Back to Journals » OncoTargets and Therapy » Volume 11
The incidence and risk factors of hepatotoxicity induced by perioperative hyperthermic intraperitoneal chemotherapy in gastrointestinal carcinoma patients: a retrospective study
Authors Zheng Z, Yu H, Xiong B, Shen S, Yang H, Zhou YF
Received 7 April 2018
Accepted for publication 4 July 2018
Published 11 September 2018 Volume 2018:11 Pages 5715—5722
DOI https://doi.org/10.2147/OTT.S170398
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Zhewen Zheng,1,2,* Haijun Yu,1,2,* Bin Xiong,3 Shuangting Shen,1,2 Hui Yang,1,2 Yunfeng Zhou1,2
1Department of Radiation and Medical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China; 2Hubei Key Laboratory of Tumor Biological Behaviors and Hubei Cancer Center, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China; 3Department of Gastrointestinal Surgical Oncology, Zhongnan Hospital of Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Aim: To investigate the incidence and risk factors of hepatotoxicity induced by perioperative hyperthermic intraperitoneal chemotherapy (HIPEC) in gastrointestinal carcinoma patients.
Patients and methods: Patients with gastrointestinal cancers treated with surgery in the presence or absence of HIPEC at a single institution were retrospectively reviewed. The patients received the treatment of surgery + HIPEC or surgery alone. The incidence of hepatotoxicity induced by HIPEC was recorded and risk factors were analyzed.
Results: In total, 301 eligible patients were included in the study, with 201 cases in the surgery + HIPEC group and 100 cases in the surgery group alone. The incidence of hepatotoxicity in the surgery + HIPEC group was higher than that in the surgery-alone group (57.71% vs 42%, P<0.05). In univariate analysis, HIPEC regimens, HIPEC techniques, HIPEC duration, and gastrointestinal complications were associated with the incidence of hepatotoxicity (P<0.05), while patient age, gender, tumor type, clinical stage, pathological type, blood transfusion, hepatitis B virus infection status, long-term alcohol use, and surgical techniques were not (P>0.05). Multivariate analysis showed that HIPEC regimen was the main risk factor of hepatotoxicity induced by HIPEC, with cisplatin + docetaxel being an independent risk factor of the HIPEC-induced hepatotoxicity. Open HIPEC techniques and HIPEC duration more than 60 minutes tend to increase the incidence of hepatotoxicity.
Conclusion: Surgery + HIPEC increases the incidence of hepatotoxicity. HIPEC regimen is the main risk factor for hepatotoxicity induced by HIPEC. Further prospective study is needed to confirm our conclusion.
Keywords: hepatotoxicity, HIPEC, gastrointestinal tumors, perioperative
Introduction
Peritoneal dissemination from gastrointestinal cancers is common and occurs in 5%–20% of patients explored for potential curative resection. Peritoneal relapse or metastasis is the major failure pattern of advanced gastrointestinal cancers and is a significant cause of death in colorectal cancer, gastric cancer, and pancreatic cancer patients.1 Gastrointestinal cancer associated-carcinomatosis was regarded as a terminal disease and could only be treated with palliative strategies. However, in the recent decade, new curative treatment options for selected patients with peritoneal dissemination from gastrointestinal cancers have emerged and bring new hope for these advanced patients.
The novel therapeutic strategies for peritoneal carcinomatosis (PC) that have emerged that combine cytoreductive surgery (CRS) and peritonectomy procedures2 with perioperative intraperitoneal chemotherapy (PIC), including hyperthermic intraperitoneal chemotherapy (HIPEC), and/or early postoperative intraperitoneal chemotherapy.3,4 The first study documenting a combination of intraperitoneal chemotherapy with long-term intraperitoneal thermotherapy was reported by Spratt et al5 in 1980. Theoretically, cytoreductive surgery is performed to treat macroscopic disease, and PIC is used to treat microscopic residual disease with the objective of removing disease completely in a single procedure. It was considered standard of care for diseases such as pseudomyxoma peritonei or peritoneal mesothelioma.1,6–8 Moreover, it is the only treatment that has brought curative results for carcinomatosis in many clinical trials.7,9,10
However, oncologists remain skeptical regarding the high toxicity of this combined therapeutic approach despite many of these studies suggesting a survival benefit. Several studies have reported the clinical and treatment-related risk factors for perioperative morbidity and mortality in patients who underwent CRS and PIC.11–13 The incidence of perioperative mortality ranged from 1% to 3%.11–13 Most studies mainly emphasized on the complications and risk factors of surgery, while little was known about the complications of the HIPEC. Specifically, to our knowledge, there are few studies about the incidence and risk factors of hepatotoxicity induced by the HIPEC in gastrointestinal carcinoma patients who received a combined treatment of CRS and HIPEC.
