Back to Journals » International Medical Case Reports Journal » Volume 15
The Importance of Initial Epidurography Prior to Any Drug Administration in Three-Day Adhesiolysis Procedure
Authors Diebels OR, Baheri B, Gios J, Dierick A, Hans G
Received 20 April 2022
Accepted for publication 1 September 2022
Published 3 November 2022 Volume 2022:15 Pages 615—620
DOI https://doi.org/10.2147/IMCRJ.S370185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Owen Ray Diebels,1 Babak Baheri,2 Jens Gios,2 Ann Dierick,2 Guy Hans2
1University of Antwerp (UA), Antwerp University Hospital (UZA), Edegem, Belgium; 2Department of Anesthesiology and Pain Management, Antwerp University Hospital (UZA), Edegem, Belgium
Correspondence: Babak Baheri, Email [email protected]
Abstract: Percutaneous epidural adhesiolysis (PEA) is a minimal invasive procedure to relieve sciatalgia caused by post lumbar surgery syndrome (PLSS). Fluoroscopic-guided contrast-epidurography is essential to ensure a safe procedure. We present a case of a 28-year-old male patient who underwent a PEA which was complicated by a dural puncture. We highlight the dangers of such complications and discuss associated risk factors.
Keywords: failed back surgery syndrome, FBSS, fluoroscopy, percutaneous epidural adhesiolysis, PEA, subarachnoid space
Introduction/Background
Chronic lower back pain is a prevalent condition affecting a great number of patients. The lifetime prevalence of lower back pain is reported to be 65–80% wherein 35–75% will be prevalent for twelve months after the first episode.1 Post lumbar surgery syndrome (PLSS), defined as chronic lower back and leg pain following lumbar surgery, occurs in 10–40% of the surgeries.2,3 Aside from recurrent disc prolapse, the formation of epidural scarring and adhesions play an important role in the pathogenesis of the pain.4
The most important causal factor of PLSS is epidural scarring, being the main factor in 20–36% of PLSS cases. While multiple treatment options for PLSS are available, such as transforaminal or epidural infiltrations, most of these are ineffective. Transforaminal infiltrations are estimated to have an efficacy of around 80% and provide only short-term relief.5,6 Spinal cord stimulation is proven to give long-term relief; however, it is reserved as a last-resort modality.7
Percutaneous epidural adhesiolysis and neuroplasty is considered to be a minimally invasive intervention to treat the patients suffering from persisting leg pain after a prior back surgery. Since its introduction in 1985, the Racz procedure (also known as adhesiolysis) has gained widespread acceptance among the pain management interventionalists.8
Any inward displacement of epidural catheter, in subarachnoidal or dural space, can be a potentially life-threatening condition by inadvertent administration of epidural drug dosages into the subdural space.9
We present a case report of a patient who underwent percutaneous epidural adhesiolysis (PEA) and had a timely detection of epidural catheter displacement into the subdural space.
This case report reveals the importance of percutaneous diagnostic epidurography in avoidance of an aberrant injection during a therapeutic adhesiolysis procedure.
Case Report
A 28-year-old, non-obese, male patient presented with chronic lumbar pain combined with a sciatalgia in the left leg, persisting for two years after a previous lumbar discectomy surgery on level L4-L5. The pain was described as a burning, stabbing sensation with electric shocks running down the posterior leg. Using a numeric pain rating scale (NRS), the patient noted a score of 8 on 10.
On physical examination, anteflexion of the spine was limited and painful. Retroflexion was painful, but not limited. Straight leg raise was positive at 40°.
An MRI of the lumbar spine showed degenerative disc disease of level L4-L5 with disc-bulging and a medial fissure of the annulus fibrosus. The latest postoperative electromyography (EMG) study showed no signs of peripheral neuropathy in the lower left extremity.
The conservative treatment included physiotherapy and painkillers, failed to relieve the pain complications. Subsequently, different interventional therapies, including facet joint infiltrations, sacroiliac joint infiltrations, transforaminal epidural infiltrations and erector spinae plane blocks, provided a little pain relief for only one week.
