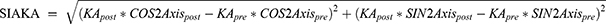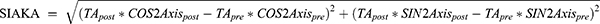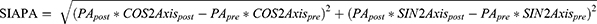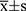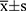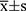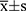Back to Journals » International Journal of General Medicine » Volume 15
The Impact of Posterior Corneal Astigmatism on Surgically Induced Astigmatism in Cataract Surgery
Received 18 July 2022
Accepted for publication 14 November 2022
Published 28 November 2022 Volume 2022:15 Pages 8417—8425
DOI https://doi.org/10.2147/IJGM.S382774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Wenjie Liu,* Lichun Yang,* Jiewei Liu
Cataract Department, Shanxi Eye Hospital, Taiyuan, Shanxi Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiewei Liu, Email [email protected]
Purpose: This study aimed to evaluate the changes in posterior corneal astigmatism after cataract surgery and provide a theoretical basis to accurately evaluate the total corneal astigmatism (TA) to be corrected before toric intraocular lens (IOL) implantation.
Patients and Methods: Sixty-two patients (89 eyes) who underwent phacoemulsification combined with toric IOL implantation (AcrySof IQ Toric SN6AT2-T9) at Shanxi Eye Hospital between January 2017 and September 2018 were enrolled. Surgically induced astigmatism of the posterior cornea (SIAPA) was analysed using vector analysis during pentacam examination.
Results: The vector variances of keratometric astigmatism (KA), TA, and posterior corneal astigmatism (PA) preoperatively and postoperatively in the “with-the-rule (WTR) astigmatism” group and “overall patient” group were statistically significant (P < 0.05). A statistically significant difference was observed between surgically induced KA (SIAKA) and surgically induced astigmatism of the total cornea (SIATA) for all patients, including those with WTR astigmatism. For all patients, SIAKA was less than SIATA by 0.05 ± 0.21 D, and for patients with WTR astigmatism, SIAKA was less than SIATA by 0.09 ± 0.22 D. For patients in the “against-the-rule (ATR) astigmatism” group, there were no statistically significant differences between SIAKA and SIATA, although SIAKA was greater than SIATA by 0.03 ± 0.18 D. When PA ≤ 0.4 D or KA ≤ 2.0 D, SIAPA can be ignored. However, when PA > 0.4 D or KA > 2.0 D, ignoring SIAPA caused by cataract surgery incision will cause SIAKA in patients with WTR astigmatism to underestimate SIATA, while SIAKA in patients with ATR astigmatism will cause an overestimation of SIATA.
Conclusion: SIA on the posterior corneal astigmatism may have a significant role on more precise planning of toric IOL implantation, especially in cases with higher preoperative anterior or posterior corneal astigmatism.
Keywords: surgically induced astigmatism, SIA, posterior corneal astigmatism, PA, keratometric astigmatism, KA, total corneal astigmatism, TA
Introduction
Procedures for enhancing visual quality after cataract surgery are constantly improving with the concept guided by refraction. Experience has shown that even astigmatism less than 1.00 D after cataract surgery can cause adverse visual symptoms, such as blurred vision, ghosting, halo, and glare.1,2 Therefore, correcting preoperative astigmatism and reducing postoperative residual astigmatism are necessary and have become the main goals of contemporary cataract surgery, with great significance for patients to obtain independence from glasses and good postoperative visual quality.
Toric intraocular lens (IOL) implantation is the most effective and stable surgical method for correcting astigmatism in patients with cataract; however, studies have shown that some patients still have residual astigmatism after toric IOL implantation. Incorrect estimation of SIA is one of the causes of postoperative refractive error.3,4 The error in surgically induced astigmatism (SIA) contributes significantly to this.5–9 Astigmatism to be corrected by surgery should be the vector sum of corneal astigmatism and SIA. A profound understanding of SIA is imperative in the evaluation of preoperative total corneal astigmatism (TA) and selection of toric IOL. SIA can vary under different conditions, including incision structure, length, position, impermeability, individual biomechanical differences in cornea, and surgeons’ habits.10–12 For a 2.2-mm clear corneal incision, SIA is reported to be 0.25 ± 0.13 D to 0.81 ± 0.54 D.13 Therefore, understanding a surgeon’s personalised centroid of the SIA is important to reduce refractive errors after toric IOL implantation.
