, four variables including sex, baseline CD4 count, WHO-stage, and TB/HIV coinfection were found to be statistically significantly associated with the hazard of getting adverse drug reactions.
For instance, the hazard of experiencing ADRs was two times higher among patients with HIV/TB coinfection as compared to those without TB coinfection (AHR=2.069, 95%CI: 1.115–3.843). For each additional cell/mm3, the hazard of experiencing ADRs decreased by 0.003% (AHR=0.997, 95%CI: 0.996–0.998). Baseline CD4 count was negatively associated with the risk of developing ADRs for adult patients on ART.
The shape parameter, P, was estimated to be 1.481 (95%CI: 1.254–1.748). The variability among individuals was also captured by parameter “theta” and estimated to be 0.306 (95%CI: 0.102–0.521) (Table 5).
 |
Table 5 The Predictors of the Incidence of ADRs Among Patients on ART, at Nigist Elleni Mohamed Memorial Comprehensive Specialized Hospital, Hossana, Ethiopia, 2021 |
Discussion
In the current study, we included 376 participants who were on ART since 2017 and were followed until mid-2021. Adverse reaction is an increasingly important issue in the management of HIV-infected patients. Therefore, we estimated the incidence and identified important predictors of ADRs. Baseline CD4 count, adherence, WHO-stage, and TB/HIV coinfection were found to be predictors of ADRs.
The baseline hazard (the effect of time when all categorical variables are at a reference category and when all continuous covariates are at zero) of experiencing ADRs was fitted with Weibull distribution with a shape parameter (P) greater than one (P=1.481, 95%CI: 1.254–1.748). The Weibull shape parameter greater than one implies that the hazard of experiencing ADRs increases with time of treatment. The univariate frailty was estimated to be significantly different from zero (theta: 0.306, 95%CI: 0.102–0.521). This shows that there are unmeasured confounders which have a significant effect on the time to the occurrence of ADRs, and the distribution of these unmeasured variables is different between individuals.
In this study, the incidence rate of ADRs was found to be 4.8 per 100 PY with 95%CI of (4.102–5.317). This finding was similar with the study conducted in northern Ethiopia and South Africa where the incidence rate of ADRs was 4.3/100 PY7 and 4.2/100 PY24 respectively. This similarity might be due to similarity in the follow-up period. However, our finding is lower than a study conducted in Northwest Ethiopia25 and Southern India26 where the rate was 10.11/100 PY and 15/100 PY respectively. This discordance might be due to the difference in follow-up time, in our case the follow-up time was five years, however, the study in India was a two years follow-up, which is less than half our study period, this might have contributed to large rates of ADRs as most of ADRs are likely to occur in the first one to two years of the follow-up period. Besides, the finding was higher than the study conducted in Northwest Ethiopia27 where the incidence rate was 3/100 PY. This difference might be due to the difference in the definition of ADRs, in our case ADRs was defined as anemia, skin rash, pain (tingling) of extremities, vomiting, hepatotoxicity, jaundice; However, the study in Northwest Ethiopia defined ADRs as at least one of the following events: hospitalization, or switch/discontinuation of drug because of adverse drug reaction, this tight definition might contributed to lower rates of ADRs.
From sociodemographic characteristics, only sex was identified as an independent predictor of ADRs among patients on ART. According to the findings of the current study, females were more likely to experience adverse reaction. This is concordant with the research conducted in Southern Ethiopia10 and Tanzania28 where females were found to be more prone to ADRs than males. The difference in ADRs between males and females could be due to differences in body mass index and fat content.
The WHO clinical stage of the patients was an important predictor of ADRs, where patients in the advanced clinical stage at the initiation of HAART were at higher risk of developing ADRs at any time compared to patients in clinical stage I. The finding is supported by the study conducted in Bahir Dar, Northwest Ethiopia7 Debre Markos, Northwest Ethiopia27 and India.26 This could be because individuals in advanced clinical stages of the disease may be unable to withstand drug adverse effects, resulting in drug modifications or hospitalizations, thereby finding themselves at higher risk of drug reactions.29 Furthermore, patients with advanced clinical stages of the disease are more likely to be on other medications, which could lead to drug interactions and overlapping toxicities between ART therapies and other medications. Another possibility is that poor adherence due to pill burden resulted in poor treatment efficacy, requiring a medicine change or hospital admission.