To treat the radicular pain, a percutaneous epidural adhesiolysis was performed in three sessions spread across three days. Before the start of each session, a diagnostic epidurography was performed by administering the contrast material through the epidural catheter. The following describes the procedures that have been performed over the three days.
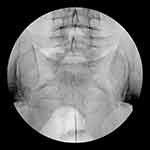
Day 1: A 16 gauge Coudé epidural needle was inserted through the sacral hiatus and an epidural catheter was advanced to S3 level, with gentle deviation of the catheter tip towards the left side (Figure 1). After confirming the optimal position of the catheter through anteroposterior (AP) and lateral imaging, 8 mL contrast medium (Omnipaque 240) was injected which showed good patency of L5 and S1 neuroforamina on both sides.
On the left side at the level of L4 neuroforamen, a filling defect was shown, confirming the occlusion of left L4 nerve root channel by fibrotic scar tissue.
After correct positioning of the catheter, a mixture of lidocaïne (1%, 10mL), hyaluronidase (1500 IU) and methylprednisolone-acetate (40mg) was injected. Through the catheter, an infusion of hypertonic saline (10% NaCl, 20mL) was administered.
Then, the catheter was flushed with lidocaine (1%, 2mL). The catheter tip was settled at the left L4 level with the external part tightly secured on the flank. To secure the catheter from dislodging, it had been fixed to the skin at the puncture site using a circular tightly adhesive tape, applying a sterile dressing thereover. We affixed the exposed portion of the catheter to the right flank with tape and transported the patient to the recovery area.
The patient was monitored and showed normal neurologic signs and stable hemodynamic parameters. After two hours, he was discharged to the wardroom.
Day 2: By injecting 8 mL of contrast medium (Omnipaque 240), an early epidurographic imaging has been taken which showed some loosening of scar tissue at the left L4 level, by revealing patchy contrast infiltration and opening up of the scar tissue through the nerve root channel. After one day of treatment, the neuroforamen became somewhat permeable to injectate. Then, the catheter was advanced cranially and a mixture of lidocaine, hyaluronidase and methylprednisolone-acetate was injected followed by hypertonic saline.
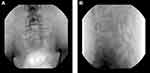
Day 3: At first an epidurography was performed, while the catheter was still in the secure position of the last day, with its tip located at left L4 level (Figure 2). A total volume of 7 mL contrast medium (Omnipaque 240) was injected without manipulating the catheter. The imaging showed a spread of contrast in the subarachnoid space, indicating a puncture of the dura mater by catheter (Figure 3). After withdrawing the catheter, its tip was retracted at S1 level where contrast was injected once again. Imaging showed no contrast dispersal in the subarachnoid space. For precaution, no medication was administered, and the epidural catheter was removed.
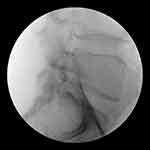 |
Figure 2 Schematic representation of lateral view shows the orientation and position of the catheter tip relative to spinal segment. Tip of the catheter is placed into the ventral epidural space. |
Discussion
Percutaneous epidural adhesiolysis (PEA) has been proven to be an effective tool in the treatment of post lumbar surgery syndrome. Manchikanti et al showed that epidural adhesiolysis had a significant effect on pain relief and improved functionality at 2 months, 6 months and 12 months following a one-day treatment in up to 72% of the patients. The best effect is achieved when hypertonic saline is used as an adjuvant.1 Epidural adhesiolysis had a significant better outcome when compared to epidural steroid injections. Adhesiolysis achieved pain relief in up to 82%, whereas steroid injections achieved pain relief in up to 12%.6 These results were confirmed by a recent study indicating that epidural adhesiolysis had a significant effect on both pain and disability scores post intervention.10
There are two types of interventions, described as percutaneous lumbar epidural adhesiolysis. It can be done once (one day), or in a series of up to three injections (three-day procedure).
