Back to Journals » Journal of Pain Research » Volume 11
The impact of prophylactic dexamethasone on postoperative sore throat: an updated systematic review and meta-analysis
Authors Jiang Y, Chen R, Xu S, Li J, Yu F, Kong L, Sun Y, Ye Y, Li Y, Yu M, Wu J
Received 27 April 2018
Accepted for publication 6 August 2018
Published 18 October 2018 Volume 2018:11 Pages 2463—2475
DOI https://doi.org/10.2147/JPR.S172419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Yaofei Jiang,1–4,* Ruoxi Chen,2,* Suming Xu,2,* Jiaxi Li,2,* Fanqi Yu,2 Lingdong Kong,2 Yuhan Sun,2 Yuan Ye,2 Yimin Li,2 Mengqi Yu,2 Jiaming Wu1
1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Jiaxing University, Jiaxing, China; 2Department of General Surgery, The Second Affiliated Hospital, Nanchang University, Nanchang, China; 3Hubei Cancer Clinical Study Centre, 4Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital, Wuhan, China
*These authors contributed equally to this work
Background/Aims: An updated systematic review and meta-analysis was conducted to assess the effect of prophylactic dexamethasone for tracheal intubation of general anesthesia on postoperative sore throat (POST).
Methods: Comprehensive literature search of databases for randomized controlled trials (RCTs), including Embase, PubMed, and Cochrane Library, which evaluate the effect of prophylactic dexamethasone on POST was conducted. RevMan 5.0 and STATA 12.0 software were used to perform meta-analyses.
Results: Fourteen RCTs totaling 1,837 patients were included for analysis. Compared with placebo, a significant reduction in the incidence of POST (OR 0.44, 95% CI 0.33–0.58, P<0.00001), hoarseness (OR 0.42, 95% CI 0.31–0.58, P<0.00001), and postoperative nausea and vomiting (PONV) (OR 0.06, 95% CI 0.03–0.14, P<0.00001) and a comparable incidence of cough (OR 0.59, 95% CI 0.19–1.89, P=0.38) was described in patients receiving dexamethasone, with or without concomitant drugs. Dexamethasone ≥0.2 mg/kg had a statistically greater impact on reducing the incidence of POST than dexamethasone 0.1–0.2 mg/kg, while dexamethasone ≤0.1 mg/kg did not. Dexamethasone was as effective as other drugs such as ondansetron, magnesium sulfate, ketamine gargle, betamethasone gel, and ketorolac for reducing POST (OR 0.70, 95% CI 0.46–1.07, P=0.10). Dexamethasone plus a different drug was more effective than dexamethasone alone for reducing the incidence of POST at 24 hours (OR 0.40, 95% CI 0.21–0.77, P=0.006). Compared with controls, a statistically higher blood glucose level was the only adverse event during the immediate postoperative period in patients receiving dexamethasone.
Conclusions: Intravenous dexamethasone ≥0.2 mg/kg within 30 minutes before or after induction of general anesthesia should be recommended as grade 1A evidence with safety and efficacy in reducing the incidence of POST, hoarseness, and PONV in patients without pregnancy, diabetes mellitus, or contraindications for corticosteroids.
Keywords: systematic review, meta-analysis, corticosteroids, dexamethasone, postoperative sore throat
Introduction
Postoperative sore throat (POST) is common after tracheal intubation of general anesthesia. Numerous factors including age, female gender, smoking history, size of the endotracheal tube, cuff pressure, time and manipulations needed to insert the tube, and time of operation and anesthesia may affect the incidence of POST, with discomfort and dissatisfaction.1–3
A previous systematic review and meta-analysis demonstrated that single low-dose corticosteroids can provide pain relief in patients with sore throat, with no increase in serious adverse effects.4 Considering the involvement of inflammation in pathophysiology of POST, the use of steroidal or nonsteroidal anti-inflammation drug may be an effective pharmacological strategy to prevent POST after tracheal intubation. As glucocorticoid, dexamethasone was supposed to have anti-inflammatory and analgesic impact.5,6 A previous systematic review and meta-analysis has demonstrated a significant reduction in the incidence of POST from intubation at 24 hours in patients treated with intravenous dexamethasone compared with placebo,7 with similar results as another one.8 However, the relatively small sample size included in both the reviews precluded the authors from drawing definitive conclusions, and the optimal timing of dexamethasone administration, and combination effect of dexamethasone with other medicines, remains unclear.
