Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
The Impact of Virologic Parameters and Liver Fibrosis on Health-Related Quality of Life in Black African Patients with Chronic Hepatitis B: Results from a High Endemic Area
Authors Mahassadi AK , Team Machekam O , Attia AK
Received 30 March 2020
Accepted for publication 31 July 2020
Published 2 October 2020 Volume 2020:13 Pages 407—418
DOI https://doi.org/10.2147/CEG.S255102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Koulaouzidis
Alassan Kouamé Mahassadi,1,2 Olga Team Machekam,1 Alain Koffi Attia1,2
1Hepatology and Gastroenterology Unit, Yopougon Teaching Hospital, Abidjan, Côte d’Ivoire; 2Faculty of Medicine, Department of Gastrointestinal Diseases, Félix Houphouët Boigny University, Abidjan, Côte d’Ivoire
Correspondence: Alassan Kouamé Mahassadi
Hepatology and Gastroenterology Unit, Yopougon Teaching Hospital, 01 B.p. V166, Abidjan 01, Côte d’Ivoire
Tel +225 22480094
Email [email protected]
Background: The effects of virologic parameters, liver fibrosis, and treatment on the HRQoL in black African patients with CHB are unknown.
Objective: To determine the magnitude and the effects of hepatitis B e antigen (HBeAg), hepatitis B surface antigenemia (HBs antigenemia), viral load, liver fibrosis and treatment on HRQoL impairment in black African patients with CHB using the SF36 (SF36) and chronic liver disease questionnaires (CLDQ).
Materials and Methods: HRQoL comparison was determined in a case–control study and enrolled 214 patients with CHB (mean age: 42 years, male: 65.9%) and 210 healthy controls subjects (mean age: 37.8 years; male: 63.8%). Control subjects were younger than those with CHB (p=0.01). Analysis of covariance, Welch test and linear regression were used to compare HRQoL between subgroups.
Results: Adjusted to age and gender, patients with CHB elicited low mean scores on the subscales of role-physical (66.9 vs 78, p=0.001), role-emotional (64 vs 77.5, p=0.01), bodily pain (70.8 vs 96.2, p=0.001), social functioning (74.6 vs 84.5, p=0.003) and general health (64.6 vs 74.4, p=0.03) in comparison with control subjects. Multivariate analysis showed that CHB impaired HRQoL on physical (β= − 16.7 (1.8), p< 0.0001) and mental component summaries (β= − 5.1 (2.0), p=0.01) adjusted to others variables. Patients with HBeAg negative CHB elicited low scores on physical (p=0.004) and mental (p=0.05) component summaries and low CLDQ’s average score (p=0.002) in comparison with those positive. Patients with low (≤ 1000 IU/mL) HBs antigenemia (p=0.03) or viral load (p=0.03) scored less on physical component summary and those with significant fibrosis or cirrhosis scored less (p=0.003) on mental component summary.
Conclusion: Black African patients with CHB expressed poor HRQoL, particularly those with HBeAg negative CHB, low viral load, or HBs Antigenemia.
Keywords: chronic hepatitis B, Hepatitis B e antigen, HBs antigenemia, viral load, liver fibrosis, health related quality of life, sub-Saharan Africa
Introduction
Chronic hepatitis B (CHB) is the most prevalent chronic liver disease affecting 257 millions of individuals worldwide mostly in Asia and Africa.1 Liver fibrosis and viral load are the main factors leading to cirrhosis and hepatocellular carcinoma, major complications of CHB accounting for 66% of 1 to 34 million annual deaths worldwide related to viral hepatitis.1–3 Therefore, the disease perception is hampered in chronically infected patients and impacts negatively their health-related quality of life (HRQoL).4,5
CHB is a continuous liver disease with quiescent and exacerbation phases that depict two subtypes according to the hepatitis B e antigen (HBeAg) profile.1,6 Patients with HBeAg positive CHB are more likely to have less fibrosis and high viral load than their counterparts.1,6,7 During the treatment, HBeAg negativity is a goal to be achieved in those previously positive that indicates a treatment successful.8 However, divergent results of the impact of HBeAg status on HRQoL were published.9,10
Moreover, recent guidelines recommend the determination of HBeAg status, viral load, and liver fibrosis before antiviral treatment.8 Among these parameters, liver fibrosis has been intensively reported to be a predictor of HRQoL impairment in patients with CHB.4,5
Several studies focusing on HRQoL, mainly conducted in Asian and Caucasian patients, have demonstrated that extended fibrosis or decompensated cirrhosis alters the HRQoL in patients with CHB and, those with compensated cirrhosis express similar perceptions of HRQoL with that of general population.4,5,9,10
The most and widely used generic questionnaire to assess the HRQoL is SF36 questionnaire (SF36) which comprises 36 items grouped into 8 subscales depicting the health perception among general population.11 Besides the SF36, specific questionnaires devoted to patients with chronic liver diseases have been published which provide reliable results on the HRQoL impairment in patients with chronic liver diseases.12,13 Among them, the chronic liver disease questionnaire (CLDQ) comprises 27 items that encompass 6 subscales of HRQoL impairment specifically in patients with chronic liver diseases.13 In our knowledge, none of them has been used in Black African patients with CHB to assess their HRQoL.