In this patient, we have chosen to perform a three-day procedure. A clinical study by Hossieni et al showed that both procedures achieved a similar efficacy rate, advocating for the one-day procedure due to reduced patient discomfort and treatment costs. However, the sample size included in this study was small and more comparative research should be performed.11
Epidural catheter migration or displacement is a known entity with an incidence up to 50%, whether inserted for anesthesia or analgesia.12
Migration of an epidural catheter can be a potential complication of the procedure. Migrations can be subcutaneous, intravascular, subdural or subarachnoid. Epidural test doses of local anesthetics immediately after catheter insertion have been used to exclude subdural placement. Serious complications caused by PEA are uncommon. In most cases, no serious complications have been reported. Some of the reported complications include post-operative numbness and paresthesia, hypotension, dural puncture and headache, difficulty to pass the catheter and infections.3
In this case, catheter got displaced into subarachnoid space leading to an intrathecal contrast dispersal. Any misjudgement of contrast-fluoroscopic imaging could lead to a life-threatening condition.
An assurance of catheter fixation along with vigilant monitoring of the puncture site is a must.
In a case report by Jaju et al, an epidural catheter got displaced leading to drug accumulation in the subcutaneous space.13
A dural puncture is the most common complication following PEA. The puncture itself does not result in any significant clinical danger; however, medication(s), especially hypertonic saline, must not be injected as another case report has shown that intrathecal injections of hypertonic saline may lead to myelopathy. If no medication is injected, no treatment is required more than conservative remedies for postdural puncture headache (PDPH).14,15
The diagnosis of a migrated subdural catheter can be made clinically by the sudden and rapid onset of dense, ascending motor blockade while on a steady epidural infusion.9
The risky consequence of an unrecognized accidental dural puncture can be a respiratory failure secondary to a high-level block by administering a large dose of local anesthetics.16
Reported factors influencing catheter migration include weight, body mass index, depth of the epidural space and patient positioning.17,18
Phillips et al studied epidural catheter migration in labor and found an incidence of 54%. Migration was 2 cm on average and tended to be directed inward.19
Crosby et al found a similar incidence of catheter migration but in an outward direction.20 It has been documented in both the obstetric and non-obstetric settings.21,22
Motamed et al showed that 45% of the epidural failures in major abdominal surgery could be attributable to catheter migration.23
In a randomized controlled trial conducted by Riveros and Barnett, comparing three types of epidural catheter dressing, no association was found between catheter migration and BMI.
However, the dressing technique could influence catheter migration and the risk of epidural failure.24
Hamilton et al showed an association between patient position change and movement of epidural catheter. When the patient transitions from a flexed position to a straight back and lateral decubitus position, a change in epidural catheter position was noted. The authors found that the magnitude of epidural catheter movement was more pronounced in patients with BMI >30 kg/m².25
Although a subdural migration of the intrathecal catheter has been reported by Sorokin et al, an initial subdural placement should always be considered during the catheter placement.26
Highest incidence of accidental dural puncture occurred during repeated attempts for epidural access, either due to difficult anatomy or anxious, uncooperative patients.27
Epidurography is the diagnostic portion of a PEA procedure without which the lysis of adhesion bands cannot be accomplished. This case highlights the importance of fluoroscopic-guided contrast-epidurography for epidural catheter displacing.
Uchino et al have evaluated the post-operative in-dwelling epidural catheter position by administering 5 mL of contrast via the catheter.28
As a diagnostic tool, when a wire-reinforced epidural catheter is used, a high-resolution spiral computed tomography scan (HRS CT-Scan), can be considered, as it has been reported to have successfully identified a wandering epidural catheter.29
Other reports have proceeded to perform CT myelograms with administration of contrast via the migrated epidural catheter to delineate the location of the catheter tip.27,29,30
The patient presented in this case was not obese, thus the patient’s weight and body mass index is not the major risk factor leading to catheter migration. In this case, catheter migration is most likely attributed to changes in patient position.
Conclusion
Percutaneous epidural adhesiolysis (PEA) is an effective and safe treatment option in the treatment of chronic lumbar pain caused by post-lumbar surgery syndrome. Although more evidence is needed to fully understand the role and indications of this treatment, it can be considered as a possible treatment modality for chronic, refractory lumbar radicular pain.