Therefore, we conducted an updated systematic review and meta-analysis of randomized controlled trials (RCTs) to assess the impact of prophylactic dexamethasone on POST for tracheal intubation of general anesthesia.
Methods
Our systematic review and meta-analysis was reported according to the recommendations of the PRISMA statement.9
Outcome measures
The incidence of POST during the immediate 24-hour postoperative period was defined as primary endpoint, while the incidence of hoarseness, postoperative nausea and vomiting (PONV), cough, and adverse events were defined as secondary endpoints.
Data collection and analysis
Keywords were searched in Embase, PubMed, and Cochrane Library from their inception to December 12, 2017. RCTs were further identified by Cochrane Highly Sensitive Search Strategies.10 Keywords and MeSH terms were used in combination as follows: 1) corticosteroid, glucocorticoid, steroid, or dexamethasone; AND 2) sore throat, or sore throats, as shown in Table S1. Additional studies were identified by searching Authors’ names, “related articles” function, and screening the reference lists.
Section criteria
RCTs written in the English language assessing the prophylactic impact of dexamethasone vs placebo without other antiemetics, dexamethasone vs placebo plus concomitant administration of a different drug, dexamethasone vs a different drug, dexamethasone plus a different drug vs dexamethasone, or comparisons using different doses of dexamethasone for POST in patients with tracheal intubation of general anesthesia undergoing surgery except tonsillectomy were included. Exclusion criteria were as follows: 1) non-RCTs; 2) studies including patients undergoing tonsillectomy or laryngeal surgery; 3) studies without available data; 4) overlapping data; or 5) letters, reviews, case reports, and expert opinions.
Data extraction and management
The information and data we obtained were extracted from included studies by by Yaofei Jiang and Ruoxi Chen, and a third investigator was used to judge any disagreements. For each study, we recorded information and data of study population, interventions, and outcomes.
Assessment of quality of evidence in included studies
Cochrane risk of bias instrument was used to assess the risk of bias for RCT quality by two reviewers independently.11 The following six items were examined including sequence generation, allocation concealment, double-blind evaluation, completeness of outcome data, selective reporting of outcome, and comparability of baseline characteristics between groups. The bias risk for each item was classified as high, low, or unclear. RCTs with no less than three items defined as high risk of bias were excluded for the meta-analysis. The seven matching criteria used to assess the baseline comparability between groups were as follows: age, gender, weight or BMI, American Society of Anaesthesiologists (ASA), intubation attempts, time of operation, and time of anesthesia. We defined baseline as incomparability if the number of nonmatching criteria was no less than 3.
Statistical analysis
RevMan 5.0 and STATA 12.0 software were used to perform statistical analyses. Dichotomous variables were calculated with ORs and 95% CIs. We used random-effects model to pool data with statistical heterogeneity determined by the inconsistency index (I2≥50%) and the Chi-squared test (P≤0.10). Subgroup analyses were conducted to determine the optimal dose and administration time. Sensitivity analyses were performed by omitting one study at a time. Begg’s rank correlation test was used to assess the publication bias, determined as positive by Pr>|z|≤0.1.12 The overall quality of evidence from available RCTs was assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.13,14
Results
Trial identification
A total of 645 articles were identified by comprehensive search. Fourteen RCTs were finally included for meta-analysis after screening of titles, abstracts, or full text articles (Figure 1).
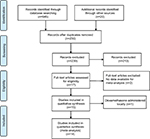  | Figure 1 Flow chart of literature search. |
Characteristics of included studies
The characteristics of the studies included are shown in Table 1. Fourteen RCTs totaling 1,837 patients undergoing general anesthesia with tracheal intubation were included.
  | Table 1 Characteristics of trials included in the meta-analysis Abbreviations: D, dexamethasone; IV, intravenous; O, ondansetron; P, paracetamol. |
Patients classified as ASA class I or II were included in majority of the RCTs. Exclusion criteria were as follows: pregnancy, diabetes mellitus, obesity, and any contraindication to corticosteroid medications. Dexamethasone ranging from 4 to 0.2 mg/kg was administered intravenously in single or combination except one RCT15 administered locally. Timing of dexamethasone administration varied from 30 minutes before tracheal intubation to 30 minutes after tracheal intubation. Controls included placebo, ondansetron,16 magnesium sulfate,17 ketamine gargle,18 betamethasone gel,19 and ketorolac20 or a combination of these medications. Regarding the methodological quality of the RCTs, all showed low overall risks of bias (Table 2).