The other predictor independently associated with ADRs was HIV/TB coinfection, where patients with TB were at higher risk of experiencing adverse reaction at any time compared to patients without TB. This finding is similar with the study conducted at Southern Ethiopia30 and Canada31 where HIV/TB coinfected patients experience a higher rate of adverse drug reactions to treatment than those without TB. This may be due to the fact that patient on ART with TB coinfection are more likely to be taking antiTB drugs, which could result in drug interactions and overlapping toxicities between ART and antiTB medications.
Likewise, CD4 at initiation of treatment was identified as one of the independent predictor contributing to the development of adverse reaction among patients on ART. For each additional CD4 count in cell/mm3, the hazard of experiencing ADRs decreased by 0.003%. The finding is supported by the fact that patients on ART having a higher CD4 count indicated a stronger immune system. People living with HIV who have a low CD4 cell count are at high risk of developing adverse reaction. A low CD4 count means that HIV has weakened your immune system and may be making you ill.
Conclusion
Most of the ADR cases occurred within two years after initiation of ART. Adverse drug reactions were not as common as they had been in other parts of Ethiopia and other African countries. Advanced clinical stage, HIV/TB coinfection, CD4 count, and sex were identified as predictors ADRs. Conversely, age, marital status, occupational status, body mass index, hemoglobin level, comorbidity, adherence to ART, functional status, ART regimen, alcohol consumption, and smoking were not associated with the incidence of adverse reaction.
Abbreviations
ADR, adverse drug reaction; AHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; CI, confidence interval; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; MRP, medical related problem; OR, odds ratio; WHO, World Health Organization.
Data Sharing Statement
Data will be provided up on request.
Ethics Approval and Consent to Participate
Ethical clearance and letter of cooperation was obtained from Institutional Review Board of Wachemo University, College of Medicine and Health Sciences and the hospital was informed about the study objectives through written letter. An informed consent was waived by Institutional Review committee of the hospital. Confidentiality was maintained at all levels of the study. Data were held on secured password protected system. All the procedures were conducted based on the principles of Declaration of Helsinki.
Acknowledgments
Our heartfelt gratitude goes to the Wachemo University, College of Health and Medical Sciences for support by all necessary services. In addition, we appreciate the support from hospital administrations and data collectors.
Disclosure
The author reports no conflicts of interest in this work.
References
1. UNAIDS. Global HIV/AIDS statistics - 2020 Fact sheet Ι UNAIDS; 2020 [
2. World Health Organization. Latest HIV estimates and updates on HIV policies uptake; 2020 [
3. Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90–90–90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):1–10.
4. Mwagomba B, Zachariah R, Massaquoi M, et al. Mortality reduction associated with HIV/AIDS care and antiretroviral treatment in rural Malawi: evidence from registers, coffin sales and funerals. PLoS One. 2010;5(5):e10452. doi:10.1371/journal.pone.0010452
5. World Health Organization. Adverse drud reactions-WHOΙ world health organization; 2017 [
6. Angamo MT, Chalmers L, Curtain CM, Bereznicki LR. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Safety. 2016;39(9):847–857. doi:10.1007/s40264-016-0444-7
7. Kindie E, Alamrew Anteneh Z, Worku E. Time to development of adverse drug reactions and associated factors among adult HIV positive patients on antiretroviral treatment in Bahir Dar City. Northwest Ethiopia. 2017;12(12):e0189322.
8. Khan K, Khan AH, Sulaiman SA, Soo CT, Akhtar AJ. Adverse drug reactions in HIV/AIDs patients at a tertiary care hospital in Penang, Malaysia. Japanese J Infect Dis. 2016;69(1):56–59. doi:10.7883/yoken.JJID.2014.246
9. Onoya D, Hirasen K, van den Berg L, Miot J, Long LC, Fox MP. Adverse drug reactions among patients initiating second-line antiretroviral therapy in South Africa. Drug Safety. 2018;41(12):1343–1353. doi:10.1007/s40264-018-0698-3
10. Tamirat T, Woldemichael K, Tewelde T, Laelago T. Anti-retro viral therapy adverse drug reaction and associated factors among human immuno deficiency virus infected adult patients at Nigist Eleni Mohammed Memorial hospital, South Ethiopia. Afr Health Sci. 2020;20(2):560–567. doi:10.4314/ahs.v20i2.3
11. Gudina EK, Teklu AM, Berhan A, et al. Magnitude of antiretroviral drug toxicity in adult HIV patients in Ethiopia: a cohort study at seven teaching hospitals. Ethiop J Health Sci. 2017;27(Suppl 1):39–52. doi:10.4314/ejhs.v27i1.5S
12. Tegegne AS, Zewotir T. Factors affecting first month adherence due to antiretroviral therapy among HIV-positive adults at Felege Hiwot Teaching and Specialized Hospital, north-western Ethiopia; a prospective study. BMC Infect Dis. 2018;18(1):1–11.