Any catheter malposition should be ruled out by contrast imaging before contemplating epidural drug administration. Analysis of fluorogram with awareness and vigilance of preventable catheter displacing complications is the first step toward mitigating risks to provide safe and effective epidural adhesiolysis for chronic pain management.
In three-day trials, performing and careful evaluation of contrast fluorogram at every visit, is an obligation before injecting any medication through the catheter.
Meticulous care must be taken during patient positioning and transfer.
An adequate knowledge of contrast-fluoroscopic imaging patterns and high index of suspicion in patients with indwelling epidural catheter are vital to avoid complications.
Disclosure
No institutional approval was required to publish the case details in this case report.
Written informed consent for publication of their details was obtained from the patient.
The authors report no conflicts of interest in this work.
References
1. Manchikanti L, Rivera JJ, Pampati V., et al. One day lumbar epidural adhesiolysis and hypertonic saline neurolysis in treatment of chronic low back pain: a randomized, double-blind trial. Pain Physician. 2004;7(2):177–186. doi:10.36076/ppj.2004/7/177
2. Sebaaly A, Lahoud MJ, Rizkallah M, Kreichati G, Kharrat K. Etiology, evaluation, and treatment of failed back surgery syndrome. Asian Spine J. 2018;12(3):574–585. doi:10.4184/asj.2018.12.3.574
3. Akbas M, Elawamy AR, Salem HH, Fouad AZ, Abbas NA, Dagistan G. Comparison of 3 approaches to percutaneous epidural adhesiolysis and neuroplasty in post lumbar surgery syndrome. Pain Physician. 2018;21(5):E501–E508.
4. Dhagat PK, Jain M, Singh SN, Arora S, Leelakanth K. Failed back surgery syndrome: evaluation with magnetic resonance imaging. J Clin Diagn Res. 2017;11(5):Tc06–tc09. doi:10.7860/JCDR/2017/24930.9817
5. Durand G, Girodon J, Debiais F. Medical management of failed back surgery syndrome in Europe: evaluation modalities and treatment proposals. Neurochirurgie. 2015;61:S57–S65. doi:10.1016/j.neuchi.2015.01.001
6. Cho JH, Lee JH, Song KS, et al. Treatment outcomes for patients with failed back surgery. Pain Physician. 2017;20(1):E29–e43. doi:10.36076/ppj.2017.1.E29
7. Geudeke MW, Krediet AC, Bilecen S, Huygen F, Rijsdijk M. Effectiveness of epiduroscopy for patients with failed back surgery syndrome: a systematic review and meta-analysis. Pain Pract. 2021;21(4):468–481. doi:10.1111/papr.12974
8. Johnson BA, Staats PS, Wetzel FT, Mathis JM. Diagnostic Epidurography and Therapeutic Epidurolysis. In: Image-Guided Spine Interventions. Springer-Verlag New York; 2014:171–202.
9. Tay YC, Abrahams MJ. Timely detection of epidural catheter migration: diagnosis and management: a case report. Int J Anesthetic Anesthesiol. 2017;4(2):548.
10. Manchikanti L, Knezevic NN, Sanapati SP, Sanapati MR, Kaye AD, Hirsch JA. Is Percutaneous adhesiolysis effective in managing chronic low back and lower extremity pain in post-surgery syndrome: a systematic review and meta-analysis. Curr Pain Headache Rep. 2020;24(6):30. doi:10.1007/s11916-020-00862-y
11. Hossieni B, Dadkhah P, Moradi S, Hashemi SM, Safdari F. The results of treating failed back surgery syndrome by adhesiolysis: comparing the one- and three-day protocols. Anesth Pain Med. 2017;7(5):e60271. doi:10.5812/aapm.60271
12. Day Y, Graham D. Epidural catheter migration. Anaesthesia. 2002;57(4):418. doi:10.1046/j.1365-2044.2002.2575_25.x
13. Jaju R, Paliwal B, Sethi P, Bhatia P. Epidural catheter displacement - A report of delayed diagnosis. Indian J Anaesth. 2018;62(12):1009–1010. doi:10.4103/ija.IJA_593_18
14. Helm S 2nd, Racz GB, Gerdesmeyer L, et al. Percutaneous and endoscopic adhesiolysis in managing low back and lower extremity pain: a systematic review and meta-analysis. Pain Physician. 2016;19(2):E245–282. doi:10.36076/ppj/2016.19.E245
15. Kim RC, Porter RW, Choi BH, Kim SW. Myelopathy after the intrathecal administration of hypertonic saline. Neurosurgery. 1988;22(5):942–945. doi:10.1227/00006123-198805000-00026