Several studies10,14–18 have reported on SIA caused by cataract surgery incision; however, most of them are based merely on anterior corneal astigmatism. Since Koch19 reported the influence of posterior corneal astigmatism (PA) on TA, an increasing number of clinicians have begun to focus on the role of PA in the implantation of toric IOL. However, most clinicians have not considered the influence of PA caused by incision when evaluating SIA. Fortunately, in recent years, it has become possible to observe changes in PA owing to the emergence of Pentacam and other instruments.
In this study, we calculated the SIA of keratometric astigmatism (KA), PA, and TA after surgery to observe the effects of cataract incision on the anterior and posterior cornea, especially the change in the posterior cornea and its influence on SIA, to provide a theoretical basis for accurately evaluating TA to be corrected before toric IOL implantation.
Materials and Methods
We enrolled 62 patients (89 eyes) who underwent phacoemulsification combined with toric IOL implantation (AcrySof IQ Toric SN6AT2-T9, Alcon Laboratories, Inc.) in the cataract department of Shanxi Eye Hospital between January 2017 and September 2018.
The inclusion criteria were as follows: (1) no previous history of eye trauma or eye surgery; (2) no corneal or other eye diseases; (3) no corneal contact lens wearing history within the last 2 weeks; (4) Pentacam showing regular corneal astigmatism; (5) no intraoperative or postoperative complications; and (6) a grade 2–3 hardness of the lens nucleus (emery grade).
The main examination indexes before and after surgery included uncorrected visual acuity, manifest refraction, best corrected visual acuity, axial position, and IOL decentration after pupil dilation 1 day, 1 week, 1 month, and 3 months postoperatively. IOL master and Pentacam examinations were performed 3 months after the surgery.
A Pentacam HR anterior segment analyser (Oculus, Germany) was used. Under the condition of natural pupil size in a dark environment, we asked the patient to blink before the examination to maintain uniform distribution of the tear film and reduce aberration measurement errors caused by the tear film. The patient opened his eyes and looked at a flashing blue light. After aligning the machine, the examiner reconstructed the three-dimensional structure of the anterior segment with a 25 images/second mode, and recorded the magnitude and steepest axis of the PA, KA, and TA. The TA was obtained using the optical path tracking method. Only cases whose quality specification window showed “OK” were selected, and this inspection was performed by the same skilled technician.
All surgeries were performed by an experienced doctor in our department using the Centurion system (Alcon, US). Before surgery, the axial position of the toric IOL and a 120° incision were marked for patients sitting in front of a slit lamp without dilated pupils. Under topical anaesthesia, a 2.2-mm scleral single plane incision was made at 120°, and a continuous circular capsulorhexis approximately 5.5 mm in diameter was created using a capsulorhexis forceps. This was followed by hydrodissection, phacoemulsification of the nucleus, and cortical aspiration, after which toric IOL SN6ATX was inserted into the capsule. The viscoelastic material behind the toric IOL was removed, and the toric IOL was rotated to the preoperative marked axial position. Finally, the incision was hydrated using balanced salt solution.