Sub-Saharan Africa displays the most elevated prevalence of CHB of 6.1% among population that accounts for nearly 88 thousand deaths annually.14 The viral infection is acquired mostly in childhood, and HBeAg positive CHB is the most prevalent profile at this stage.1,2,14 During the course of the disease, people may lose HBeAg positivity with liver damage, low viral load, fluctuating transaminases, and long-standing and fluctuating clinical symptoms such as chronic fatigue, loss of appetite, and flare up.1,2,15 Some of these patients may be in an inactive phase with mild or no liver damage, very low viral load, and HBs antigenemia.1,2,16
Furthermore, viral load is correlated with hepatitis B surface antigenemia (HBs antigenemia) and associated with an advanced liver fibrosis in patients with CHB.16,17 A Report suggests that a low viral load obtained during antiviral treatment may improve HRQoL of patients with CHB.18 However, the effect of HBs antigenemia on HRQoL is unknown and, none of these virologic parameters was reported to have an impact on HRQoL in black African patients with CHB. We hypothesized that these virologic parameters and antiviral treatment could have an impact on HRQoL in black African patients with CHB.
This study was aimed to evaluate the internal consistency and reliability of the SF36 and CLDQ and the magnitude of HRQoL impairment in patients with CHB in comparison with that of control subjects, to determine the impact of the virologic parameters, liver fibrosis and treatment on HRQoL in those with CHB.
Patients and Methods
Patients
A study was conducted from April to August 2019, in Abidjan, the economic capital of Ivory Coast, West Africa, and enrolled 232 patients prospectively who completed the SF36 and CLDQ self-administrated. These patients were the attendees of the gastroenterology units of 4 hospitals in Abidjan (Centres hospitaliers et Universitaires de Cocody, Yopougon, la clinique Danga, la polyclinique Farah). Among them, 18 patients were excluded (chronic hepatitis C: 11 patients, chronic hepatitis D: 4 patients, hepatocellular carcinoma: 3 patients). Finally, 214 patients who met the inclusion criteria of CHB (mean age: 42 years, male: 65.9%) were retained. These patients were compared with 210 previous control subjects (mean age: 37.8 years, male: 63.8%) with known and published SF36 scores. They were recruited at the main streets of three cities of Côte d’Ivoire (Abidjan, Grand Bassam, and Bonoua), using a face-to-face interview if, they declared not having jaundice in the past 5 years or suffered from chronic liver disease or functional gastrointestinal disorders.19,20
Control subjects were younger than those with CHB (p=0.01). Informed consent was obtained before the completion of the questionnaires from the enrolled subjects. Those having difficulties to understand the meaning of some items of the questionnaires received additional explanation without interference in the choice of the response.
The study was approved by the ethics committee of the Centre Hospitalier et Universitaire de Yopougon (registration number: 0054/dms/okt/yf/19) and according to the Helsinki declaration.
Study Design
In the first part of the study, we performed a case–control study to assess the internal consistency and reliability of SF36 and CLDQ among enrolled subjects, the magnitude of the HRQoL impairment in black African patients with CHB in comparison with that of control subjects. In the second part, we determined the impact of HBeAg status, HBs antigenemia, viral load, liver fibrosis and treatment on HRQoL impairment within those with CHB.