16. Bromage PR. Epidural Analgesia.
17. Shon YJ, Bae SK, Park JW, Kim IN, Huh J. Partial displacement of epidural catheter after patient position change: a case report. J Clin Anesth. 2017;37:17–20. doi:10.1016/j.jclinane.2016.10.013
18. Bishton IM, Martin PH, Vernon JM, Liu WH. Factors influencing epidural catheter migration. Anaesthesia. 1992;47(7):610–612. doi:10.1111/j.1365-2044.1992.tb02337.x
19. Phillips DC, Macdonald R. Epidural catheter migration during labour. Anaesthesia. 1987;42(6):661–663. doi:10.1111/j.1365-2044.1987.tb03096.x
20. Crosby ET. Epidural catheter migration during labour: an hypothesis for inadequate analgesia. Can J Anaesth. 1990;37(7):789–793. doi:10.1007/BF03006538
21. Burns SM, Cowa CM, Barclay PM, Wilkes RG. Intrapartum epidural catheter migration: a comparative study of three dressing applications. Br J Anaesth. 2001;86(4):565–567. doi:10.1093/bja/86.4.565
22. Hermanides J, Hollmann MW, Stevens MF, Lirk P. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–154. doi:10.1093/bja/aes214
23. Motamed C, Farhat F, Remerand F, Stephanazzi J, Laplanche A, Jayr C. An analysis of postoperative epidural analgesia failure by computed tomography epidurography. Anesth Analg. 2006;103(4):1026–1032. doi:10.1213/01.ane.0000237291.30499.32
24. Riveros Perez E, Barnett K, Jimenez E, Yang N, Rocuts A. Intrapartum epidural catheter displacement: comparison of three dressing methods (Migracion de cateter epidural intraparto: comparacion de tres metodos de fijacion). Rev Fac Cien Med Univ Nac Cordoba. 2019;76(3):170–173. doi:10.31053/1853.0605.v76.n3.22726
25. Hamilton CL, Riley ET, Cohen SE. Changes in the position of epidural catheters associated with patient movement. Anesthesiology. 1997;86(4):778–784. doi:10.1097/00000542-199704000-00007
26. Sorokin A, Annabi E, Yang WC, Kaplan R. Subdural intrathecal catheter placement: experience with two cases. Pain Physician. 2008;11(5):677–680. doi:10.36076/ppj.2008/11/677
27. Jadon A, Chakraborty S, Sinha N, Agrawal R. Intrathecal Catheterization by Epidural Catheter: management of Accidental Dural Puncture and Prophylaxis of PDPH. Indian J Anaesth. 2009;53(1):30–34.
28. Uchino T, Hagiwara S, Iwasaka H, et al. Use of imaging agent to determine postoperative indwelling epidural catheter position. Korean J Pain. 2010;23(4):247–253. doi:10.3344/kjp.2010.23.4.247
29. Brookman JC, Sair HI, Benkwitz C, Gulur P. Wandering epidural catheter. Anesthesiology. 2010;113(5):1198. doi:10.1097/ALN.0b013e3181e4f349
30. Sprigge JS, Harper SJ. Accidental dural puncture and post dural puncture headache in obstetric anaesthesia: presentation and management: a 23-year survey in a district general hospital. Anaesthesia. 2008;63(1):36–43. doi:10.1111/j.1365-2044.2007.05285.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.