Back to Journals » International Journal of Nanomedicine » Volume 14
The In Vitro Immunomodulatory Effects Of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract And Hypoxoside On Macrophage And Natural Killer Cells
Authors Elbagory AM , Hussein AA, Meyer M
Received 24 May 2019
Accepted for publication 7 August 2019
Published 19 November 2019 Volume 2019:14 Pages 9007—9018
DOI https://doi.org/10.2147/IJN.S216972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Abdulrahman M Elbagory,1 Ahmed A Hussein,2 Mervin Meyer1
1DST/Mintek Nanotechnology Innovation Centre, Department of Biotechnology, University of the Western Cape, Bellville 7535, South Africa; 2Chemistry Department, Cape Peninsula University of Technology, Bellville 7535, South Africa
Correspondence: Mervin Meyer
Department of Biotechnology, University of the Western Cape, Private Bag X17, Bellville 7535, South Africa
Tel +27 21 959 2032
Fax +27 21 959 3505
Email [email protected]
Background: Macrophages and Natural Killer (NK) cells are an integral part of the innate immune system. These cells produce pro-inflammatory cytokines in response to bacterial infections. However, prolonged inflammation can be a contributing factor in the etiology of several diseases such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, psoriasis and eczema. Reducing the secretion of pro-inflammatory cytokines is an effective treatment strategy for these conditions. Gold nanoparticles (AuNPs) have been shown to have immunosuppressive effects. Extracts of the Hypoxis hemerocallidea plant have also been shown to have immunomodulatory effects. It has been demonstrated previously that extracts of the H. hemerocallidea can be used to synthesize AuNPs.
Purpose: This study aimed to investigate whether AuNPs synthesized using H. hemerocallidea extract and its major secondary metabolite, hypoxoside, have any immunomodulatory effects in macrophages and NK cells.
Methodology: AuNPs derived from the H. hemerocallidea extract were synthesized as previously described. Using similar methodologies, this study shows for the first time the synthesis of AuNPs from hypoxoside. The AuNPs were characterized using several optical and spectroscopic techniques. The immunomodulatory effects of the aqueous extract of H. hemerocallidea, hypoxoside, as well as the AuNPs produced from the extract and hypoxoside, were investigated by measuring the cytokine levels in macrophages (IL-1β, IL-6 and TNF-α) and NK cells (IFN-γ) using solid phase sandwich ELISA technique.
Results: The results show that spherical AuNPs (average size 26 ± 2 nm) were synthesized from hypoxoside. The results also show that the four treatments (H. hemerocallidea extract, hypoxoside and their respective AuNPs can lower the pro-inflammatory cytokine levels in the macrophages cells, while only AuNPs produced from hypoxoside can reduce cytokine responses in NK cells.
Conclusion: This study shows that all four treatments investigated here could be further explored for the development of anti-inflammatory therapies.
Keywords: green nanotechnology, gold nanoparticles, Hypoxis hemerocallidea, hypoxoside, anti-inflammatory, innate immune cells, cytokines, ELISA
Introduction
The function of the immune system is to provide protection against the invasion of foreign substances such as pathogens.1 In humans, the immune system also prevents the proliferation of tissues that can potentially be harmful, such as tumors and damaged tissues.2 The immune system can be separated into the innate and adaptive immune system. Macrophages and natural killer (NK) cells are part of the innate immune system, which is considered as the first line of defense. The function of macrophage and NK cells, which is mainly to phagocytose pathogens, is orchestrated by cytokines or interleukins (ILs).3 The modulation of the immune system, by altering the secretion of ILs, is considered an integral part of new immunotherapies for the treatment of cancer, as well as bacterial and viral infections.4–9
Immune responses are carefully controlled by the collective action of molecular pathways that either suppress or activate immune activation. A disturbance in this balance can result in increased susceptibility to infections, chronic inflammation or autoimmune diseases depending on whether the immune system is overly active or suppressed.10 Immunomodulatory drugs that can stimulate or suppress immune responses can be used for the treatment of such immune disorders. For instance, drugs that suppress the immune system can be useful in the treatment of inflammatory disorders, such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, psoriasis and eczema.11
Bacterial infections stimulate the macrophages and NK cells to secrete pro-inflammatory cytokines, e.g. IL-1β, TNF-α and IFN-γ, to aid in the infiltration of the immune cells into the infected tissue.12 In some cases however, persistent inflammation (chronic inflammation) can lead to undesired complications.13 In chronic inflammation, continual recruitment of innate and adaptive immune cells leads to the production of high levels of pro-inflammatory modulators.14 Patients with chronic inflammation can be susceptible to several diseases such as diabetes, cancer, inflammatory bowel syndrome and rheumatoid arthritis.15 Anti-inflammatory agents, therefore, can prove useful in the management of bacterial infections.16 Several nanomaterials are emerging as promising agents for application immune modulation.17 Gene expression analysis showed that the expression levels of several cytokines including IL-1β, IL-6 and TNF-α were affected in rats after the animals were injected with Gold nanoparticles (AuNPs).18 Citrate-AuNPs, on the other hand, exhibited anti-inflammatory responses by downregulating the cellular response induced by IL-1β both in vivo and in vitro.19