Administration of the Questionnaires
All patients fulfilling the inclusion criteria or having liver disease related to chronic infection of viral hepatitis B were asked to participate in the study after they read the informed consent and received additional verbal explanations. Those who accepted to participate received the observation chart that comprised 2 parts: the first one retrieved the sociodemographic and biochemical parameters, done by the interviewer, and the second part included the SF36 and CLDQ items.11,13
The SF36 comprises 36 items grouped into 8 subscales of HRQoL that are: physical functioning, role limitation due to physical problems (role-physical), role limitation due to emotional problems (role-emotional), bodily pain, social functioning, vitality and mental health. All subscales are scored from 0 (worse) to 100 (better score) points and summarized into 2 composites scales: physical (PCS) and mental component summaries (MCS) depicting, respectively, the physical and mental perceptions of HRQoL.11
The CLDQ comprises 27 items grouped into 6 subscales depicting specifically the HRQoL impairment in patients with the chronic liver disease that are abdominal symptoms, fatigue, systemic symptoms, activity, emotional functioning and Worry. All subscales are scored from 1 (worse) to 7 points (better score) in HRQoL. The global score is the average score obtained from all 6 subscales.13
Data Collection and Definitions
The parameters retrieved were age, gender, body mass index, marital status (single, married, widowed, divorced or living alone) remunerated activity, past medical history or comorbidities, status of chronic hepatitis B viral infection, treatment regimen and scores obtained from SF36 and CLDQ after calculation accordingly.11,13
The virologic and biochemical parameters retrieved were HBeAg, HBs antigenemia, viral load, alanine aminotransferase (ALT), and liver fibrosis assessed by individual liver stiffness (Fibroscan, Echosens®) measurement.
CHB is defined as the persistence of hepatitis B surface antigen positivity on blood test more than 6 months at 2 measurements with ALT elevation above the upper limit of normal (40 IU/L).7 Those with HBeAg positivity are named HBeAg positive CHB or, negative if not.7,8
Fibrosis stage is defined as follows: no significant fibrosis [F0F1]: LS <7.3 Kpa, significant fibrosis [F2F3] 7.3 ≤ LS < 11 and cirrhosis [F4] ≥11 Kpa as previously reported by Bonnard et al, in Burkina Faso, West Africa and according to METAVIR score system.21,22
Statistical Analysis
Continuous variables were reported as mean with standard deviation or standard error otherwise, as median with range and categorical variables as number and percentage. The reliability of SF36 and CLDQ was assessed using a Cronbach’s alpha measurement.23 We used an analysis of covariance adjusted to age and gender to compare SF36 scores between patients with CHB and control subjects.24 To assess the impact of virologic parameters and liver fibrosis on HRQoL subscales within patients with CHB, we used an analysis of variance with the Welch test for non-homogeneous variances and the Games-Howell test for post hoc comparison.25,26 Stepwise multiple linear regression analysis with PCS, MCS, and CLDQ’s average score as dependent variables were computed to retrieve factors associated with HRQoL impairment adjusted to sociodemographic factors. The beta (β) regression coefficient (with its standard error) is the estimate of the association that indicates the magnitude of the expected change in the outcome when the factor increases by one unit.27 Statistical analysis was performed with the SPSS software version 22 with a two-sided significant p-value less than 0.05.
Results
Baseline Characteristics of Patients with Chronic Hepatitis B
The baseline characteristics are summarized in Table 1. Among patients with CHB, 53.1% and 29.1% achieved a university and secondary degrees respectively, 77.3% have a remunerated activity and 48.6% declared having health insurance. Among the 209 patients who completed their marital status, 47.3% were married, 36.8% singles, 2.9% widowed. Overall, 114 (53.3%) have comorbidities mainly chronic constipation or irritable bowel syndrome (35%), arterial hypertension, or heart diseases (29%). Overall, HBeAg negative CHB was found in 182 (89.2%%) patients whereas 22 were HBeAg positive. The duration of CHB was less than 5 years in 62.9% of cases and, respectively, 35.5% and 23.9% of patients with CHB have shown a high level of HBs antigenemia (≥10,000 UI/mL) or viral load (>1000 UI/mL). According to liver stiffness measurement, significant fibrosis (F2F3) and cirrhosis (F4) were found in 19.1% and 12% of patients, respectively. Treatment was administered in 110 patients with CHB mainly with Tenofovir among them 16 were HBeAg positive.