The calculation methods were as follows: (1) Classification of astigmatism. For KA and TA, the orientation of with-the-rule (WTR) astigmatism ranged from 60° to 120°, the orientation of against-the-rule (ATR) astigmatism was 180 ± 30°, and that of oblique astigmatism was 45 ± 15° or 135 ± 15°. For PA, because the posterior cornea specifically acts as a minus lens, the definitions of WTR astigmatism and ATR astigmatism are completely opposite that of SIA; that is, the orientation of WTR is 180 ± 30°, that of ATR is 90 ± 30°, and that of oblique astigmatism is 45 ± 15° or 135 ± 15°. (2) Calculation method for astigmatism: The arithmetic mean and vector analysis methods were adopted. The vector analysis method decomposes the astigmatism value into the x- and y-axes, x = cylinder * cos (2 * axis) and y = cylinder * sin (2 * axis), where the cylinder represents the value of cylinder power, and the axis represents the steepest axis. After synthesis, cylinder = 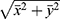 , and angle =
, and angle = 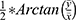 . If x > 0 and y > 0, axis = angle; If x < 0, axis = angle + 90°; If x > 0 and y < 0, axis = angle + 180°. The positive axis of the x axis represents a 180° axis astigmatism, and the negative axis represents a 90° axis astigmatism; the positive axis of the y axis represents a 45° axis astigmatism, and the negative axis represents a 135° axis astigmatism. (3) Before the data were converted into power vector components and to ensure a consistent coordinate system, the angle of the axis of astigmatism in the left eye was converted using the following formula: transformed angle =180 – angle. (4) The SIA calculation method:
. If x > 0 and y > 0, axis = angle; If x < 0, axis = angle + 90°; If x > 0 and y < 0, axis = angle + 180°. The positive axis of the x axis represents a 180° axis astigmatism, and the negative axis represents a 90° axis astigmatism; the positive axis of the y axis represents a 45° axis astigmatism, and the negative axis represents a 135° axis astigmatism. (3) Before the data were converted into power vector components and to ensure a consistent coordinate system, the angle of the axis of astigmatism in the left eye was converted using the following formula: transformed angle =180 – angle. (4) The SIA calculation method:
The results of the quantitative data are expressed as 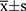 , and the Kolmogorov–Smirnov test was used to test for normality. If data were normally distributed and had homogeneity of variance, the paired t-test was used to compare various types of astigmatism, including SIA, before and after surgery. Otherwise, the nonparametric Wilcoxon rank-sum test was used. For correlation analysis, if the data were normally distributed, Pearson linear correlation analysis was used; however, if the distribution was not normal, Spearman rank correlation analysis was used. The chi-square test or Fisher’s exact method was used to count the data. Statistical significance was set at P < 0.05.
, and the Kolmogorov–Smirnov test was used to test for normality. If data were normally distributed and had homogeneity of variance, the paired t-test was used to compare various types of astigmatism, including SIA, before and after surgery. Otherwise, the nonparametric Wilcoxon rank-sum test was used. For correlation analysis, if the data were normally distributed, Pearson linear correlation analysis was used; however, if the distribution was not normal, Spearman rank correlation analysis was used. The chi-square test or Fisher’s exact method was used to count the data. Statistical significance was set at P < 0.05.
Results
In this study, 89 eyes of 62 patients (women accounted for 53.2% and men accounted for 46.8%) were evaluated prospectively, including 42 eyes of 29 men and 47 eyes of 33 women. The average age was 54.47 ± 14.29 years old. The average preoperative KA was 0.97±2.35 D. The WTR astigmatism group consisted of 61 eyes; the ATR astigmatism group, 26 eyes; and the oblique astigmatism group, 2 eyes.
Table 1 shows the mean, 95% confidence interval, and maximum and minimum values before and after cataract surgery. As shown in the table, there were 72 eyes in the ATR group before surgery, accounting for 80.90% of all eyes evaluated in this study, and 75 eyes in the ATR group after surgery, accounting for 84.27% of all eyes evaluated in this study (three eyes that were preoperatively in the oblique astigmatism group and preoperatively similar to ATR astigmatism had transformed into ATR astigmatism).
 |
Table 1 Distribution Characteristics of PA Preoperatively and Postoperatively (D, |
Tables 2–4 show the vector values of KA, TA, and PA and the statistical comparison of the magnitude of the x- and y-axes before and after surgery. The results showed a significant difference in the y-axis of KA, TA, and PA (P < 0.05), except for the PA of ATR astigmatism. There was a significant difference in the x-axis for KA in ATR astigmatism (P = 0.01).
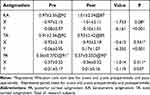 |
Table 2 Comparison of KA, PA and TA of All Patients (D, |
 |
Table 3 Comparison of KA, PA and TA of Anterior WTR Astigmatism Group (D, |
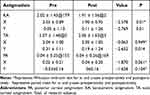 |
Table 4 Comparison of KA, PA, and TA of the Anterior ATR Astigmatism Group (D, |
Table 5 shows the characteristics of SIAPA, SIAKA, and SIATA in all patients and in the WTR and ATR groups. The centroid SIAPA value was 0.09 ± 0.06 D, including 57 eyes < 0.1 D (64%), 28 eyes = 0.1–0.2 D (31.5%), and 4 eyes > 0.2 D (4.5%). Table 6 shows that for all patients, there was a significant difference between SIAKA and SIATA (t = 2.185, P = 0.032), and SIAKA was less than SIATA by 0.05 ± 0.21 D. For patients in the WTR group, the difference between SIAKA and SIATA was statistically significant (t = 3.010, P = 0.004), and SIAKA was less than SIATA by 0.09 ± 0.22 D. For patients in the ATR group, there was no significant difference between SIAKA and SIATA (t = 0.713, P = 0.482), and SIAKA was greater than SIATA by 0.03 ± 0.18 D.