Nanoparticles can be formulated using several physical and chemical techniques.20 However, the green synthesis of nanoparticles using plants is gaining interest to avoid using toxic materials.21 The synthesis of biogenic gold nanoparticles (Hypoxis-AuNPs) from Hypoxis hemerocallidea extracts was previously described.22 These AuNPs were found to inhibit the growth of several bacterial strains known to cause chronic wound infections. It is likely that nanoparticles synthesized from the plant extract could have similar bioactivities to the extract. This depends on whether phytochemicals responsible for the bioactivities of the plant extract are also involved in the synthesis of the nanoparticles. Plant-derived nanoparticles can enhance the bioavailability and biological activity of the phytochemicals responsible for the bioactivity.23–25
H. hemerocallidea, commonly known as African Potato, is a wild tuberous plant that is native to the southern parts of Africa.26 The plant has several medicinal uses and is considered the best-known medicinal plant among South Africans.27 It is claimed that H. hemerocallidea can be used as an immune modulation phytotherapy in the management of immune-related diseases such as the common cold, flu, rheumatic arthritis, cancer and HIV/AIDS.28 H. hemerocallidea is also used by traditional healers to treat hypertension, diabetes, psoriasis, ulcers, urinary infections, tuberculosis, asthma and central nervous system disorders.29,30 Researchers reported that H. hemerocallidea showed antibacterial, antioxidant and anticancer activities.31,32 Animal studies have shown that the aqueous extract of H. hemerocallidea has anti-inflammatory activities. This was demonstrated by the ability of H. hemerocallidea to inhibit the acute inflammation in rats induced by egg albumin.29 The anti-inflammatory activity of H. hemerocallidea was attributed to the ability of extracts from this plant to inhibit the synthesis of inflammatory mediators.33
Hypoxoside is an important and well-studied phytochemical that is found in the corms of H. hemerocallidea.34 This unique norlignan diglucoside can be found abundantly in several Hypoxis spp. and was used as an analytical marker to investigate the authenticity of the Hypoxis spp. and their products.35 Hypoxoside is a prodrug, which, upon its hydrolysis by the gastric lysosomal enzyme, β-glucosidase, is converted to the biologically active aglycon form, rooperol.33 This conversion of hypoxoside to rooperol can also occur in tumor tissues and inflammatory sites, where lysosomal enzymes including β-glucosidase can be released by cancer cells or the activation of macrophages.36 Studies have shown that rooperol exhibits strong antioxidant, anticancer and antibacterial activities.37–40 Rooperol was also shown to have potent anti-inflammatory action. It has been suggested that the increased production of reactive oxygen species (ROS) and nitric oxide (NO) in response to rooperol treatment is responsible for its anti-inflammatory activity.41 Derivatives of rooperol have been shown to inhibit the production of several pro-inflammatory ILs in stimulated human macrophages.42
Since extracts of H. hemerocallidea have been shown to have immune modulatory activities, it is possible that the nanoparticles produced from this plant may also have immune modulatory activities. This study reports for the first time the synthesis of AuNPs from hypoxoside (Hy-AuNPs) as well as the immune modulatory activities of the H. hemerocallideaextract, hypoxoside, and their AuNPs using cell culture models of macrophages and NK cells. The study also compares the immune modulatory activities the AuNPs to H. hemerocallidea extract and hypoxoside.
Materials And Methods
Isolation Of Hypoxoside
An ethanolic extract of the H. hemerocallidea was acquired from Afriplex Pty Ltd. (Paarl, South Africa). This extract was concentrated and partitioned with ethyl acetate. The ethyl acetate fraction (5.0 g) was separated by chromatography on a Sephadex LH-20 column using a Methanol: H2O mixture 1:1 (v/v) to isolate the fraction containing hypoxoside. The fraction containing hypoxoside was further purified using Prep-HPLC (Waters model, Milford, MA, USA) equipped with Waters 2535 Quaternary Gradient Module pump, a manual injector and variable wavelength detector (Waters 2489 UV-Vis detector). A C18 YMC column (5 µM, 250 × 30 mm) was used and the separation was monitored at 254 nm. The elution was performed using Methanol: H2O (1:1, v/v) solvent mixture at a flow rate of 5 mL/min. The 1H and 13C NMR spectra of the isolated hypoxoside correlated with the spectra described by Nair and Kanfer.35
Biosynthesis And Characterization Of AuNPs
The synthesis of Hypoxis-AuNPs (AuNPs synthesized from H. hemerocallidea extract) was done using a method previously described.22 A similar protocol was followed for the synthesis of Hy-AuNPs (AuNPs synthesized using hypoxoside) with slight modification. Sodium tetrachloroaurate (III) dihydrate (Sigma Aldrich, St. Louis, MO, USA) was mixed with hypoxoside (1.0 mg/mL) in a ratio of 5:1 (v/v). The mixture was stirred (40 rpm) for 2 hrs, centrifuged and the AuNPs were obtained in the pellet. The AuNPs were washed trice with distilled water. A POLARstar Omega microtitre plate reader (BMG Labtech, Ortenberg, Germany) was used to monitor the characteristic Localized Surface Plasmon Resonance (LSPR) peaks of the AuNPs. The morphology and the elemental composition of the AuNPs were investigated by High Resolution Transmission Electron Microscope (HRTEM) using the FEI Tecnai G2 20 field-emission gun (FEG) equipped with an Electron Diffraction X-ray (EDX) liquid nitrogen cooled Lithium doped Silicon detector. A suspension of AuNPs’ was deposited onto copper grids, which was then air-dried. The images obtained were analyzed using ImageJ 1.50b version 1.8.0_60 (http://imagej.nih.gov/ij). Dynamic Light Scattering (DLS) analysis was done using a Malvern Zetasizer Instrument (Malvern Ltd., UK) at 25°C and a 90° angle. The chemical functional groups of hypoxoside and Hy-AuNPs were studied using a PerkinElmer spectrum two Fourier-Transform Infrared (FTIR) spectrophotometer (Waltham, MA, USA) as described previously.43
Evaluating The Stability Of The AuNPs
The stability of Hypoxis-AuNPs and Hy-AuNPs in RPMI 1640 and α-MEM (Gibco, UK) cell culture media was tested as described previously.22 The stability of the AuNPs was evaluated by mixing the AuNPs and the cell culture medium at 1:1 ratio and incubating the mixture at 37°C for 24 hrs. The mixtures of the AuNPs and the cell culture medium were subjected to UV-Vis analysis after the 24 hrs period.