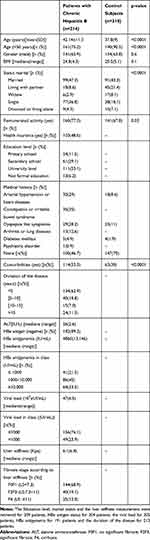 |
Table 1 Baseline Characteristics |
Baseline Characteristics Comparison with Control Subjects
In comparison with chronically infected patients, control subjects were younger (p<0.0001), less married (p<0.0001), had a remunerated activity (p=0.03), or fewer comorbidities (p<0.0001) in comparison with those having CHB (Table 1).
The Internal Consistency of the SF36 Questionnaire and CLDQ and Domains Distribution
The Cronbach’s alpha estimates of SF36 reliability were high among patients with CHB (0.85) and control subjects (0.76) as for CLDQ in those with CHB (0.89). Adjusted to age, the average SF36 scores elicited by patients with CHB were low on the subscales of role-physical (66.9 vs 78, p=0.001), role-emotional (64 vs 77.5; p=0.01), bodily pain (70.8 vs 96.2, p=0.001), social functioning (74.6 vs 84.5, p=0.003) and general health (64.6 vs 74.5, p=0.03). Overall PCS (71.5 vs 86.1, p<0.0001) and MCS (66.3 vs 68.4, p=0.02) decreased significantly in patients with CHB compared with that of control subjects (Figure 1). Meanwhile, in those with CHB, the mean (with standard deviation) scores obtained with CLDQ were greater than the average on the subscales of abdominal symptoms (5.4±1.5), fatigue (5.2±1.2), systemic symptoms (5.6±1.1), activity (5.6±1.4), emotional functioning (5.4±1.2) and worry (4.9±1.7).
Factors Associated with Health Quality in Comparison with Control Subjects
Factors associated with PCS in multivariate regression analysis (beta coefficient (standard error) estimates) were age (β= −0.23 (0.1); p=0.01), remunerated activity (β= 4.8 (1.8); p=0.01), comorbidities (β= −0.82 (1.9); p<0.0001) and CHB (β=−16.7 (1.8); p<0.0001). Those associated with MCS were remunerated activity (β= 6.1 (2.04), p=0.003), comorbidities (β= −7.5 (2.2), p=0.001), widowed subject (β= −9.6 (4.1), p=0.02), and those living alone (β= 10.9 (4.1), p=0.01) and CHB (β= −5.1 (2.0); p=0.01) (Table 2).
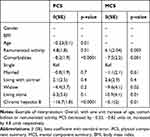 |
Table 2 Multivariate Analysis of Factors Affecting Health Quality Assessed with SF36: Comparison with Healthy Control Subjects |
The Influence of Virologic Parameters and Liver Stiffness on Health-Related Quality of Life Impairment in Patients with Chronic Hepatitis B
As summarized in Tables 3 and 4 using SF36 and CLDQ, regression analysis demonstrated that HBeAg status was positively associated with HRQoL enhancement more importantly on PCS (β= 8.97, p=0.04) and affected positively the subscales of role-physical (p=0.03), role-emotional (p=0.03) and general health (p=0.05). With CLDQ, HBeAg status enhanced the CLDQ’s average score (β= 0.55, p=0.02) and was positively associated with the subscales of activity (p=0.01) and worry (p=0.04). HBs antigenemia as a continuous variable did not correlate with all SF36 or CLDQ subscales while viral load exerted a negative impact on the subscale of mental health (p=0.001). The degree of liver stiffness affected negatively both PCS (β=−0.44, p=0.03) and MCS (β= −0.63, p=0.05). In particular, liver stiffness decreased the magnitude of HRQoL on the subscales of role-physical (p=0.03), social functioning (p=0.04), vitality (p=0.03), mental health (p=0.02) and general health (p=0.01) with SF36 and, on the subscale of worry (p=0.02) with CLDQ.