  | Table 2 Quality of evidence in included studies |
Treatment effects
Primary endpoints
Incidence of POST: dexamethasone vs placebo, with or without concomitant drugs
The incidence of POST comparing dexamethasone vs placebo with or without concomitant drugs was reported in nine trials.15–25 Compared with placebo, a statistical decrease in the incidence of POST at 24 hours was found in patients treated with dexamethasone, with or without concomitant drugs (OR 0.44, 95% CI 0.33–0.58, P<0.00001; Figure 2). No statistical heterogeneity was found among studies (P=0.06, I2=42%).
  | Figure 2 Incidence of POST at 24 hours grouped by concomitant drugs. Abbreviations: PONV, postoperative nausea and vomiting; POST, postoperative sore throat; M-H, Mantel-Haenszel. |
Incidence of POST: dexamethasone vs a different drug
The incidence of POST in patients receiving dexamethasone vs a different drug, including ondansetron,16 magnesium sulfate,17 ketamine gargle,18 betamethasone gel,19 or ketorolac,20 was described in five RCTs. Compared with these different drugs, no statistical difference in the incidence of POST at 24 hours was found in patients receiving dexamethasone (OR 0.70, 95% CI 0.46–1.07, P=0.10; Figure 3). No statistical heterogeneity was found among studies (P=0.49, I2=0%).
  | Figure 3 Comparison of dexamethasone with other drugs. Abbreviation: M-H, Mantel-Haenszel. |
Incidence of POST: dexamethasone plus a different drug vs dexamethasone
Data on incidence of POST comparing dexamethasone plus a different drug vs dexamethasone were reported in three trials.16,18,26 Compared with dexamethasone, a statistical decrease in the incidence of POST at 24 hours was found in patients treated with dexamethasone plus a different drug (OR 0.40, 95% CI 0.21–0.77, P=0.006; Figure 4). No statistical heterogeneity was found among studies (P=0.24, I2=31%).
  | Figure 4 Comparison of dexamethasone plus a different drug with dexamethasone. Abbreviation: M-H, Mantel-Haenszel. |
Secondary endpoints
Incidence of hoarseness: dexamethasone vs placebo, with or without concomitant drugs
The incidence of hoarseness was reported in six RCTs.15,17,18,20,24,25,27 Compared with placebo, a significant reduction in the incidence of hoarseness was found in patients treated with dexamethasone with or without concomitant drugs (OR 0.42, 95% CI 0.31–0.58, P<0.00001; Figure 5). No statistical heterogeneity was found among studies (P=0.16, I2=32%).
  | Figure 5 Incidence of hoarseness. Abbreviation: M-H, Mantel-Haenszel. |
Incidence of PONV: dexamethasone vs placebo, with or without concomitant drugs
The incidence of PONV was described in five RCTs.16,20–22,27 Compared with placebo, a significant reduction in the incidence of PONV was found in patients treated with dexamethasone with or without concomitant drugs (OR 0.06, 95% CI 0.03–0.14, P < 0.00001; Figure 6). No statistical heterogeneity was found among studies (P=0.19, I2=35%).
  | Figure 6 Incidence of PONV. Abbreviations: PONV, postoperative nausea and vomiting; M-H, Mantel-Haenszel. |
Incidence of cough: dexamethasone vs placebo, with or without concomitant drugs
The incidence of cough was reported in three RCTs.19,25,27 Compared with placebo, comparable incidence of cough was found in patients treated with dexamethasone with or without concomitant drugs (OR 0.59, 95% CI 0.19–1.89, P=0.38; Figure 7). Statistical heterogeneity was found among studies (P=0.02, I2=71%).
  | Figure 7 Incidence of cough. Abbreviation: M-H, Mantel-Haenszel. |
Incidence of adverse events: dexamethasone vs placebo with or without concomitant drugs
Data on postoperative blood glucose in patients receiving dexamethasone were described in one RCT.17 Compared with magnesium sulfate, statistically higher level of postoperative blood glucose was described in patients treated with dexamethasone. Compared with controls, comparable incidence of other adverse events such as dysphonia, dysphagia, itching, wound infection, diarrhea, headache, muscle pain, dizziness, drowsiness, urinary retention, and abdominal distension was found in patients treated with dexamethasone.