Cell Culture
The human leukemic monocyte cell line (THP1) (kindly provided by Prof. Samantha Sampson, from the Host-Pathogen Mycobactomics group at Stellenbosch University) was maintained in RPMI 1640 containing 50% Fetal Bovine Serum (FBS) (Lonza, Walkersville, MD, USA) and 1% penicillin–streptomycin (Lonza, Walkersville, MD, USA). The human Natural Killer cell line, NK92, was obtained from American Type Culture Collection (ATCC) and maintained in α-MEM supplemented with 12.5% FBS, 12.5% horse serum (Sigma-Aldrich, Cape Town, South Africa), 200 U/mL recombinant IL-2 (R&D systems, MN, USA), 0.1 mM 2-mercaptoethanol and 1% penicillin–streptomycin. The cells were cultured in a 37°C humified incubator at 5% CO2 saturation.
Differentiation Of THP1 Cells
Differentiation of THP1 cells into macrophage-like cells was done using 25 nM phorbol 12-myristate 13-acetate (PMA) (Sigma Aldrich, St. Louis, MO, USA) as previously described.44 The cells were seeded in a 96-well plate (Greiner Bio-one GmbH, Frickenhausen, Germany) at a density of 2 × 105 cells/100 µL/well. The cells were treated for 2 days with 25 nM PMA to induce cell differentiation. PMA induced macrophage-like phenotypic changes were confirmed by optical microscopy.
Determining The Toxicity Of Extracts And AuNP In THP1 And NK92 Cells
The effects of the H. hemerocallidea aqueous extract, hypoxoside, Hypoxis-AuNPs and Hy-AuNPs on the cell viability of NK92 and PMA differentiated THP1 cells were determined using the WST-1 Cell Proliferation Assay as recommended by the manufacturer (Roche Diagnostics GmbH, Mannheim, Germany). The cells were treated for 24 hrs with increasing concentrations of H. hemerocallidea extract, hypoxoside and the AuNPs. The concentrations of the H. hemerocallidea extract and hypoxoside were 30, 60, 120, 240 and 480 µg/mL, while the concentrations of Hypoxis-AuNPs and Hy-AuNPs were 1, 2, 4, 8, 16, 32 nM. To compensate for any interference from the AuNPs on the assay, background controls were included as previously described.22
Measurement Of Cytokine Responses In THP1 And NK92 Cells
NK92 and PMA differentiated THP1 cells were seeded at a density of 2 × 105 cells/100 µL/well in 24-well cell culture plates. After 24 hrs, the THP1 cells were exposed for 6 hrs to cell culture medium containing 10 µg/mL Lipopolysaccharide (LPS). Based on the results of the WST-1 Cell Proliferation Assay, concentrations of H. hemerocallidea aqueous plant extract (240 µg/mL), hypoxoside (240 µg/mL), Hypoxis-AuNPs (16 nM) and Hy-AuNPs (16 nM) that were not toxic to the cells were identified. The NK92 and LPS activated THP1 cells were subjected to these treatments for 18 hrs after removing the LPS containing medium. The negative controls were cells that were not treated any way, while the positive controls for THP1 cells were cells that were treated for 6 hrs with LPS, which was replaced afterwards with cell culture medium for 18 hrs. After 24 hrs, the supernatants were collected from all wells. The samples were centrifuged at 10,000 rpm for 15 mins using an Eppendorf 5417 R Centrifuge equipped with F-45-30-11 rotor (Eppendorf, Hamburg, Germany) to remove any cells and the AuNPs. The presence of IL-1β, IL-6 and TNF-α in the supernatants collected from PMA differentiated THP1 cells was assessed using MaxDiscovery™ ELISA kits (Bioo Scientific, TX, USA). The cytokines concentrations were measured according to the manufacturer’s protocol. Supernatants from NK92 cells were analyzed for the presence of IFN-γ.
Statistical Analysis
Data were expressed as the mean ± standard deviation of the three replicates. Statistical analysis was conducted using the GraphPad Prism 6 and two-tailed Student’s t-test. Differences among three or more groups were analyzed by one- or two-way analysis of variance. Differences with p < 0.05 were considered to be statistically significant.
Results And Discussion
Biosynthesis Of AuNP Using Hypoxoside
The synthesis and characterization of Hypoxis-AuNPs using the water extract of H. hemerocallidea to reduce gold(III) chloride were previously reported.22 Hypoxoside is a unique glycoside and a major phytochemical component of H. hemerocallidea extracts. It was reported that glycosides can reduce gold(III) chloride to AuNPs,45 and it is, therefore, possible that hypoxoside was involved in the synthesis of Hypoxis-AuNPs. Here, the study investigated whether hypoxoside can reduce gold(III) chloride and found that hypoxoside was able to reduce gold(III) chloride resulting in the formation of Hy-AuNPs. The optimal synthesis conditions (temperature, concentration of hypoxoside and the time of synthesis) were established using the same methods described previously for the synthesis of AuNPs from plant extracts.46 The UV-Vis spectrum for Hy-AuNP showed absorption maximum (λmax) at around 534 nm (Figure 1), which is within the range of the visible spectrum (500–600 nm) and the characteristic λmax for spherical AuNPs.47 The optimal synthesis time was established by measuring changes in λmax over time (Figure 1A). A sharp increase in absorbance (up to about 1.1 absorbance units) was detected between 0 and 20 mins, however the increase in absorbance from 20 to 120 mins was moderate since the absorbance units only increased from 1.1 to 1.25 over this time period (Figure 1B). Yulizar et al, suggested that an increase in absorbance reflects an increase in AuNP number.48 This implies that the number of Hy-AuNPs increased over the 120-min time period, but that the formation of new seeds (nucleation) of Hy-AuNPs occurs mainly within 20 mins from the start of the reaction. While λmax at 60 and 120 mins is around 534 nm, the λmax at earlier time points 10, 20 and 40 mins appears to be red-shifted. Moreover, the absorbance peaks produced at 10, 20 and 40 mins also appeared to be much broader. Yulizar et al, also suggested that the shape of absorbance peaks is mainly determined by the morphological characteristics of the AuNPs.48 It can, therefore, be postulated that the growth of Hy-AuNPs, which follows the nucleation step, occurs after 20 mins, reaching the final energetically stable form at 120 mins.
