Back to Journals » Infection and Drug Resistance » Volume 12
The Impact Of Pharmaceutical Interventions On The Use Of Carbapenems In A Chinese Hospital: A Pre–Post Study
Received 28 August 2019
Accepted for publication 10 October 2019
Published 15 November 2019 Volume 2019:12 Pages 3567—3573
DOI https://doi.org/10.2147/IDR.S229009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Chuanwei Xin, Zhongni Xia, Gonghua Li
Department of Pharmacy, Zhejiang Academy of Traditional Chinese Medicine, Tongde Hospital of Zhejiang Province, Hangzhou, People’s Republic of China
Correspondence: Gonghua Li
Department of Pharmacy, Zhejiang Academy of Traditional Chinese Medicine, Tongde Hospital of Zhejiang Province, Gucui Road, Hangzhou, Zhejiang Province, People’s Republic of China
Tel +86 571 8997 2240
Email [email protected]
Background: The challenge of drug resistance to carbapenems is of international concern with leading to increased hospital lengths of stay, costs, and mortality rates. How to get rid of the vicious cycle of drug resistance, new drugs, and re-resistance, and even the emergence of all-drug-resistant bacteria that humans cannot cope with, are the major challenges we face. To date, data about pharmaceutical interventions on the use of carbapenems are currently limited.
Patients and methods: A retrospective cohort study was conducted to compare pre- and post-intervention in Tongde Hospital of Zhejiang Province. Pharmaceutical interventions were performed in the post-intervention group, including real time monitoring of medication orders, educative group activities, and making interventions to physicians. Intervention acceptance and outcomes, including the length of hospital stay, readmission rates, 30-day mortality, and utilization of carbapenems, which was evaluated by the daily defined doses (DDDs), the days of therapy (DOTs), and the cost of carbapenems, were reviewed.
Results: During the study, 593 interventions were provided by clinical pharmacists with an average acceptance rate of 82.79%. Compared with the pre-intervention group, prescriptions of carbapenems for pathogen-directed therapy were improved significantly in the post-intervention group (59.27% vs 21.74%, p=0.022). The DDDs decreased from 281.96 to 174.28 and DOTs decreased from 9.19 to 5.18 after pharmaceutical intervention, and the pharmaceutical interventions had significantly lower mean total cost of carbapenems ($13,828.8 vs $8137.1, p=0.004) and length of hospital stay (9.3±1.5 vs 15.9±2.2, p=0.014). There was a significant reduction in 30-day mortality in the post-intervention group (9.46% vs 17.86%, p=0.013) while there were no differences found in the 30-day readmission (20.19% vs 20.66%, p=0.99).
Conclusion: Implementation of pharmaceutical interventions in our hospital successfully improved the appropriateness of carbapenem prescribing overall, and reduced the DDDs, DOTs, length of hospital day, and cost of carbapenems.
Keywords: carbapenems, pharmaceutical interventions, readmission rates, daily defined doses, days of therapy
Background
Antimicrobial resistance is one of the top 10 threats to global health in 2019,1,2 according to the World Health Organization. The challenge of drug resistance to carbapenems is of international concernwith leading to increased hospital lengths of stay, costs, and mortality rates.3 How to get rid of the vicious cycle of drug resistance, new drugs, and re-resistance, and even the emergence of all-drug-resistant bacteria that humans cannot cope with, are the major challenges we face.
China has a high rate of antibiotic use for hospital inpatients and the overuse of antimicrobials is common;4 it is estimated that 75% of patients with seasonal influenza are prescribed antibiotics and the rate of antibiotic prescription for inpatients is 80%.5,6 In order to ensure the effectiveness of current antibiotics, especially the broad-spectrum antibiotics, such as carbapenems, it is essential for clinical pharmacists to improve the inappropriate use of carbapenems. However, the development of clinical pharmacy in China has not been popularized, and the working mode of clinical pharmacists has not been determined either, which hinders the development of clinical pharmacy.
Based on successful experiences of the WHO, the Ministry of Health in China has established a series of policies to improve the intelligent use of antibiotics, including a 3-year nationwide campaign with a special task force to overhaul the use of antibiotics in health-care settings, principles for clinical use of antimicrobial agents in 2015 and a special treatment plan for carbapenems in 2017. To date, data about pharmaceutical interventions on the rational use of carbapenems in China are currently limited. Therefore, the aim in our study was to evaluate the impact of pharmaceutical interventions on the rational use of carbapenems about the following outcomes: the acceptance rate of intervention; the daily defined doses (DDDs); the days of therapy (DOTs); the total cost of carbapenems; the length of hospital stay; the overall 30-day mortality and readmission.
Methods
Study Design And Setting
Patients admitted to Tongde Hospital of Zhejiang Province, a 1900-bed teaching hospital located in Hangzhou of the People's Republic of China, which includes 52 clinical departments and approximately 700,000 admissions annually, from January 2017 to December 2017 (pre-intervention group) and from January 2018 to December 2018 (post-intervention group) were included in the study. Inclusion criteria were patients who have taken carbapenems since admission, defined as one who had not used carbapenems during the previous 30 days. The ethical research committee approval was obtained from Tongde Hospital of Zhejiang Province. All participants provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki. We performed the same policy of screening during the two study periods. We also had taken executive infection control measures during this study period in our hospital. All of the patients were treated by the attending physician.