 |
Table 3 Impact of Virologic Parameters on SF 36 Subscales in Black African Patients with Chronic Hepatitis B: Univariate Linear Regression Analysis |
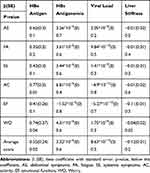 |
Table 4 Impact of Virologic Parameters on CLDQ Subscales in Black African Patients with Chronic Hepatitis B: Univariate Linear Regression Analysis |
Trends and Post Hoc Analysis of SF36 and CLDQ Subscales According to Virologic Parameters, Stages of Liver Fibrosis, and the Treatment in Patients with Chronic Hepatitis B
We performed an analysis of variance with post hoc analysis with all virologic parameters and sociodemographic factors dichotomized accordingly within patients with CHB and expressed the mean scores of all subscales. In comparison with those positive, patients with HBeAg negative CHB scored less on the subscales of role-physical (65.8 vs 83, p=0.02), role-emotional (62.1 vs 83.3, p=0.01), vitality (59.8 vs 66.6, p=0.04) and general health (64.1 vs 72.7, p=0.02) with SF36 (Figure 2A). The same observation was made on the subscales of abdominal symptoms (5.4 vs 6.0, p=0.04), systemic symptoms (5.6 vs 6.0, p=0.03), activity (5.5 vs 6.3, p<0.0001) and worry (4.7 vs 5.6, p=0.04) with CLDQ (Figure 2B). Overall, these patients elicited low score on PCS (71.1 vs 80.1, p=0.004), MCS (66.1 vs 74.1, p=0.05) and CLDQ’s average score (5.3 vs 5.9, P=0.002) in comparison with those positive (Table 5). Patients with low HBs antigenemia (≤1000 IU/mL) scored less on the subscale of general health (57.2 vs 67.4, p=0.02) in comparison with those having HBs antigenemia within 1000 and 10,000 IU/mL (p=0.02) (Figure 2C). Patients with low viral load (≤1000 IU/mL) scored less on the subscale of physical functioning (82.3 vs 89.3, p=0.03) than those with a viral load >1000 IU/mL (Figure 2D). Overall, patients with low (≤1000 IU/mL) HBs antigenemia or viral load scored less on PCS than those with value >1000 UI/mL (Table 5).
We found a significant statistical effect of the stages of liver fibrosis on the subscales of role-emotional (F=3.5, df =2, p=0.03) and mental health (F=3.3, df=2, p=0.04) with SF36 but not in the post hoc analysis. However, those categorized as having significant fibrosis or cirrhosis elicited low mean scores on the subscales of role-emotional (52.5 vs 69.4, p=ns) and mental health (64.3 vs 69.1, p=ns) in comparison with those having no significant fibrosis (Figure 2E). Overall, MCS was low in those with significant fibrosis or cirrhosis (60.5 vs 69.5, p=0.003) or receiving antiviral treatment (63.5 vs 69.6, p=0.03) than their counterparts (Table 5). Finally, neither age and gender nor the duration of the disease influenced the HRQoL assessed with SF36 or CLDQ (Table 5).
Multivariate Analysis of Virologic Factors Associated with Health Quality Impairment in Patients with CHB Adjusted to Sociodemographic Factors
Table 6 summarizes the virologic factors associated with HRQoL impairment adjusted to sociodemographic factors in patients with CHB. With PCS as a dependent variable, factors associated with physical HRQoL enhancement was HBs antigenemia level within 1000 and 1000 IU/mL (β= 9.43 (3.7); p=0.01), while the impairment was found in those having reached the secondary school (β=−11.45 (3.3); p=0.01). With MCS as a dependent variable, significant fibrosis had a negative impact on mental HRQoL (β= −9.04 (3.5); p=0.01) and, nearly significant negative association with cirrhosis (Table 6). With the CLDQ’s average score, factors associated with HRQoL were only comorbidities (β=−0.7 (0.2); p=0.001) and ALT (p=0.05) (not shown).
Discussion
HRQoL was altered in black African patients with CHB in our study in comparison with controls subjects and affected the physical and mental perception of HRQoL assessed with the SF36. Globally liver fibrosis was the main factor that deteriorated the mental perception of HRQoL in univariate and multivariate analysis. The degree of liver fibrosis provoked fear in patients with CHB (negative impact of liver stiffness on the subscale of worry: ß= −0.04, p=0.02). Furthermore, HBeAg positivity and high level of HBs Antigenemia or viral load correlated with good HRQoL in black African patients with CHB even though the effect of the former did not remain in multivariate analysis. Finally, patients with low viral load or HBs Antigenemia exhibited an impaired physical HRQoL with a persistent effect of HBs antigenemia in multivariate analysis while those receiving an antiviral treatment exhibited a mental impairment of HRQoL.