In the present study, we retrospectively analyzed the incidence of hepatotoxicity induced by HIPEC in gastrointestinal carcinoma patients. We also identified the associated risk factors of the hepatotoxicity induced by perioperative HIPEC, which may help to reduce hepatotoxicity and to improve HIPEC in future studies.
Patients and methods
Patient population
All patients with gastrointestinal carcinoma treated with surgery or surgery + HIPEC at Zhongnan Hospital of Wuhan University from January 2013 to December 2016 were selected for evaluation in this study. The study was approved by Ethics Committee of Zhongnan Hospital of Wuhan University.14 All patients signed informed consent prior to HIPEC treatment, which included allowing their data to be used for further research. All patient data was treated with strict confidentiality. Owing to the retrospective nature of the present study, it was granted an exemption in writing by the Ethics Committee of Zhongnan Hospital of Wuhan University. Inclusion criteria were age between 18 and 70 years, good performance status (World Health Organization Performance Status ≤2), and a histological diagnosis of gastrointestinal carcinoma. Patients with extra-abdominal metastasis and abnormality of liver function, renal function, blood count, and electrocardiogram before operation and HIPEC were excluded. Patients receiving any abdominal operations, including liver surgery, liver radiofrequency ablation, and other liver-damaging interventions, within 1 month of the surgery or HIPEC were also excluded.
Surgical treatment
All patients received surgical treatment including radical surgical resection, CRS, tumor excision, or exploratory laparotomy. Locally advanced gastrointestinal carcinoma patients with high risk of peritoneal metastasis received radical surgical resection. CRS in these patients was performed to visible clear abdominal and pelvic cancer nodules, while normal peritoneum and normal visceral structures were not resected during CRS. Tumor excision and exploratory laparotomy were used to reduce the symptoms and tumor burden with a palliative purpose. In all patients, a mechanical bowel preparation was used. Patients received antibiotic prophylaxis within 1 hour prior to abdominal incision. Prophylaxis for venous thrombosis and pulmonary embolus during the cytoreductive surgery was limited to sequential compression devices.
PIC
The detailed procedures were carried out according to previous reports.15,16 Briefly, the chemotherapeutic drugs were dissolved in 3 L saline. The perfusion solution was infused at a rate of 400 mL/min when the perfusion saline was kept at 43.0°C±0.5°C and monitored with temperature sensors in real time by an automatic hyperthermia chemotherapy perfusion device (ES-6001, Wuhan E-sea Digital Engineering, Wuhan, People’s Republic of China). The total HIPEC time was 60 minutes.
Assessment of liver function
Liver function was detected before and after HIPEC in each patient. Data of AST, ALT, TBIL, and PT were collected and assessed using the Common Terminology Criteria for Adverse Events version 4.0 at baseline and 3 days after the completion of HIPEC.
Review of the clinical record
The history of alcohol abuse and blood transfusion and the status of hepatitis B virus infection were reviewed and recorded for each patient. The occurrence of vomiting of degree II or higher (Common Terminology Criteria for Adverse Events version 4.0) induced by HIPEC was also recorded.
Statistical analysis
Data was analyzed using the Statistical Package for Social Sciences version 22.0 (IBM Corporation, Armonk, NY, USA). The numerical data were directly recorded, and the categorical data were recorded into different categories. The χ2 test was used for univariate comparisons. All variables associated with the hepatotoxicity with a P-value <0.05 in univariate analysis were examined consecutively with multivariate analysis using a forward stepwise binary logistic regression model. Significance was defined as P<0.05.