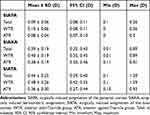 |
Table 5 Distribution Characteristics of Different Types of SIA |
 |
Table 6 Comparison of Different Types of SIA |
As shown in Table 7, the linear correlation analysis between SIAPA and various factors shows that preoperative PA is positively correlated with SIAPA (r = 0.228, P = 0.032).
 |
Table 7 Linear Correlation Analysis Between SIAPA and Other Factors |
We divided patients into two groups according to PA: the “PA ≤ 0.4 D” group (n = 43) and the “PA > 0.4 D” group (n = 46). When PA was ≤ 0.4 D (SIATA: 0.38 ± 0.22 D and SIAKA: 0.38 ± 0.19 D), there was no significant difference between the two groups (t = −0.110, P = 0.913). However, when PA was > 0.4 D (SIATA: 0.50 ± 0.26 D, SIAKA: 0.40 ± 0.20 D), the difference was statistically significant (t = 2.939, P = 0.005);
We divided patients into two groups according to KA: the “KA ≤ 2 D” group (n = 38) and the “KA > 2 D” group (n = 51). When KA was ≤ 2 D (SIATA: 0.37 ± 0.23 D and SIAKA: 0.39 ± 0.16 D), there was no significant difference between the two groups (t = −0.555, P = 0.582). However, when KA was > 2 D (SIATA: 0.49 ± 0.26 D, SIAKA: 0.39 ± 0.21 D), the difference was statistically significant (t = 3.287, P = 0.002).
Discussion
With the change in purpose of cataract surgery from vision restoration to refractive design, accurate preoperative evaluation of TA is vital for patients diagnosed with cataract and corneal astigmatism when using toric or multifocal IOL.20–23 The corneal astigmatism to be corrected by surgery should be the vector sum of corneal astigmatism and intraoperative astigmatism. In recent years, the importance of PA in the TA has been confirmed. This study aimed to analyse the effect of a 2.2 supero-temporal scleral incision on PA. Since SIA is a vector, the x- and y-axes directions are calculated simultaneously in this study, which is helpful to statistically compare the values of different types of astigmatism before and after surgery.
The results showed that the steepest meridian of the PA hardly changed after the surgical incision, and most of them were ATR astigmatism cases. In all patients and patients with WTR astigmatism, the differences in postoperative vector values for KA, TA, and PA were statistically significant, and all showed a change in the y-axis direction. Although there was no statistically significant difference in PA in the ATR astigmatism group postoperatively, there was also a trend in the y-axis from negative to positive. This conclusion is similar to the research results of Cheng et al24 in which the surgical incision close to the y-axis, which is at 120°, could explain the transformation, which mainly affects the refractive power in the y-axis direction but has little effect on the refractive power in the x-axis direction. In contrast, Kim et al25 reported that astigmatism along the x- and y-axes did not show any statistically significant difference. The authors found that the small difference due to postoperative changes had no direction. This may have been related to the surgery.
The average SIAPA values reported in different studies vary considerably. The SIAPA value obtained in this study was 0.09 ± 0.06 D (95% CI: 0.08, 0.11 D). Nemeth et al26 reported that the SIAPA of a 2.8-mm biplane corneal incision was 0.32 ± 0.29 D, and 25% of patients had values ≥ 0.5 D; however, Klijn27 observed 2.2-mm corneal and scleral incisions and reported that the SIAPA caused by a cataract incision was considered very small and can be ignored. Kim et al obtained a SIAPA of 0.20 ± 0.17 D through a temporal 2.2-mm corneal incision. Zhou28 reported SIAPA values of 0.09 ± 0.47 D (2.2-mm group) and 0.09 ± 0.06 D (3.0-mm group), which were similar to the results of this study. The reasons for the great differences among various studies included differences in sample size, preoperative astigmatism range, time elapsed since surgery, orientation of surgical incision (temporal side, upper side, upper temporal side, and steepest meridian), incision width (2.2 mm, 2.6 mm, 2.8 mm, and 3.0 mm), and incision structures (single plane, biplane, and triplane). Even if they had the same orientation, width, and structure, the incision lengths and internal incision widths could differ due to different surgery habits. Therefore, it is of vital significance to calculate personalised intraoperative astigmatism with different incision orientations, incision widths, and lens nucleus hardness.