The Z-average size of Hy-NPs was 26 ± 2 nm as determined by the DLS analysis. The size distribution by intensity was bimodal. Small nanoparticles with an average diameter of 1.5 ± 0.5 nm and larger nanoparticles with an average diameter of 56 ± 33 nm can be observed in Figure 2. The zeta potential of Hy-NPs was −23. Such negative zeta potential value has been shown to indicate colloidal stability of AuNPs due to the repulsive force between the particles.49 It can thus be concluded that the Hy-NPs are stable in solution.
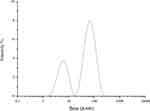 |
Figure 2 The hydrodynamic diameter of Hy-AuNPs displayed by intensity as determined by the DLS analysis. |
The HRTEM images in Figure 3 show the quasi-spherical shape of Hy-AuNPs (Figure 3A and B). The particle size distribution of Hy-AuNPs determined from the HRTEM images (using ImageJ software) showed that most of the particles are between 24 and 28 nm in diameter (Figure 3C), which is in accordance with the DLS analysis. Moreover, the Selected Area Electron Diffraction (SAED) pattern of Hy-AuNPs confirmed their polycrystalline nature as indicated by the presence of the bright rings corresponding to (111), (200), (210), (311) and (222) of the face-centered cubic structure of gold (Figure 3D), as previously reported.50 The lattice fringes of the Hy-AuNPs had a distance of 0.271 nm, which corresponds to the (111) orientation of gold (Figure 3E).51 Additional confirmation about the bio-reduction of gold(III) chloride to form AuNPs was presented by the appearance of elemental Au peaks in the EDX profile of Hy-AuNPs (Figure 3F).
FTIR analysis was applied to investigate the possible functional groups in the structure of hypoxoside that is involved in the synthesis of Hy-AuNPs. Figure 4 shows the FTIR spectra of both hypoxoside and Hy-AuNPs. The FTIR spectrum of hypoxoside is similar to the spectrum reported previously.35 The C–H rocking vibration is shown by the absorption bands at 801 and 890 cm−1 and the C–H bending at 2884 cm−1. The absorption bands at 1246 and 3280 cm−1 represent the bending and stretching alcoholic O–H, respectively. The peaks at 890 and 1070 cm−1 denote the out of plane C–H attached to a carbon through a double bond (C=C–H). The sharp absorption bands at 1510 and 1586 cm−1 indicate the C=C in aromatic ring. It is expected that hypoxoside will undergo oxidation to reduce gold(III) chloride, which should be manifested in the FTIR spectrum of Hy-AuNPs. It was reported that C–6–OH in the sugar unit of glycosides is readily oxidized into carboxylic acid upon reaction with gold(III) chloride.45 Interestingly, the FTIR spectrum of Hy-AuNPs showed a very broadband starting from 3000 to 1750 cm−1, which may be a result of the stretching the O–H group of carboxylic acid, which is a characteristic band of carboxylic acids that overlap other peaks of other functional groups.52 Moreover, Hy-AuNPs showed two bands at 3770 and 3703 cm−1, which may be the result of the shift of both the alcoholic O–H and the bending C–H groups (from 3280 and 2884 cm−1, respectively, as shown in the FTIR spectrum of hypoxoside). This huge shift is caused by strong hydrogen bonding of the functional groups involved and is sometimes detectable in systems that are in a complex.53
 |
Figure 4 FTIR spectra of hypoxoside and Hy-AuNPs. |
From the above, it can be hypothesized that the reduction of gold(III) chloride by hypoxoside was mainly mediated through the oxidation site at C–6–OH, which is converted into carboxylic acid. Secondly, the alcoholic O–H groups of hypoxoside were responsible for the stabilization of Hy-AuNPs by means of chemisorption with the growing Au seeds.
Furthermore, the results indicate that hypoxoside might have contributed largely to the synthesis of AuNPs from the H. hemerocallidea water extract. This is evident from the similar physicochemical characteristics reported here for Hy-AuNPs and those reported previously for Hypoxis-AuNPs.22 However, because of the longer incubation time needed for the synthesis of Hy-AuNPs and for the difference in the average size, hypoxoside may not be the sole contributor in the synthesis of Hypoxis-AuNPs.
The Immunomodulation Activity Of H. Hemerocallidea, Hypoxoside And Their AuNPs
H. hemerocallidea is used in traditional medicine for the treatment of immune-related diseases and it has been shown that extracts of H. hemerocallidea have anti-inflammatory activity.29 It has been suggested that the immunomodulatory effects of H. hemerocallidea can be ascribed to the bioactivity of rooperol, which is formed when hypoxoside, a major constituent of the H. hemerocallidea plant, is hydrolyzed.33 It is demonstrated here for the first time that hypoxoside can reduce gold(III) chloride to form Hy-AuNPs. It has also been demonstrated that metal-based nanoparticles have immune modulatory effects.17 Herein, the immunomodulatory effects the AuNP that has been synthesized from H. hemerocallidea (i.e. Hypoxis-AuNPs) and Hy-AuNPs are investigated using macrophage and NK cell cultures. In vitro immunomodulatory assays require that the treatments with the AuNPs be done in cell culture medium. Cell culture media contain proteins, peptides, amino acids, carbohydrates, minerals and buffering agents. If any of these media constituents react with the AuNPs, the biophysical properties of the AuNPs will be altered. This can also cause the AuNPs to aggregate. For this reason, the stability of the AuNPs was investigated in α-MEM and RPMI cell culture media. This involved placing Hypoxis-AuNPs and Hy-AuNPs in α-MEM and RPMI medium for a period of 24 hrs and to determine if the UV-Visspectra of the AuNPs change over this period as described previously.54 Changes in the UV-Vis of AuNPs, more specifically the red shift or broadening of the adsorption peaks, can indicate increase or decrease in the size of AuNPs or their aggregation.55 Figure 5 shows that α-MEM and RPMI media did not affect the UV-Vis spectra of Hypoxis-AuNPs and Hy-AuNPs. This implies that Hypoxis-AuNPs and Hy-AuNPs were stable in these media.