Results
Major clinical and pathological characteristics of the patients
A total of 301 consecutive gastrointestinal cancer patients who underwent surgery with or without HIPEC between January 2013 and December 2016 were evaluated. In the surgery + HIPEC group, 201 cases were treated with HIPEC + radical operation, or cytogenetic surgery, or tumor resection, or laparotomy, and 100 patients underwent surgery alone (tumor resection or radical surgery) in the surgery-alone group. Major clinical and pathological characteristics were balanced between the 2 groups. The detailed clinical and pathological characteristics and treatment information of patients in the 2 groups are listed in Table 1. The number of patients receiving different HIPEC regimen was as follows: 112 patients were treated with cisplatin (Cis) and docetaxel (Doc), 48 patients were treated with 5-fluorouracil (5-FU) and Doc, 21 patients were treated with 5-FU and Cis, and 20 patients were treated with 5-FU alone.
  | Table 1 The basic characteristics of the 301 patients in current study |
Incidence of hepatotoxicity in the surgery + HIPEC and surgery-alone groups
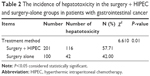
A total of 116 out of the 201 patients (57.71%) in the surgery + HIPEC group developed hepatotoxicity, in which grades I, II, III, and IV hepatotoxicity were seen in 94 (94/201, 46.77%), 19 (19/201, 9.45%), 3 (3/201, 1.49%), and 0 (0.00%) patients, respectively. Forty-two out of the 100 (42.00%) patients in the surgery-alone group had hepatotoxicity, in which grade I, II, and III of hepatotoxicity was seen in 33 (33%), 8 (8%), and 1 (1%) patients, respectively, and there were no cases of grade IV hepatotoxicity. No grade IV hepatotoxicity was noticed in both groups. The surgery + HIPEC group showed a higher incidence of hepatotoxicity than that of the surgery-alone group (P<0.05), especially in the category of grade I hepatotoxicity. The detailed incidence of hepatotoxicity in each group is indicated in Table 2.
Univariate analysis of risk factors associated with the incidence of hepatotoxicity in the surgery + HIPEC group
Table 3 shows the influence of 9 investigated clinical factors on the incidence of hepatotoxicity in the surgery + HIPEC group. Gastrointestinal complications ≥II was associated with the incidence of hepatotoxicity (P=0.016). There was no correlation between the incidence of hepatotoxicity and clinical factors such as patient age, gender, tumor type, clinical stage, pathological pattern, interpretative blood transfusion, hepatitis B virus infection status, and long-term alcohol use.
  | Table 3 The main clinicopathological features of 201 patients in surgery + HIPEC group |
Table 4 indicates the influence of 4 treatment-related factors on the incidence of hepatotoxicity in the surgery + HIPEC group. There was no correlation between the surgical approaches and the incidence of hepatotoxicity. Three treatment-related factors were associated with the incidence of hepatotoxicity: HIPEC regimes (platinum + Doc regiments, P<0.001), HIPEC methods (open-abdomen technique, P=0.004), duration of HIPEC (≥60 minutes, P=0.047).
Multivariable analysis of risk factors associated with the incidence of hepatotoxicity in the surgery + HIPEC group
Table 5 shows the results of the multivariable analysis, where only HIPEC regimen was found to be independently associated with the incidence of hepatotoxicity in the surgery + HIPEC group. The regiment of docetaxel + Cis was associated with highest risk of HIPEC-induced hepatotoxicity (Cis + Doc vs 5-Fluorouracil + Doc: odds ratio =2.393, P=0.028; Cis + Doc vs Cis +5-Fluorouracil, odds ratio =4.225, P=0.007). Patients with different methods of HIPEC, different durations of HIPEC, and different gastrointestinal complications showed no significant difference in the incidence of hepatotoxicity.
Discussion
Currently, CRS + HIPEC proposed by Verwaal et al17 is the most effective strategy to treat PC. This comprehensive treatment strategy takes full advantages of surgical resection, locoregional chemotherapy, and hyperthermal therapy. CRS can maximally remove the visible abdominopelvic tumors, and HIPEC can eradicate invisible residual tumor nodules, micrometastases, and free cancer cells. We have finished experimental18 and clinical15,16,19 studies to confirm the safety and effectiveness of CRS + HIPEC for PC. In the present study, we retrospectively investigated the incidence of hepatotoxicity and analyzed the risk factors. We found that HIPEC can significantly increase the incidence of hepatotoxicity, especially grade I hepatotoxicity. The HIPEC regimen of Cis + Doc is an independent risk factor of the HIPEC-induced hepatotoxicity.
The common complications of CRS + HIPEC are grouped into gastrointestinal, pulmonary, hematological complications, and others. Small bowel perforations and anastomotic leaks are the most common and clinically significant gastrointestinal complications after CRS and HIPEC.20 Postoperative pleural effusions and pneumonia are common pulmonary complications after CRS and HIPEC.21 Hematological toxicity represented the commonest cause of complications in 13% of patients11 after CRS and HIPEC. Other complications such as renal insufficiency, vascular access infections, hepatotoxicity, and venous thromboembolism are less frequent, and are not adequately explored in most studies. In the present study, the incidence of hepatotoxicity in the group of surgery + HIPEC was much higher than that in the group of surgery alone. Each patient diagnosed with hepatotoxicity induced by surgery + HIPEC received liver-protective treatment in our study. Therefore, the incidence and severity of hepatotoxicity induced by surgery + HIPEC may be higher than the reported results in the present study. Our results suggest that we need to pay more attention to HIPEC-induced hepatotoxicity, and a prophylactic liver-protective treatment may be reasonable.