In this study, the difference between SIAKA and SIATA was statistically significant (P < 0.05) in all patients and in the WTR astigmatism group. For all patients, SIAKA was less than SIATA by 0.05 ± 0.21 D, whereas for the WTR astigmatism group, SIAKA was less than SIATA by 0.09 ± 0.22 D. However, for the ATR astigmatism group, there were no significant differences between SIAKA and SIATA (t = 0.713, P = 0.482) although SIAKA was greater than SIATA by 0.03 ± 0.18 D. As the steepest meridian of the posterior corneal surface is mostly vertical and the refractive power is a negative value, for the WTR astigmatism group, the surgical incision has a relaxing effect on the anterior corneal surface and a reinforcing effect on the posterior corneal surface. This reduces the postoperative TA, that is, ignoring SIAPA will lead to overestimation of the postoperative corneal astigmatism in the WTR astigmatism group. Therefore, SIATA will be greater than SIAKA in the WTR group. The incision strengthened the astigmatism of both the anterior and posterior surfaces in the ATR astigmatism group, which increased the postoperative TA value. Therefore, if SIAPA is ignored, the postoperative corneal astigmatism value of the ATR astigmatism group will be underestimated, and for the ATR astigmatism group, SIATA will be less than SIAKA. Hayashi29 confirmed that the corneal incision steepened the posterior corneal surface through iTrace examination, further proving the aforementioned theory. Therefore, we can speculate that when evaluating the TA to be corrected before surgery, for patients with WTR astigmatism, considering the influence of SIAPA, the actual astigmatism to be corrected should be greater than the measured value, whereas for patients with ATR astigmatism, the actual astigmatism to be corrected should be less than the measured value.
In the correlation analysis of SIAPA, we found that the magnitude of PA was positively correlated with SIAPA, but not with corneal thickness, age, SIATA, and other factors; this is consistent with the reports of Cheng.24 Therefore, we divided PA into two groups according to the numerical value. The results showed that the best dividing point of PA was 0.4 D. When PA was ≤ 0.4 D, SIATA: 0.38 ± 0.22 D, and SIAKA: 0.38 ± 0.19 D, there was no significant difference between the two groups (P = 0.913); however, when PA was > 0.4 D, SIATA: 0.50 ± 0.26 D, and SIAKA: 0.40 ± 0.20 D, the difference between them was statistically significant (P = 0.005). These results show that when PA ≤ 0.4 D, both SIATA and SIAKA are similar and SIAPA can be ignored, whereas when PA > 0.4 D, ignoring SIAPA will lead to an underestimation of SIAKA.
In addition, we divided patients into two groups according to preoperative KA. After statistical analysis, we observed that the best dividing point was KA of 2.0 D. When KA was ≤ 2.0 D, SIATA: 0.37 ± 0.23 D, and SIAKA: 0.39 ± 0.16 D, the difference between them was not statistically significant (P = 0.582), and SIAPA could be ignored; however, when KA was > 2.0 D, SIATA: 0.49 ± 0.26 D, and SIAKA: 0.39 ± 0.21 D, the difference between them was statistically significant (P = 0.002), and SIAKA will be underestimated if SIAPA is ignored. However, due to the limitation of the number of cases, WTR / ATR groupings were not performed, and this will be improved in further studies.
At present, we have a deeper understanding of the importance of TA in toric IOL implantation. However, our calculation of SIA still depends only on KA. In this study, we found that there is still a certain deviation between the real SIA we need to correct (because PA also affects the real SIA) and the previously used astigmatism value. For WTR astigmatism, SIAKA is less than SIATA; therefore, the real corneal astigmatism value to be corrected is smaller. However, for ATR astigmatism, SIAKA is greater than SIATA; therefore, the real value to be corrected is larger.