Several studies on HRQoL have reported different scores of SF36 or CLDQ in various patients with CHB in comparison with healthy control subjects.4,5,9,10 Even though divergent results were published, it is clear that CHB impairs the HRQoL.9,10 We found a trend towards decreasing the average scores of the subscales of SF36 related to the stage of liver fibrosis without an impaired liver function in our study quite similar to that of Wo et al, in biopsy-proven non-cirrhotic and compensated cirrhotic patients.9 Moreover, our study demonstrated that among the 8 subscales of SF36, patients with CHB had similar scores with the control group adjusted to age and gender, on the subscales of physical functioning, vitality, and mental health. Our findings were similar to that of Lam et al, in Chinese patients with uncomplicated CHB, except for the subscale of bodily pain28 meanwhile the study of Ong et al, reported a lower score on the subscales of physical functioning and general health.5 Overall, black African patients in our study as, Chinese patients with CHB exhibited similar impairment of physical and mental HRQoL contrasting with the findings of Bondini et al in the United States, in which Western patients with CHB and healthy subjects have a similar pattern of HRQoL measured by the SF36.29 Meanwhile, CHB affected mostly mental HRQoL as reported by Ong et al, in Singaporean patients compared with healthy subjects.5
Physical or mental distress did not depend on the educational level as we found in our study in which those having reached the secondary school level expressed physical HRQoL impairment when chronically infected.30 Overall, our findings suggest that patients suffering from CHB display different coping behavior strategies varying from high and lower endemic areas that must be considered in the care policy.28,29
CHB is a stressful condition related to the potential risk of complications such as cirrhosis or hepatocellular carcinoma that patients with CHB acknowledge and do not want to reach.31 These patients may elicit anxiety, grief reaction, worry and stigma whatever the education level and may explain the negative impact of CHB on mental HRQoL in black African patients.15,31 Moreover, the explanations given to these patients by the care providers and the difficulties encountered by some of them for a better health-care facility may potentiate their HRQoL impairment.32,33 Our study demonstrated that these patients expressed HRQoL impairment and worry regarding the stage of liver fibrosis and paradoxically those with HBeAg positivity were less worried about liver fibrosis probably related to the quiescent aspect of this profile.2,6
Better was the outcome (low viral load and HBs Antigenemia) and more deteriorated was the HRQoL in black African patients with CHB even they received an antiviral treatment. This finding was probably related to the negative impact of the disease on daily base activities, the anxiety resulting from the persistence of the viral infection despite the antiviral treatment and, the lassitude feeling as the consequences of a long period of treatment with nucleotide analogues leading to better virologic outcome.15,30 Xue et al found that antiviral treatment with nucleotide analogues did not alleviate the impairment of mental HRQoL in patients with CHB after discontinuation of therapy.30
On the opposite side, HBs antigenemia, HBeAg positivity, and high viral load were associated with good HRQoL. The effect of the former remained in multivariate analysis enhancing physical HRQoL. Moreover, patients with HBeAg positivity elicited better physical and mental HRQoL in our study, contrasting with the findings of Karacaer et al in the Turkish population that reported mental HRQoL impairment in comparison with those negative.10 The most valuable explanation could be the fact that most patients in our study harbored HBeAg negative CHB. These patients may elicit more symptoms (ie, chronic fatigue, jaundice) and significant liver fibrosis in the immunoreactive phase or, experience more flare-up in comparison with those positive in whom, viral replication is active with no or mild fibrosis, high HBs antigenemia and infrequent symptoms defining the immunotolerant phase.2,6,34 In our study, the 22 patients with HBeAg positive CHB were probably asymptomatic and those receiving an antiviral treatment (16 among 20) fit probably the guidelines that recommend to initiate antiviral treatment in case of familial history of cirrhosis or hepatocellular carcinoma, both highly prevalent in sub-Saharan Africa.8,14