Most chemotherapy drugs can induce a certain degree of hepatotoxicity. Hepatotoxicity associated with HIPEC is also closely related to the selection of chemotherapeutic regiments. Commonly used chemotherapies include platinum-based drugs (including Cis, carboplatin, oxaliplatin, laboplatin), taxanes (including paclitaxel and Doc), mitomycin, and 5-FU, among others. Regimens can consist of a single drug or multiple drugs. Presently, the dose of chemotherapeutic drugs is based on the standard doses of intravenous chemotherapy. Yet, high-dose regimes absorbed into the portal vein can damage normal stem cells. Moreover, the peritoneum is around 90 μm in thickness, whereas highly concentrated intraperitoneal chemotherapy can reach cytotoxic concentrations 1–3 mm below the peritoneum and up to 3–6 mm when used in conjunction with hyperthermia.22 Chemotherapeutic drugs are thus able to penetrate the peritoneal and visceral layers, causing damage to hepatic cells. The 4 groups of chemotherapy drugs cause different extents of hepatotoxicity. Among them, the combined regimen using Cis + Doc has the highest incidence of hepatotoxicity. Hence, when selecting chemotherapeutic drugs for HIPEC, the incidence of hepatotoxicity should also be taken into account in addition to the treatment efficacy. On the other hand, when the combination of Cis + Doc is used as HIPEC regimen, perioperative hepatoprotective measures could be taken to prevent possible hepatotoxicity.
Intraoperative HIPEC can be performed by 2 methods: open-abdomen technique (before abdominal closure) or closed-abdomen technique (after abdominal closure). The open-abdomen technique can evenly distribute chemotherapy drugs and heat in the abdominal cavity, but there is a risk of heat loss and the exposure to chemotherapy drugs for the surgical team. In general, the longer the HIPEC duration and the higher the HIPEC temperature are, the greater its effect in tumor cell killing is, although the corresponding effects on normal cells also intensify. To our knowledge, there is no study that has investigated the effect of HIPEC methods on hepatotoxicity to date. Our study indicated that HIPEC techniques (open or closed procedures) and HIPEC duration are closely related with the incidence of hepatotoxicity in patients treated with surgery + HIPEC in univariate analysis. The incidence of hepatotoxicity in the group that underwent open HIPEC techniques and had HIPEC duration longer than 60 minutes tended to show an increase in the incidence of hepatotoxicity, although with no statistical significance in multivariable analysis. So, it may be necessary for the patients undergoing open HIPEC techniques and with HIPEC duration longer than 60 minutes to receive a hepatoprotective treatment before HIPEC.
In the present study, we also found that the gastrointestinal complications induced by surgery + HIPEC have a close relationship with the incidence of hepatotoxicity in univariate analysis. We did not detect the cause and effect relationship between the gastrointestinal complications and hepatotoxicity. Nausea, vomiting, loss of appetite, diarrhea, and constipation are all adverse reactions associated with standard chemotherapeutic treatment. In severe cases, digestive system dysfunction can lead to poor treatment compliance and lower quality of life. Perioperative HIPEC can alter gastrointestinal reactivity, possibly by altering the postoperative structure of the gastrointestinal tract. According to some reports, more than 60% of patients receiving postoperative adjuvant chemotherapy for gastrointestinal tumors experience gastrointestinal complication of grade II or higher.23,24 On the contrary, our research shows that perioperative HIPEC patients do not experience a high incidence of gastrointestinal complication (28/201, 13.93%), but the incidence of concomitant hepatotoxicity among patients who underwent grade II or more gastrointestinal complication was significantly increased (22/28, 78.57%). Thus, concern must be raised on gastrointestinal complication during HIPEC treatment. Liver function tests ought to be performed at the onset of gastrointestinal complication, and hepatoprotective treatment should be commenced as soon as possible.