One of the limitations of this study is that there was no continuous observation of changes in early postoperative SIA. Another limitation is the failure to accurately measure the length and size of the internal incision. In addition, Goggin30 found that there was about 0.14 D error between the two measurement results of corneal astigmatism. Therefore, it is speculated that the results of SIA do not exclude the fluctuation of the results of remeasurement, and this study did not carry out the repeatability analysis of remeasurement. And the measurement of posterior astigmatism is less accurate than IOL Master 700, we will make further observations in future studies.
Conclusion
SIA on the posterior corneal astigmatism may have a significant role on more precise planning of toric IOL implantation, especially in cases with higher preoperative anterior or posterior corneal astigmatism.
Abbreviations
IOL, Intraocular lens; PA, Posterior corneal astigmatism; KA, Keratometric astigmatism; TA, Total corneal astigmatism; WTR, With the rule; ATR, Against the rule; SIA, Surgically induced astigmatism; SIAKA, Surgically induced keratometric astigmatism; SIATA, Surgically induced astigmatism of the total cornea; SIAPA, Surgically induced astigmatism of the posterior cornea; CCT, Central corneal thickness.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Funding
Science Foundation of Health and Family Planning Commission of Shanxi Province (2015069). Foundation of Shanxi Eye Hospital for Young Scholars (Q201801).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Teus MA, Arruabarrena C, Hernandez-Verdejo JL, Sales-Sanz A, Sales-Sanz M. Correlation between keratometric and refractive astigmatism in pseudophakic eyes. J Cataract Refract Surg. 2010;36(10):1671–1675. doi:10.1016/j.jcrs.2010.05.010
2. Kaur M, Shaikh F, Falera R, Titiyal JS. Optimizing outcomes with toric intraocular lenses. Indian J Ophthalmol. 2017;65(12):1301–1313. doi:10.4103/ijo.IJO_810_17
3. Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39(4):624–637. doi:10.1016/j.jcrs.2013.02.020
4. Potvin R, Kramer BA, Hardten DR, Berdahl JP. Factors associated with residual astigmatism after toric intraocular lens implantation reported in an online toric intraocular lens back-calculator. J Refract Surg. 2018;34(6):366–371. doi:10.3928/1081597X-20180327-01
5. Choi A, Kwon H, Jeon S. Accuracy of total corneal power calculation for multifocal toric intraocular lens implantation: swept-source OCT-based biometer vs scheimpflug tomographer. J Refract Surg. 2021;37(10):686–692. doi:10.3928/1081597X-20210610-01
6. Holladay JT. Calculation of total surgically induced astigmatism with a toric intraocular lens. J Cataract Refract Surg. 2020;46(5):793–794. doi:10.1097/j.jcrs.0000000000000124
7. Hirnschall N, Findl O, Bayer N, et al. Sources of error in toric intraocular lens power calculation. J Refract Surg. 2020;36(10):646–652. doi:10.3928/1081597X-20200729-03
8. Holladay JT, Pettit G. Improving toric intraocular lens calculations using total surgically induced astigmatism for a 2.5 mm temporal incision. J Cataract Refract Surg. 2019;45(3):272–283. doi:10.1016/j.jcrs.2018.09.028
9. Yoon CH, Kim MK. Improving the toric intraocular lens calculation by considering posterior corneal astigmatism and surgically-induced corneal astigmatism. Korean J Ophthalmol. 2018;32(4):265–272. doi:10.3341/kjo.2017.0108
10. Barequet IS, Yu E, Vitale S, Cassard S, Azar DT, Stark WJ. Astigmatism outcomes of horizontal temporal versus nasal clear corneal incision cataract surgery. J Cataract Refract Surg. 2004;30(2):418–423. doi:10.1016/S0886-3350(03)00492-9
11. Pakravan M, Nikkhah H, Yazdani S, Shahabi C, Sedigh-Rahimabadi M. Astigmatic outcomes of temporal versus nasal clear corneal phacoemulsification. J Ophthalmic Vis Res. 2009;4(2):79–83.