HRQoL is currently assessed in western and Asian populations but rarely in Africa as a tool for the determination of the beneficial effect of antiviral treatment on HRQoL in patients with CHB.5,13 Our study demonstrated that Black African patients with CHB receiving antiviral treatment have elicited probably psychological distress as reported by Ichikawa et al, in patients with chronic hepatitis C.35 Furthermore, we confirmed as other previous publications that subjects having subsidies experienced better HRQoL whereas comorbidities diminish HRQoL as did CHB in comparison with control subjects in our study.4,5,9,19,36 We also demonstrated that BMI was positively correlated with good HRQoL in black Africans probably since our patients were not under or overweighted.19,37 Furthermore, mental HRQoL impairment in widowed subjects could be attributable to the psychological reaction related to the loss of a loved one.38 Meanwhile, the absence of conflictual relationship may explain the better HRQoL in those living alone in our study.39
The limitations of the study were the absence of histologic assessment of liver fibrosis in patients with CHB in our study even though, the stiffness assessment of liver fibrosis is accurate, reliable, and responsive in comparison with the histologic one.40 The younger age of the control subjects may have introduced a selection bias.41 However, the age distribution of control subjects probably reflects the demographic profile of the population in Sub-Saharan African countries, younger than that of western countries.42 The absence of translated versions of SF36 and CLDQ in local languages limited the generalizability of our results to a large sample of patients with CHB in Côte d’Ivoire.43 Finally, we did not find in our study, the same responsiveness of the impact of virologic parameters and liver fibrosis on HRQoL assessment with SF36 and CLDQ indicating probably a low correlation between their corresponding subscales.13 However, for the first time, our study provided new insights on HRQoL in black African patients with CHB from high endemic areas quite different with that of previous reports regarding the role of HBeAg profile, HBs antigenemia, and viral load on HRQoL. Furthermore, our study suggests that black African patients with HBeAg negative CHB, low HBs antigenemia, or viral load may have physical and psychological distress even they received antiviral treatment. Psychological treatment may be helpful in these patients. Further studies using large sample of black African patients with CHB are needed to ascertain accurately the magnitude of HRQoL impairment.
Conclusion
Black African patients with CHB expressed an impaired HRQoL more deteriorated in those with HBeAg negative CHB, low viral load or HBs antigenemia. The assessment of HRQoL must be integrated in the treatment of black African patients with CHB. Caregivers must pay attention to those with better outcome as some of them may have poor HRQoL.
Acknowledgment
Many thanks to Gadafi Iddrisu Balali of the department of theoretical and applied biology of Kwame Nkrumah University, Kumasi, Ghana, for having corrected the English transcription.
Disclosure
Alassan Kouamé Mahassadi reports grants from Gilead, during the conduct of the study. The authors of the manuscript declare no other potential conflict of interest regarding this work.
References
1. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–2324.
2. Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B; special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352.
3. Chen CJ, Yang HI. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26(4):628-638.
4. Levy AR, Kowdley KV, Iloeje U, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health. 2008;11(3):527–538.
5. Ong SC, Mak B, Aung MO, Li SC, Lim SG. Health‐related quality of life in chronic hepatitis B patients. Hepatol. 2008;47(4):1108–1117.
6. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatol. 2009;49(S5):S45–55.
7. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatol. 2006;43(S1):S173–S181.
8. European Association For The Study Of The Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398.
9. Woo G, Tomlinson G, Yim C, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol Hepatol. 2012;26(7):445–451.
10. Karacaer Z, Cakir B, Erdem H, et al. Quality of life and related factors among chronic hepatitis B-infected patients: a multi-center study, Turkey. Health Qual Life Outcomes. 2016;14(1):153.
11. Ware JE. SF-36 health survey update. Spine. 2000;25(24):3130–3139.
12. Cutteling JJ, de Man RA, Bussschbach JJV, Darlington ASE. Overview of research on health related quality of life in patients with chronic liver disease. J Med. 2007;65(7):227–234.
13. Younossi ZM, Guyatt G, Buparai N, King D. Development of a disease specific questionnaire to measure health quality of life in patients with chronic liver disease. Gut. 1999;45:295–300.
14. Spearman CW, Afihene M, Ally R, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol. 2017;2(12):900–909.
15. Hann HW, Han SH, Block TM, et al. Symptomatology and health attitudes of chronic hepatitis B patients in the USA. J Viral Hepatitis. 2008;15(1):42–51.
16. Su TH, Hsu CS, Chen CL, et al. Serum hepatitis B surface antigen concentration correlates with HBV DNA level in patients with chronic hepatitis B. Antivir Ther. 2010;5(8):1133–1139.
17. Martinot-Peignoux M, Carvalho-Filho R, Lapalus M, et al. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naïve, e antigen-positive patients. J Hepatol. 2013;58(6):1089–1095.