Subgroup analyses
Incidence of POST: dose of dexamethasone
Subgroup analyses according to the dose of dexamethasone ranging from 4 to 0.2 mg/kg indicated that dexamethasone ≥0.2 mg/kg and 0.1–0.2 mg/kg both significantly reduced the POST incidence compared with controls (0.1–0.2 mg/kg: OR 0.60, 95% CI 0.43–0.82; ≥0.2 mg/kg: OR 0.33, 95% CI 0.21–0.50), while dexamethasone ≤0.1 mg/kg did not (OR 0.57, 95% CI 0.31–1.03), as shown in Figure 8. Dexamethasone ≥0.2 mg/kg had a significantly greater effect on reducing the POST incidence than dexamethasone 0.1–0.2 mg/kg (0.1–0.2 mg/kg: OR 0.23, 95% CI 0.18–0.31; ≥0.2 mg/kg: OR 0.40, 95% CI 0.28–0.55; P<0.00001), as shown in Figure S1.
  | Figure 8 POST according to dexamethasone dose. Abbreviations: POST, postoperative sore throat; M-H, Mantel-Haenszel. |
Incidence of POST: timing of dexamethasone administration
Patients in most of the trials were administered with dexamethasone that varied from 30 minutes to immediately before induction of anesthesia. The incidence of POST in patients treated with dexamethasone administered 30 minutes prior to intubation vs 30 minutes after tracheal intubation was described in two RCTs.28,29 Comparable incidence of POST at 24 hours was found in patients receiving dexamethasone administered 30 minutes prior to intubation vs 30 minutes after tracheal intubation (OR 0.91, 95% CI 0.44–1.86, P=0.79; Figure S2). No statistical heterogeneity was found among studies (P=0.66, I2=0%).
Sensitivity analysis
Sensitivity analyses omitting one study at a time demonstrated a significantly different result of meta-analysis on POST incidence comparing dexamethasone with a different drug.
Publication bias
No statistical publication bias was found among studies by Begg’s rank correlation test, except for the data on POST incidence in patients treated with dexamethasone vs placebo, with or without concomitant drugs (Pr>|z|=0.008) (Table S2).
Quality of evidence
As shown in Table 3, quality of GRADE evidence from available RCTs, upgraded by large-effect or dose–response gradient, and downgraded by publication bias (Pr>|z|≤0.1), indirectness (variations in study setting), imprecision (sensitivity analysis with a significantly different conclusion), or inconsistency (statistical heterogeneity among studies).
Reliability and conclusiveness of composite outcome result
For conclusive and reliable meta-analysis, we calculated the optimal sample size by assuming a 40% control event rate of POST and a 25% relative risk reduction with 80% power and a two-sided a=0.01. Our calculations with sequential monitoring boundary crossing indicated that the cumulative evidence is reliable and conclusive (Figure S3).
Discussion
For patients experiencing tracheal intubation of general anesthesia, POST is a common but unpleasant complication. As glucocorticoid, dexamethasone has anti-inflammatory and analgesic impact. Therefore, prophylactic dexamethasone may be beneficial.
Our study demonstrated the safety and efficacy of prophylactic dexamethasone for patients undergoing surgery requiring tracheal intubation of general anesthesia in reducing the incidence of POST, hoarseness, and PONV compared with placebo, with or without contaminant drugs. It may be explained that dexamethasone is anti-inflammatory and immunosuppressive agent, and it may exert its therapeutic actions through central inhibition of prostaglandin synthesis, by decreasing serotonin turnover in the central nervous system, and by influencing the systemic inflammatory response in favor of anti-inflammatory mediators.5,6,30
Besides, our meta-analysis demonstrated comparable efficacy of dexamethasone in reducing POST as other drugs including ondansetron, magnesium sulfate, ketamine gargle, betamethasone gel, and ketorolac in this patient population. Among these latter, ondansetron exerts positive effects on pain relief as a Na channel blocker, a 5-HT3 receptor antagonist, and μ-opioid agonist,31 while magnesium sulfate32,33 and ketamine gargle34 both as N-methyl-d-aspartate (NMDA) receptor antagonists, betamethasone gel as corticosteroids, and ketorolac as an NSAID with analgesic and anti-inflammatory activities by inhibiting cyclooxygenase. Dexamethasone in combination with other drugs is most effective in reducing POST, perhaps due to its synergistic effects of pain relief through NMDA receptor antagonists.34,35 Although without other serious adverse events, dexamethasone was associated with increased level of postoperative blood glucose.