12. He Q, Huang J, He X, Yu W, Yap M, Han W. Effect of corneal incision features on anterior and posterior corneal astigmatism and higher-order aberrations after cataract surgery. Acta Ophthalmol. 2021;99(7):e1027–e1040. doi:10.1111/aos.14778
13. Ho JD, Tsai CY, Liou SW. Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am J Ophthalmol. 2009;147(5):788. doi:10.1016/j.ajo.2008.12.020
14. Rho CR, Joo CK. Effects of steep meridian incision on corneal astigmatism in phacoemulsification cataract surgery. J Cataract Refract Surg. 2012;38(4):666–671. doi:10.1016/j.jcrs.2011.11.031
15. Masket S, Wang L, Belani S. Induced astigmatism with 2.2- and 3.0-mm coaxial phacoemulsification incisions. J Refract Surg. 2009;25(1):21–24.
16. Akura J, Kaneda S, Hatta S, Matsuura K. Controlling astigmatism in cataract surgery requiring relatively large self-sealing incisions. J Cataract Refract Surg. 2000;26(11):1650–1659. doi:10.1016/S0886-3350(00)00484-3
17. Kohnen T, Dick B, Jacobi KW. Comparison of the induced astigmatism after temporal clear corneal tunnel incisions of different sizes. J Cataract Refract Surg. 1995;21(4):417–424. doi:10.1016/S0886-3350(13)80532-9
18. Holladay JT, Cravy TV, Koch DD. Calculating the surgically induced refractive change following ocular surgery. J Cataract Refract Surg. 1992;18(5):429–443. doi:10.1016/S0886-3350(13)80095-8
19. Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080–2087. doi:10.1016/j.jcrs.2012.08.036
20. Sheoran K, Arya SK, Bansal RK, Jinagal J, Jha UP. Surgically induced astigmatism and posterior corneal curvature changes following phacoemulsification. Indian J Ophthalmol. 2022;70(2):406–412. doi:10.4103/ijo.IJO_882_21
21. Abulafia A, Koch DD, Holladay JT, Wang L, Hill W. Pursuing perfection in intraocular lens calculations: IV. Rethinking astigmatism analysis for intraocular lens-based surgery: suggested terminology, analysis, and standards for outcome reports. J Cataract Refract Surg. 2018;44(10):1169–1174. doi:10.1016/j.jcrs.2018.07.027
22. Carvalho MJ, Suzuki SH, Freitas LL, Branco BC, Schor P, Lima AL. Limbal relaxing incisions to correct corneal astigmatism during phacoemulsification. J Refract Surg. 2007;23(5):499–504. doi:10.3928/1081-597X-20070501-14
23. Abu-Ain MS, Al-Latayfeh MM, Khan MI. Do limbal relaxing incisions during cataract surgery still have a role? BMC Ophthalmol. 2022;22(1):102. doi:10.1186/s12886-022-02327-9
24. Cheng LS, Tsai CY, Tsai RJ, Liou SW, Ho JD. Estimation accuracy of surgically induced astigmatism on the cornea when neglecting the posterior corneal surface measurement. Acta Ophthalmol. 2011;89(5):417–422. doi:10.1111/j.1755-3768.2009.01732.x
25. Kim YJ, Knorz MC, Auffarth GU, Choi CY. Change in anterior and posterior curvature after cataract surgery. J Refract Surg. 2016;32(11):754–759. doi:10.3928/1081597X-20160816-01
26. Nemeth G, Berta A, Szalai E, Hassan Z, Modis L
27. Klijn S, Sicam VA, Reus NJ. Long-term changes in intraocular lens position and corneal curvature after cataract surgery and their effect on refraction. J Cataract Refract Surg. 2016;42(1):35–43. doi:10.1016/j.jcrs.2015.08.015
28. Zhou Y, Chang P, Wang D, Zhao Y. Comparison of the effects of two different length limbal incisions on anterior and posterior corneal surface astigmatism after phacoemulsification of cataracts. Chin J Exp Ophthalmol. 2012;30(6):543–547.
29. Hayashi K, Yoshida M, Hirata A, Yoshimura K. Changes in shape and astigmatism of total, anterior, and posterior cornea after long versus short clear corneal incision cataract surgery. J Cataract Refract Surg. 2018;44(1):39–49.
30. Goggin M, Patel I, Billing K, Esterman A. Variation in surgically induced astigmatism estimation due to test-to-test variations in keratometry. J Cataract Refract Surg. 2010;36(10):1792–1793. doi:10.1016/j.jcrs.2010.07.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.