In the present study, we did not find association between hepatotoxicity induced by surgery + HIPEC and hepatitis B virus infection status, long-term drinking history, blood transfusion, and surgical methods. Surgery can also cause abnormal liver function, but its degree is generally lower and mostly transient. According to reports, hepatitis B virus25 and alcohol26 will increase the incidence of hepatotoxicity in chemotherapy patients. These conclusions are mostly arrived at from the data of systemic chemotherapy studies but not HIPEC studies. The different risk factors of hepatotoxicity induced by HIPEC and systemic chemotherapy are yet to be further studied.
Taken together, we retrospectively analyzed the incidence and risk factors of hepatotoxicity induced by HIPEC in gastrointestinal carcinoma in a central China cancer center. We found that HIPEC can significantly enhance the incidence of hepatotoxicity. The HIPEC regimen of Cis + Doc is an independent risk factor for HIPEC-induced hepatotoxicity. Open HIPEC techniques and HIPEC duration for more than 60 minutes tend to increase the incidence of HIPEC-induced hepatotoxicity. The gastrointestinal complications during HIPEC may reflect the hepatotoxicity induced by HIPEC. This preliminary conclusion is yet to be clarified by randomized control studies.
Acknowledgment
This research was supported by National Natural Science Foundation of China (81472799).
Disclosure
The authors report no conflicts of interest in this work.
References
Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat Rev. 2016;48:42–49. | ||
Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 2007;134:247–264. | ||
Elias D, Benizri E, di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007;14(2):509–514. | ||
Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol. 2008;98(4):242–246. | ||
Spratt JS, Adcock RA, Muskovin M, Sherrill W, Mckeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40(2):256–260. | ||
Huang Y, Alzahrani NA, Liauw W, Morris DL. Repeat cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2015;41(10):1373–1378. | ||
Mi DH, Li Z, Yang KH, et al. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: a systematic review and meta-analysis of randomised controlled trials. Int J Hyperthermia. 2013;29(2):156–167. | ||
Sun J, Song Y, Wang Z, et al. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials. BMC Cancer. 2012;12:526. | ||
Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. | ||
Lam JY, Mcconnell YJ, Rivard JD, Temple WJ, Mack LA. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am J Surg. 2015;210(3):424–430. | ||
Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116(24):5608–5618. | ||
Saxena A, Yan TD, Chua TC, Morris DL. Critical assessment of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2010;17(5):1291–1301. | ||
Saxena A, Yan TD, Morris DL. A critical evaluation of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. World J Surg. 2010;34(1):70–78. | ||
Wu HT, Peng KW, Ji ZH. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: results from a Chinese center. Eur J Surg Oncol. 2016;42(7):1024–1034. | ||
Wu HT, Yang XJ, Huang CQ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel improves survival for patients with peritoneal carcinomatosis from abdominal and pelvic malignancies. World J Surg Oncol. 2016;14(1):246. | ||
Sun JH, Ji ZH, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat advanced/recurrent epithelial ovarian cancer: results from a retrospective study on prospectively established database. Transl Oncol. 2016;9(2):130–138. | ||
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. | ||
Tang L, Mei LJ, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: evidence from an experimental study. J Transl Med. 2011;9:53. | ||
Huang CQ, Yang XJ, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase II study from a Chinese center. PLoS One. 2014;9(9):e108509. | ||
Casado-Adam A, Alderman R, Stuart OA, Chang D, Sugarbaker PH. Gastrointestinal complications in 147 consecutive patients with peritoneal surface malignancy treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Int J Surg Oncol. 2011;2011:468698. | ||
Preti V, Chang D, Sugarbaker PH. Pulmonary complications following cytoreductive surgery and perioperative chemotherapy in 147 consecutive patients. Gastroenterol Res Pract. 2012;2012:635314. | ||
Beaujard AC, Glehen O, Caillot JL, et al. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer. 2000;88(11):2512–2519. | ||
McWhirter D, Kitteringham N, Jones RP, Malik H, Park K, Palmer D. Chemotherapy induced hepatotoxicity in metastatic colorectal cancer: a review of mechanisms and outcomes. Crit Rev Oncol Hematol. 2013;88(2):404–415. | ||
Vincenzi B, Imperatori M, Picardi A, et al. Liver toxicity in colorectal cancer patients treated with first-line FOLFIRI-containing regimen: a single institution experience. Expert Rev Anticancer Ther. 2015;15(8):971–976. | ||
Bahirwani R, Reddy KR. Drug-induced liver injury due to cancer chemotherapeutic agents. Semin Liver Dis. 2014;34(2):162–171. | ||
Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62(3):299–307. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.