To determine the optimal dose of dexamethasone, subgroup analyses stratified by dose indicated more efficacies of higher doses of dexamethasone (≥0.2 mg/kg) compared with lower doses (0.1–0.2 mg/kg). In almost all trials included, dexamethasone was administered that varied from 30 minutes before tracheal intubation to 30 minutes after tracheal intubation. One of the concerns of dexamethasone administration is the potential for poor glycemic control. Postoperative glucose level measured at immediate postoperative period was higher in the dexamethasone group, as described by Park et al.17 Considering the results of the evaluated blood glucose, magnesium sulfate could be a valuable option in the prevention of POST when the use of dexamethasone may not be appropriate for its potential side effects.
Several limitations still exist in this meta-analysis. First, statistical heterogeneity on postoperative cough was found among studies, while publication bias was found on POST. Second, the RCTs in this meta-analysis mostly included healthy patients and excluded patients with pregnancy, diabetes mellitus, or contraindications for corticosteroids. Therefore, the prophylactic impact of dexamethasone on these patients is still unknown.
Conclusion
Our meta-analysis demonstrated safety and efficacy of prophylactic dexamethasone in reducing the incidence of POST, hoarseness, and PONV in patients requiring tracheal intubation of general anesthesia compared with placebo, with or without concomitant drugs. Prophylactic dexamethasone ≥0.2 mg/kg administered intravenously within 30 minutes before or after induction of general anesthesia should be recommended as grade 1A evidence with safety and efficacy for patients without pregnancy, diabetes mellitus, or contraindications for corticosteroids.
Disclosure
The authors report no conflicts of interest in this work.
References
Lakhe G, Sharma SM. Evaluation of endotracheal tube cuff pressure in laparoscopic cholecystectomy and postoperative sore throat. J Nepal Health Res Counc. 2018;15(3):282–285. | ||
Gemechu BM, Gebremedhn EG, Melkie TB. Risk factors for postoperative throat pain after general anaesthesia with endotracheal intubation at the University of Gondar Teaching Hospital, Northwest Ethiopia, 2014. Pan Afr Med J. 2017;27:127. | ||
Higgins PP, Chung F, Mezei G. Postoperative sore throat after ambulatory surgery. Br J Anaesth. 2002;88(4):582–584. | ||
Sadeghirad B, Siemieniuk RAC, Brignardello-Petersen R, et al. Corticosteroids for treatment of sore throat: systematic review and meta-analysis of randomised trials. BMJ. 2017;358:j3887. | ||
Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. | ||
Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48(2):168–178. | ||
Sun L, Guo R, Sun L. Dexamethasone for preventing postoperative sore throat: a meta-analysis of randomized controlled trials. Ir J Med Sci. 2014;183(4):593–600. | ||
Zhao X, Cao X, Li Q. Dexamethasone for the prevention of postoperative sore throat: a systematic review and meta-analysis. J Clin Anesth. 2015;27(1):45–50. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. | ||
Carol Lefebvre EM, Glanville J. Chapter 6: searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. London: The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. Accessed October 5, 2018. | ||
Higgins JPT, Altman DG, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. London: The Cochrane Collaboration; 2011. Available from: www.cochrane-handbook.org. Accessed October 5, 2018. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Atkins D, Best D, GRADE Working Group, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. | ||
Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest. 2006;129(1):174–181. | ||
Lee JH, Kim SB, Lee W, et al. Effects of topical dexamethasone in postoperative sore throat. Kor J Anesthesiol. 2017;70(1):58–63. | ||
Gautam B, Shrestha BR, Lama P, Rai S. Antiemetic prophylaxis against postoperative nausea and vomiting with ondansetron–dexamethasone combination compared to ondansetron or dexamethasone alone for patients undergoing laparoscopic cholecystectomy. Kathmandu Univ Med J. 2008;6(23):319–328. | ||