 |
Figure 5 UV-Vis spectra of Hypoxis-AuNPs and Hy-AuNPs before and after 24 hrs incubation in the presence of α-MEM and RPMI cell growth media. |
The immunomodulatory effects of the H. hemerocallidea plant extract, hypoxoside, Hypoxis-AuNPs and Hy-AuNPs were evaluated in differentiated THP1 and NK92 cell lines. Monocytes and NK cells are an integral part of the innate immune system, in part by producing several ILs that exert their functions on other cells and thus control immune responses to infections.56 The toxicity of the H. hemerocallidea plant extract, hypoxoside, Hypoxis-AuNPs and Hy-AuNPs to differentiated THP1 and NK92 cells was determined using the WST-1 viability assay (Figure 6). This was done to determine if the AuNPs, extract and hypoxoside were toxic to these cells. The concentrations of Hypoxis-AuNPs and Hy-AuNPs ranged from 1 to 32 nM, while the concentrations of H. hemerocallidea plant extract and hypoxoside, ranged from 30 to 480 µg/mL. The viability of differentiated THP1 cells was unaffected by Hypoxis-AuNPs and Hy-AuNPs, while the viability of NK92 cells was only significantly affected by Hypoxis-AuNPs and Hy-AuNPs at the highest dose, 32 nM. The viability of NK92 cells was reduced to around 15% when treated with 32 nM of Hypoxis-AuNPs or Hy-AuNPs. NK92 cells were unaffected by H. hemerocallidea plant extract and hypoxoside, while THP1 cells were only significantly affected by hypoxoside at the highest dose, 480 µg/mL. Based on these data 16 nM of Hypoxis-AuNPs or Hy-AuNPs and 240 µg/mL of hypoxoside or H. hemerocallidea plant extract were selected to investigate their immunomodulatory effects on PMA differentiated THP1 and NK92 cells. This is the first time that the immune modulatory effects of the H. hemerocallidea plant extract and hypoxoside have been studied in this way using in vitro culture of macrophages and NK cells.
Prior to treatment with the extract, hypoxoside and AuNPs, THP1 cells were induced to differentiate into macrophage-like cells using PMA. After exposure to PMA, the differentiated THP1 cells were treated with LPS, a bacterial endotoxin that has been used to trigger pro-inflammatory response in macrophage-like THP1 cells.57 It has been shown that LPS can activate the secretion of pro-inflammatory cytokines, which includes IL-1β, IL-6, TNF-α and IFN-γ.58 IL-1β is considered a key mediator between the innate and the adaptive immune cells as it activates the antigen-presenting cells, which in turn leads to the production of aggressive adaptive immune cells against infections.59 TNF-α is one of the early cytokines released from macrophages after infections and is considered a “master regulator” of other pro-inflammatory cytokines.60 IL-6 is another important immune system regulator, but with both pro- and anti-inflammatory actions.61 It was shown that the pleiotropic effect of IL-6 depends on the activation of either one of its two signaling pathways.61 The relationship between IL-6 and bacterial infections is well established. Indeed, increased IL-6 levels are considered a diagnostic marker for early bacterial infections.62 Differentiated THP1 cells treated with LPS for 6 hrs, showed a significant increase in IL-1β, IL-6 and TNF-α levels, when compared to THP1 cells that were not treated with LPS (negative control) (Figure 7). Differentiated THP1 cells that were also stimulated with LPS for 6 hrs and then treated for 18 hrs with Hypoxis-AuNPs, Hy-AuNPs, hypoxoside or H. hemerocallidea plant extract showed a significant decrease in IL-1β and TNF-α levels when compared to differentiated THP1 cells that were treated with LPS only (LPS control). It was shown that at these doses of Hypoxis-AuNPs, Hy-AuNPs, hypoxoside and H. hemerocallidea plant extract are not toxic to the cells, which suggests that these treatments exert an anti-inflammatory response in THP1 macrophage-like cells.
IL-6 responses in differentiated THP1 cells were also significantly reduced when the cells were treated with the H. hemerocallidea plant extract. However, treatments with Hypoxis-AuNPs, Hy-AuNPs and hypoxoside did not significantly affect IL-6 responses in THP1 cells. In fact, treatment of differentiated THP1 cells with Hy-AuNPs and hypoxoside resulted in a moderate increase in IL-6 level when compared to cells that were treated with LPS only for 6 hrs. However, this increase was not significant. Higher doses of Hy-AuNPs and hypoxoside or a longer treatment may result in significant increase of the IL-6 response.