13. Mocroft A, Phillips A, Soriano V, et al. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Human Retroviruses. 2005;21(9):743–752. doi:10.1089/aid.2005.21.743
14. Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acq Imm Def Synd. 2003;34(3):281–288. doi:10.1097/00126334-200311010-00004
15. Ejigu A, Gehzu M, Haileselassie WJ. Adverse drug reactions causing treatment change among patients taking highly active antiretroviral therapy in health care facilities of Mekelle, Ethiopia. J Appl Pharm Sci. 2018;8(03):104–110.
16. Wasti SP, Simkhada P, Randall J, Freeman JV, van Teijlingen E. Factors influencing adherence to antiretroviral treatment in Nepal: a mixed-methods study. PLoS One. 2012;7(5):e35547. doi:10.1371/journal.pone.0035547
17. World health organization. Medicines: safety of medicines—adverse drug reactions; 2015.
18. Formica D, Sultana J, Cutroneo PM, et al. The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin Drug Saf. 2018;17(7):681–695. doi:10.1080/14740338.2018.1491547
19. El Morabet N, Uitvlugt EB, van den Bemt BJF, van den Bemt P, Janssen MJA, Karapinar-çarkit F. Prevalence and preventability of drug-related hospital readmissions: a systematic review. J Am Geriatr Soc. 2018;66(3):602–608. doi:10.1111/jgs.15244
20. ATLAS WD. Ethiopia - prevalence of HIV as a share of population aged 15–49; 2020. Available from: https://knoema.com/atlas/Ethiopia/HIV-prevalence?compareTo=.
21. Mitkie AA, Bekele FB, Debiso AT. Predictors of adverse drug reaction among adult HIV-infected patients on antiretroviral therapy in government hospitals of Kaffa Zone, Ethiopia; November 2018: a retrospective cohort. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.181.19915
22. Sherfa A, Haile D, Yihune M, Sako S. Incidence and predictors of Adverse Drug Reaction (ADR) among adult HIV positive patients on anti-retroviral treatment in Arba Minch town public health facilities, southern Ethiopia: a retrospective cohort study, 2020. PLoS One. 2021;16(5):e0251763. doi:10.1371/journal.pone.0251763
23. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503.
24. Masenyetse LJ, Manda SO, Mwambi HG. An assessment of adverse drug reactions among HIV positive patients receiving antiretroviral treatment in South Africa. AIDS Res Ther. 2015;12(1):1–8.
25. Anlay DZ, Alemayehu ZA, Dachew BA. Rate of initial highly active anti-retroviral therapy regimen change and its predictors among adult HIV patients at University of Gondar Referral Hospital, Northwest Ethiopia: a retrospective follow up study. AIDS Res Ther. 2016;13(1):1–8.
26. Shet A, Antony J, Arumugam K, Kumar Dodderi S, Rodrigues R, DeCosta A. Influence of adverse drug reactions on treatment success: prospective cohort analysis of HIV-infected individuals initiating first-line antiretroviral therapy in India. PLoS One. 2014;9(3):e91028. doi:10.1371/journal.pone.0091028
27. Kibret GD, Ayele TA, Tesfahun A. Incidence and predictors of severe adverse drug reactions among patients on antiretroviral therapy at debre markos referral hospital, Northwest Ethiopia; 2019.
28. Reuben WNM. Prevalence and severity of adverse drug reactions among adult patients using default first line and modified antiretroviral combinations in Mbeya region. Tanzania: Muhimbili University of Health and Allied Sciences; 2012.
29. AETC. Guide for HIV/AIDS Clinical Care. HIV Classification: CDC and WHO Staging Systems; 2014 [
30. Mesfin Menza Jaldo HF, Lemma Derseh G. The incidence of adverse drug reaction and its association with change in viral load among adult patients on anti-retro viral therapy in Ethiopia; Joint Modelling; 2021.
31. Yee D, Valiquette C, Pelletier M, et al. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167(11):1472–1477. doi:10.1164/rccm.200206-626OC
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