Park JH, Shim JK, Song JW, et al. A randomized, double-blind, non-inferiority trial of magnesium sulphate versus dexamethasone for prevention of postoperative sore throat after lumbar spinal surgery in the prone position. Int J Med Sci. 2015;12(10):797–804. | ||
Safavi M, Honarmand A, Fariborzifar A, Attari M. Intravenous dexamethasone versus ketamine gargle versus intravenous dexamethasone combined with ketamine gargle for evaluation of post-operative sore throat and hoarseness: a randomized, placebo-controlled, double blind clinical trial. Adv Biomed Res. 2014;3:212. | ||
Tabari M, Soltani G, Zirak N, Alipour M, Khazaeni K. Comparison of effectiveness of betamethasone gel applied to the tracheal tube and IV dexamethasone on postoperative sore throat: a randomized controlled trial. Iran J Otorhinolaryngol. 2013;25(73):215–220. | ||
Yang C, Jung SM, Bae YK, Park SJ. The effect of ketorolac and dexamethasone on the incidence of sore throat in women after thyroidectomy: a prospective double-blinded randomized trial. Kor J Anesthesiol. 2017;70(1):64–71. | ||
Thomas S, Beevi S. Dexamethasone reduces the severity of postoperative sore throat. Can J Anaesth. 2007;54(11):897–901. | ||
Singh MA, Singh LD, Majaw HW, et al. Prophylactic antiemetic therapy with dexamethasone in patients undergoing laparoscopic cholecystectomy. JMS. 2008;22(3):111–116. | ||
Ruangsin S, Wanasuwannakul T, Pattaravit N, Asim W. Effectiveness of a preoperative single dose intravenous dexamethasone in reducing the prevalence of postoperative sore throat after endotracheal intubation. J Med Assoc Thai. 2012;95(5):657–660. | ||
Park SH, Han SH, do SH, et al. Prophylactic dexamethasone decreases the incidence of sore throat and hoarseness after tracheal extubation with a double-lumen endobronchial tube. Anesth Analg. 2008;107(6):1814–1818. | ||
Bagchi D, Mandal MC, das S, et al. Efficacy of intravenous dexamethasone to reduce incidence of postoperative sore throat: a prospective randomized controlled trial. J Anaesthesiol Clin Pharmacol. 2012;28(4):477–480. | ||
Lee J, Park HP, Jeong MH, Kim HC. Combined intraoperative paracetamol and preoperative dexamethasone reduces postoperative sore throat: a prospective randomized study. J Anesth. 2017;31(6):869–877. | ||
de Oliveira GS, Ahmad S, Fitzgerald PC, et al. Dose ranging study on the effect of preoperative dexamethasone on postoperative quality of recovery and opioid consumption after ambulatory gynaecological surgery. Br J Anaesth. 2011;107(3):362–371. | ||
Eidi M, Seyed Toutounchi SJ, Kolahduzan K, Sadeghian P, Seyed Toutounchi N. Comparing the effect of dexamethasone before and after tracheal intubation on sore throat after tympanoplasty surgery: a randomized controlled trial. Iran J Otorhinolaryngol. 2014;26(75):89–98. | ||
Park SY, Kim SH, Lee AR, et al. Prophylactic effect of dexamethasone in reducing postoperative sore throat. Kor J Anesthesiol. 2010;58(1):15–19. | ||
Hong D, Byers MR, Oswald RJ. Dexamethasone treatment reduces sensory neuropeptides and nerve sprouting reactions in injured teeth. Pain. 1993;55(2):171–181. | ||
Zahedi H, Maleki A, Rostami G. Ondansetron pretreatment reduces pain on injection of propofol. Acta Med Iran. 2012;50(4):239–243. | ||
Tramer MR, Schneider J, Marti RA, Rifat K. Role of magnesium sulfate in postoperative analgesia. Anesthesiology. 1996;84(2):340–347. | ||
Koinig H, Wallner T, Marhofer P, et al. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg. 1998;87(1):206–210. | ||
Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-d-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007;21(3):259–271. | ||
Yang YL, Chao PK, Ro LS, Wo YY, Lu KT. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacology. 2007;32(5):1042–1051. |
Supplementary materials
  | Figure S1 Incidence of POST stratified according to dexamethasone dose: 0.1–0.2 mg/kg and ≥0.2 mg/kg. Abbreviations: POST, postoperative sore throat; M-H, Mantel-Haenszel. |
  | Figure S2 Incidence of POST with regard to timing of dexamethasone administration. Abbreviations: POST, postoperative sore throat; M-H, Mantel-Haenszel. |
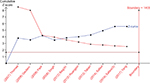  | Figure S3 Trial sequential analysis for POST. Abbreviation: POST, postoperative sore throat. |
  | Table S1 Search strategy from its inception to December 2017 |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