The Immunomodulation Effect Of H. Hemerocallidea, Hypoxoside And Their AuNPs On NK92
The effects of Hypoxis-AuNPs, Hy-AuNPs, hypoxoside and H. hemerocallidea plant extract on cytokine responses in NK92 cells, more specifically IFN-γ, were also investigated. IFN-γ is a major effector cytokine that is mainly produced by NK cells in response to viral and bacterial infections.63 The treatment of NK92 cells with Hypoxis-AuNPs, hypoxoside and H. hemerocallidea plant extract did not alter the production of IFN-γ levels when compared to the untreated negative control cells (Figure 7). However, IFN-γ production was significantly reduced in NK92 cells treated with Hy-AuNPs. The IFN-γ levels in Hy-AuNPs-treated cells were 13 ± 7 pg/mL, compared to 84 ± 17 pg/mL for the untreated cells. It has been reported that IFN-γ increases the production of pro-inflammatory cytokines, including IL-1β and TNF-α.64 In this context, the effects of Hy-AuNPs on NK92 cells can be viewed as an anti-inflammatory response. Interestingly, the free hypoxoside or any of the other AuNPs did not downregulate the production of IFN-γ compared to the Hy-AuNPs. It can be postulated that hypoxoside might have undergone chemical modification upon oxidation with the gold(III) chloride, which might have converted it to a more active form. It is known from the literature for example that hypoxoside can be converted to rooperol upon hydrolysis. The FTIR data in this study also suggest major modification in the chemistry of hypoxoside after AuNPs synthesis. This can only be confirmed by performing more sophisticated spectroscopic analysis of the hypoxoside and the Hy-AuNPs. It is also worth noting that IL-2, used in culturing the NK92 cells, can cause elevated IFN-γ production, and that might be the reason for the moderately elevated levels of this cytokine in the negative controls in our study.65,66
Inflammation is considered an essential mechanism of the innate immune system to control pathogens and bacterial infections.67 The increased production of pro-inflammatory cytokines during infections triggers a cascade of events that result in the infiltration of infected tissues by innate immune cells.13 However, the overproduction of the pro-inflammatory cytokines in response to infections may also have severe adverse health effects. Irreversible damage to the inflamed tissue can occur due to the release of lytic enzymes or through oxidative stress.13 Elevated IL-1β levels are associated with several inflammatory disorders including rheumatoid arthritis and psoriasis.19 Lowering TNF-α production is also a key therapy for rheumatoid arthritis.68 Inflammatory disorders, such as rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, psoriasis and eczema can benefit from anti-inflammatory therapies. This study demonstrated the anti-inflammatory responses of Hypoxis-AuNPs, Hy-AuNPs, hypoxoside and H. hemerocallidea plant extract in THP1 cells. It also found that Hy-AuNPs has an anti-inflammatory response in NK92 cells.
Conclusion
This study demonstrates for the first time the biosynthesis of AuNPs using hypoxoside, which was isolated from H. hemerocallidea. The physicochemical properties of Hy-AuNPs were similar to that of Hypoxis-AuNPs, which was synthesized from the water extracts of H. hemerocallidea. This may suggest that hypoxoside is involved in the synthesis of Hypoxis-AuNPs. Water extracts of H. hemerocallidea and hypoxoside reduced the secretion of pro-inflammatory cytokines in the macrophage cell line THP1. This finding lends support to the traditional use of the plant in the treatment of inflammation. Hypoxis-AuNP and Hy-AuNP also reduced the secretion of pro-inflammatory cytokines in PMA differentiated THP1 cells. Hy-AuNP was the only treatment that affected cytokine responses in NK92 cells by significantly reducing IFN-γ secretion. These AuNPs can be explored further as anti-inflammatory treatments. Metallic nanoparticles are commonly used for skin care and dermatological treatments. Considering that this study shows that Hypoxis-AuNP and Hy-AuNP have anti-inflammatory properties, these nanoparticles can potentially be used in the development of topical treatments for the autoimmune skin conditions such as psoriasis and eczema. To fully understand their activities on the immune system, it is recommended to evaluate the immunomodulatory effects of H. hemerocallidea, hypoxoside and their AuNPs on immune cells isolated from blood and in animal models.
Ethical Approval
The authors declare that the original source of the gifted THP-1 cells was a commercial entity and the University of the Western Cape does not require ethics approval for cell culture experiments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi:10.1128/CMR.00046-08
2. Candeias SM, Gaipl US. The immune system in cancer prevention, development and therapy. Anticancer Agents Med Chem. 2016;16(1):101–107.
3. Jiao Q, Li L, Mu Q, Zhang Q. Immunomodulation of nanoparticles in nanomedicine applications. Biomed Res Int. 2014;2014:426028. doi:10.1155/2014/426028
4. Péchiné S, Bruxelle JF, Janoir C, Collignon A. Targeting clostridium difficile surface components to develop immunotherapeutic strategies against clostridium difficile infection. Front Microbiol. 2018;9:1009. doi:10.3389/fmicb.2018.01009
5. Barthelemy A, Sencio V, Soulard D, et al. Interleukin-22 immunotherapy during severe influenza enhances lung tissue integrity and reduces secondary bacterial systemic invasion. Infect Immun. 2018;86(7):e00706–17. doi:10.1128/IAI.00706-17
6. Manglani M, McGavern DB. New advances in CNS immunity against viral infection. Curr Opin Virol. 2018;28:116–126. doi:10.1016/J.COVIRO.2017.12.003
7. Walker LM, Burton DR. Passive immunotherapy of viral infections: “super-antibodies” enter the fray. Nat Rev Immunol. 2018;18(5):297–308. doi:10.1038/nri.2017.148
8. Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. doi:10.1101/cshperspect.a028472
9. Ripley RT, Ayabe RI. Immunotherapy: the power of perseverance. J Thorac Cardiovasc Surg. 2018;155(4):1775–1776. doi:10.1016/j.jtcvs.2017.12.002
10. Haase D, Starke M, Puan KJ, Lai TS, Rotzschke O. Immune modulation of inflammatory conditions: regulatory T cells for treatment of GvHD. Immunol Res. 2012;53(1–3):200–212. doi:10.1007/s12026-012-8267-9
11. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi:10.1016/j.mce.2010.04.005
12. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi:10.3389/fimmu.2014.00491
13. Rauch I, Müller M, Decker T. The regulation of inflammation by interferons and their STATs. JAK-STAT. 2013;2(1):e23820. doi:10.4161/jkst.23820
14. Meirow Y, Baniyash M. Immune biomarkers for chronic inflammation related complications in non-cancerous and cancerous diseases. Cancer Immunol Immunother. 2017;66(8):1089–1101. doi:10.1007/s00262-017-2035-6