18. Kim JH, Kwon SY, Lee YS, Lee JH, Lee YS, Lee CH. Virologic response to therapy increases health-related quality of life for patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10(3):291–296.
19. Mahassadi AK, Ebela PC, Bangoura AD, Attia AK. The burden of irritable bowel syndrome and chronic constipation on health-related quality of life in black Africans: a comparison with healthy control subjects in Côte d’Ivoire, West Africa. Clin Exp Gastroenterol. 2019;12:355–365.
20. Mathers N, Fox N, Hunn A. Surveys and Questionnaires. In: The NIHR RDS for the East Midlands/Yorkshire & the Humber. 2007.
21. Bonnard P, Sombié R, Lescure FX, et al. Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)-infected patients in Burkina Faso. Am J Trop Med Hyg. 2010;82(3):454–458.
22. Goodman Z. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607.
23. Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi:10.5116/ijme.4dfb.8dfd
24. Kim HY. Statistical notes for clinical researchers: analysis of covariance (ANCOVA). Restor Dent Endod. 2018;43(4):e43. doi:10.5395/rde.2018.43.e43
25. Larson MG. Analysis of variance. Circulation. 2008;117:115–121.
26. Lee S, Lee DK. What is the proper way to apply the multiple comparison test? Korean J Anesthesiol. 2018;71(5):353–360.
27. Schneider A, Hommel G, Blettner M. Linear regression analysis: part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2010;107(44):776.
28. Lam ET, Lam CL, Lai C, et al. Health-related quality of life of Southern Chinese with chronic hepatitis B infection. Health Qual Life Outcomes. 2009;7:52. doi:10.1186/1477-7525-7-52
29. Bondini S, Kallman J, Dan A, et al. Health-related quality of life in patients with chronic hepatitis B. Liver Int. 2007;27(8):1119–1125.
30. Xue X, Cai S, Ou H, Zheng C, Wu X. Health-related quality of life in patients with chronic hepatitis B during antiviral treatment and off-treatment. Patient Prefer Adherence. 2017;11:85–93.
31. Valizadeh L, Zamanzadeh V, negarandeh R, Zamani F, Hamidia A, Zabihi A. Psychological reactions among patients with chronic hepatitis B: a qualitative study. J Caring Sci. 2016;5(1):57–66.
32. Hang Pham TT, Le TX, Nguyen DT, et al. Knowledge, attitudes and medical practice regarding hepatitis B prevention and management among healthcare workers in Northern Vietnam. PLoS One. 2019;14(10):e0223733. doi:10.1371/journal.pone.0223733
33. Mandeville KL, Krabshuis J, Ladep NG, Mulder CJ, Quigley EM, Khan SA. Gastroenterology in developing countries: issues and advances. World J Gastroenterol. 2009;15(23):2839–2854.
34. Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26(2):130–141.
35. Ichikawa T, Miyaaki H, Miuma S, et al. Hepatitis C virus‐related symptoms, but not quality of life, were improved by treatment with direct‐acting antivirals. Hepatol Res. 2018;48(3):E232–E239.
36. Fortin M, Lapointe L, Hudon C, Vanasse A, Ntetu AL, Maltais D. Multimorbidity and quality of life in primary care: a systematic review. Health Qual Life Outcomes. 2004;4:51. doi:10.1186/1477-7525-2-51
37. Doll HA, Petersen S, Stewart-Brown SL. Well-being: associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res. 2000;8(2):165–170.
38. Wilcox S, Evenson KR, Aragaki A, Wassertheil-Smoller S, Mouton CP, Loevinger BL. The effects of widowhood on physical and mental health, health behaviors, and health outcomes: the women’s health initiative. Health Psychol. 2003;22(5):513–522.
39. Diener E, Gohm CL, Suh E, Oishi S. Similarity of the relation between marital status and subjective well-being across cultures. J Cross Cult Psychol. 2000;31(4):419–436.
40. Marcellin P, Ziol M, Bedossa P, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29(2):242–247.
41. Bonita R, Beaglehole R, Kjellström T. Basic Epidemiology.
42. Unites Nations. Economic commissions for Africa. The demographic profile of African countries; March 2016. Available from: https://www.uneca.org/publications/demographic-profi%1Fle-african-countries.
43. Sperber AD. Translation and validation of study instruments for cross-cultural research. Gastroenterol. 2004;126:S124–S128.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