15. Pahwa R, Jialal I. Chronic Inflammation. StatPearls Publishing; 2018.
16. Sheth AN. Can anti-inflammatory drugs fight infection? Sci Transl Med. 2013;5(192):192ec110. doi:10.1126/scitranslmed.3006879
17. Luo YH, Chang LW, Lin P. Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications. Biomed Res Int. 2015;2015:143720. doi:10.1155/2015/143720
18. Khan HA, Abdelhalim MAK, Alhomida AS, Al Ayed MS. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet Mol Res. 2013;12(4):5851–5857. doi:10.4238/2013.November.22.12
19. Sumbayev VV, Yasinska IM, Garcia CP, et al. Gold nanoparticles downregulate interleukin-1β-induced pro-inflammatory responses. Small. 2013;9(3):472–477. doi:10.1002/smll.201201528
20. Ghosh Chaudhuri R, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev. 2012;112(4):2373–2433. doi:10.1021/cr100449n
21. Souri M, Hoseinpour V, Shakeri A, Ghaemi N. Optimisation of green synthesis of MnO nanoparticles via utilising response surface methodology. IET Nanobiotechnol. 2018;12(6):822–827. doi:10.1049/iet-nbt.2017.0145
22. Elbagory A, Meyer M, Cupido C, Hussein A. Inhibition of bacteria associated with wound infection by biocompatible green synthesized gold nanoparticles from south african plant extracts. Nanomaterials. 2017;7(12):417. doi:10.3390/nano7120417
23. Rao PV, Nallappan D, Madhavi K, Rahman S, Jun Wei L, Gan SH. Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid Med Cell Longev. 2016;2016:3685671. doi:10.1155/2016/3685671
24. Park Y, Hong YN, Weyers A, Kim YS, Linhardt RJ. Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011;5(3):69–78. doi:10.1049/iet-nbt.2010.0033
25. Lee J, Park EY, Lee J. Non-toxic nanoparticles from phytochemicals: preparation and biomedical application. Bioprocess Biosyst Eng. 2014;37(6):983–989. doi:10.1007/s00449-013-1091-3
26. Drewes SE, Elliot E, Khan F, Dhlamini JTB, Gcumisa MSS. Hypoxis hemerocallidea—not merely a cure for benign prostate hyperplasia. J Ethnopharmacol. 2008;119(3):593–598. doi:10.1016/j.jep.2008.05.027
27. Nair VDP, Kanfer I. South African Journal of Science. Vol. 104. Academy of Science of South Africa; 2008.
28. Mills E, Cooper C, Seely D, Kanfer I. African herbal medicines in the treatment of HIV: hypoxis and Sutherlandia. An overview of evidence and pharmacology. Nutr J. 2005;4:19. doi:10.1186/1475-2891-4-19
29. Ojewole JAO. Antinociceptive, anti-inflammatory and antidiabetic properties of Hypoxis hemerocallidea Fisch. & C.A. Mey. (Hypoxidaceae) corm [“African Potato”] aqueous extract in mice and rats. J Ethnopharmacol. 2006;103(1):126–134. doi:10.1016/J.JEP.2005.07.012
30. Steenkamp V, Gouws MC, Gulumian M, Elgorashi EE, van Staden J. Studies on antibacterial, anti-inflammatory and antioxidant activity of herbal remedies used in the treatment of benign prostatic hyperplasia and prostatitis. J Ethnopharmacol. 2006;103(1):71–75. doi:10.1016/J.JEP.2005.07.007
31. Oguntibeju OO, Meyer S, Aboua YG, Goboza M. Hypoxis hemerocallidea significantly reduced hyperglycaemia and hyperglycaemic-induced oxidative stress in the liver and kidney tissues of streptozotocin-induced diabetic male wistar rats. Evid Based Complement Altern Med. 2016;2016:8934362. doi:10.1155/2016/8934362
32. Katerere DR. Hypoxis hemerocallidea (African potato): a botanical whose time has come? In: Juliani HR, Simon JE, Ho C-T, editors. African Natural Plant Products Volume II: New Discoveries and Challenges in Chemistry and Quality. Washington: American Chemical Society; 2013:51–61. doi:10.1021/bk-2013-1127.ch004
33. Owira PMO, Ojewole JAO. “African potato” (Hypoxis hemerocallidea corm): a plant-medicine for modern and 21st century diseases of mankind? - a review. Phyther Res. 2009;23(2):147–152. doi:10.1002/ptr.2595
34. Nsibande BE, Gustavsson K-E, Zhu L-H. Analysis of health-associated phytochemical compounds in seven Hypoxis species. Am J Plant Sci. 2018;9(4):571–583. doi:10.4236/ajps.2018.94044
35. Nair VDP, Kanfer I. High-performance liquid chromatographic method for the quantitative determination of hypoxoside in African potato (Hypoxis hemerocallidea) and in commercial products containing the plant material and/or its extracts. J Agric Food Chem. 2006;54(8):2816–2821. doi:10.1021/jf052418s
36. Albrecht C. Hypoxoside: a putative, non-toxic prodrug for the possible treatment of certain malignancies, HIV-infection and inflammatory conditions. Hypoxoside: a putative, non-toxic prodrug for the possible treatment of certain malignancies, HIV-infection and inflam. In:
37. Nair VDP, Dairam A, Agbonon A, Arnason JT, Foster BC, Kanfer I. Investigation of the antioxidant activity of African potato (Hypoxis hemerocallidea). J Agric Food Chem. 2007;55(5):1707–1711. doi:10.1021/jf0619838
38. Laporta O, Funes L, Garzón MT, Villalaín J, Micol V. Role of membranes on the antibacterial and anti-inflammatory activities of the bioactive compounds from Hypoxis rooperi corm extract. Arch Biochem Biophys. 2007;467(1):119–131. doi:10.1016/j.abb.2007.08.013
39. Kabanda MM. Antioxidant activity of rooperol investigated through Cu (I and II) chelation ability and the hydrogen transfer mechanism: a DFT study. Chem Res Toxicol. 2012;25(10):2153–2166. doi:10.1021/tx300244z
40. Ali Azouaou S, Emhemmed F, Idris-Khodja N, et al. Selective ROS-dependent p53-associated anticancer effects of the hypoxoside derivative rooperol on human teratocarcinomal cancer stem-like cells. Invest New Drugs. 2015;33(1):64–74. doi:10.1007/s10637-014-0182-6
41. Boukes GJ, van de Venter M. Rooperol as an antioxidant and its role in the innate immune system: an in vitro study. J Ethnopharmacol. 2012;144(3):692–699. doi:10.1016/J.JEP.2012.10.014
42. Guzdek A, Niżankowska E, Allison AC, Kruger PB, Koj A. Cytokine production in human and rat macrophages and dicatechol rooperol and esters. Biochem Pharmacol. 1996;52(7):991–998. doi:10.1016/0006-2952(96)00386-3
43. Khan M, Khan M, Adil SF, et al. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine. 2013;8:1507. doi:10.2147/IJN.S43309
44. Lund ME, To J, O’Brien BA, Donnelly S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J Immunol Methods. 2016;430:64–70. doi:10.1016/J.JIM.2016.01.012
45. Jung J, Park S, Hong S, et al. Synthesis of gold nanoparticles with glycosides: synthetic trends based on the structures of glycones and aglycones. Carbohydr Res. 2014;386(1):57–61. doi:10.1016/j.carres.2013.12.012
46. Elbagory AM, Cupido CN, Meyer M, Hussein AA. Large scale screening of Southern African plant extracts for the green synthesis of gold nanoparticles using microtitre-plate method. Molecules. 2016;21(11):1498. doi:10.3390/molecules21111498
47. Prevo BG, Esakoff SA, Mikhailovsky A, Zasadzinski JA. Scalable routes to gold nanoshells with tunable sizes and response to near-infrared pulsed-laser irradiation. Small. 2008;4(8):1183–1195. doi:10.1002/smll.200701290
48. Yulizar Y, Utari T, Ariyanta HA, Maulina D. Green method for synthesis of gold nanoparticles using polyscias scutellaria leaf extract under UV light and their catalytic activity to reduce methylene blue. J Nanomater. 2017;2017:1–6. doi:10.1155/2017/3079636
49. Noruzi M. Biosynthesis of gold nanoparticles using plant extracts. Bioprocess Biosyst Eng. 2015;38(1):1–14. doi:10.1007/s00449-014-1251-0
50. Ndeh NT, Maensiri S, Maensiri D. The effect of green synthesized gold nanoparticles on rice germination and roots. Adv Nat Sci Nanosci Nanotechnol. 2017;8. doi:10.1088/2043-6254/aa724a.
51. Gardea-Torresdey JL, Parson JG, Gomez E, et al. Formation and growth of au nanoparticles in live side live alfalfa plants. Nano Lett. 2002;2:397–401. doi:10.1021/nl015673+
52. Max J-J, Chapados C. Infrared spectroscopy of aqueous carboxylic acids: comparison between different acids and their salts. J Phys Chem A. 2004;108(16):3324–3337. doi:10.1021/JP036401T
53. Fornaro T, Burini D, Biczysko M, Barone V. Hydrogen-bonding effects on infrared spectra from anharmonic computations: uracil–water complexes and uracil dimers. J Phys Chem A. 2015;119(18):4224–4236. doi:10.1021/acs.jpca.5b01561
54. Bano S, Nazir S, Nazir A, et al. Microwave-assisted green synthesis of superparamagnetic nanoparticles using fruit peel extracts: surface engineering, T 2 relaxometry, and photodynamic treatment potential. Int J Nanomedicine. 2016;11:3833–3848. doi:10.2147/IJN.S106553
55. Rouhana LL, Jaber JA, Schlenoff JB. Aggregation-resistant water-soluble gold nanoparticles. Langmuir. 2007;23(26):12799–12801. doi:10.1021/la702151q
56. Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118(1):9–18. doi:10.1182/blood-2010-08-265892
57. Juskewitch JE, Platt JL, Knudsen BE, Knutson KL, Brunn GJ, Grande JP. Disparate roles of marrow- and parenchymal cell-derived TLR4 signaling in murine LPS-induced systemic inflammation. Sci Rep. 2012;2:918. doi:10.1038/srep00918
58. Rossol M, Heine H, Meusch U, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31(5):379–446. doi:10.1615/CritRevImmunol.v31.i5.20
59. Loiarro M, Ruggiero V, Sette C. Targeting TLR/IL-1R signalling in human diseases. Mediators Inflamm. 2010;2010:1–12. doi:10.1155/2010/674363
60. Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87–103.
61. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta - Mol Cell Res. 2011;1813(5):878–888. doi:10.1016/J.BBAMCR.2011.01.034
62. Le Moine O, Devière J, Devaster JM, et al. Interleukin-6: an early marker of bacterial infection in decompensated cirrhosis. J Hepatol. 1994;20(6):819–824. doi:10.1016/s0168-8278(05)80155-2
63. González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12(2):125–135. doi:10.1038/nri3133
64. Gessani S, Belardelli F. IFN-γ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998;9(2):117–123. doi:10.1016/S1359-6101(98)00007-0
65. Ye J, Ortaldo JR, Conlon K, Winkler-Pickett R, Young HA. Cellular and molecular mechanisms of IFN-gamma production induced by IL-2 and IL-12 in a human NK cell line. J Leukoc Biol. 1995;58(2):225–233. doi:10.1002/jlb.58.2.225
66. Bream JH, Curiel RE, Yu CR, et al. IL-4 synergistically enhances both IL-2- and IL-12-induced IFN-γ expression in murine NK cells. Blood. 2003;102:207–214. doi:10.1182/blood-2002-08-2602
67. Ohkusa T, Nomura T, Sato N. The role of bacterial infection in the pathogenesis of inflammatory bowel disease. Intern Med. 2004;43(7):534–539. doi:10.2169/internalmedicine.43.534
68. Feldmann M, Maini RN. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–1250. doi:10.1038/nm939
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.