The Administration Of Carbapenems
According to the principles for clinical use of antimicrobial agents in China, carbapenems were those antibacterial drugs that should not be used at will, or need to be protected in clinical practice to prevent the bacteria from developing drug resistance too quickly and causing serious consequences. There are three kinds of carbapenems in our hospital: Mmeropenem, Imipenem, and Biapenem. Whether the use of carbapenems is reasonable depends on the drug instructions, principles for clinical use of antimicrobial agents, and the reference to the 2016 guidelines.7 Patients with indications of carbapenems should emphasize etiological diagnosis. It is necessary to send the specimens for pathogenic examination before the application of carbapenems. The appropriate carbapenems should be selected according to the pathogen type and the pharmacokinetics/pharmacodynamics of antibacterial agents. Carbapenems are mainly excreted through the kidney, therefore patients with renal insufficiency or the elderly with decreased renal function need to use less.
Pharmaceutical Interventions
Two trained clinical pharmacists began to intervene in the use of carbapenems in our hospital on January 1, 2018. The clinical pharmacists made the primary review of medication orders, screened cases for suitability, and performed interventions such as dosage adjustment or de-escalation based on culture results, had a discussion with the doctor about the pharmacological characteristics of antibiotics, the pharmacokinetics/pharmacodynamics of carbapenems, and explained the possible adverse drug reaction and drug interactions. After each intervention, the records about the acceptance rates of the clinical pharmacist’s interventions were established. These interventions were reviewed by the administration team in our hospital within 72 h; the workflow is shown in Figure 1.
 |
Figure 1 Flow of participants through the study. |
Data Collection And Definitions
The data included age, sex, and previous antimicrobial use within 3 months. All interventions were recorded on a standardized form. The cost of carbapenems was extracted from our hospital’s electronic medication record, and DDDs and DOTs per 100 occupied bed-days were adopted to indicate antibiotic utilization in our hospital. The DDD was obtained from the anatomical therapeutic chemical classification index from the WHO:8 DDDs/per 100 occupied bed-days=annual consumption of antimicrobials (g)×100/DDD (g/day)×total hospitalization days. DOTs were defined as each day that an antibiotic was administered normalized by 100 occupied bed-days.9 To identify any change in clinical outcomes, the length of stay and mortality rate before and after intervention were analyzed.
Statistical Analysis
The data were analyzed using statistical analysis software (SPSS© version 19.0). Data are presented as mean±SD or as percentages within groups. Pre-to-post-intervention comparisons were made by the chi-square test for proportions and t-test for means; the P-value for statistical significance was set at <0.05.
Results
A total of 1033 patients were involved in our study, among whom 515 patients were involved in the pre-intervention group and 518 patients were involved in the post-intervention group. The patient characteristics are shown in Table 1. There were no statistically significant differences in terms of age, previous antimicrobial use within 3 months, and mean number of complications between two groups.
 |
Table 1 Patient Demographics And Clinical Characteristics Pre- And Post-Intervention |
During the post-intervention period, there were 593 interventions provided with an average acceptance rate of 82.79%. The majority of interventions were dose optimization, optimization of frequency, and switch from intravenous to oral, followed by de-escalation. The dose optimization (95.17%) and optimization of frequency (92.71%) based on pharmacokinetic and dynamics were the most accepted intervention, followed by switching from intravenous to oral antibiotics with an acceptance rate of 80.43%. (Table 2).
 |
Table 2 Types Of Interventions Recommended By The Pharmacists |
Of the 515 patients in the pre-intervention group, 375 patients (72.82%) were initiated for empirical treatment, 112 patients (21.74%) were initiated for pathogen-directed therapy, and 28 patients (5.43%) for prophylactic use. While during the post-intervention period, patients for empirical treatment were 204 (39.38%) and only 7 (1.35%) patients for prophylactic use. Compared with the patients in the pre-intervention group, prescriptions for pathogen-directed therapy were improved significantly (59.27% vs 21.74%, p=0.022), as is shown in Table 3.
 |
Table 3 The Distribution Of Medication Orders Of Carbapenems |
The DDDs and DOTs per 100 occupied bed-days before and after pharmaceutical interventions are shown in Tables 4 and 5. With the pharmaceutical interventions in our hospital, the DDDs per 100 occupied bed-days decreased from 281.96 to 174.28 (mean difference −107.68, P=0.038, Table 4). Moreover, there was a significant reduction in DOTs per 100 occupied bed-days from 9.19 to 5.18 after post-intervention (mean difference −4.01, P=0.032, Table 5). Figures 2 and 3 show the time trends of DDDs and DOTs per 100 occupied bed-days of carbapenems before and after intervention implementation, respectively.
 |
Table 4 Defined Daily Doses Per 100 Occupied Bed-Days During Pre- And Post-Interventions |
 |
Table 5 Days Of Therapy Per 100 Occupied Bed-Days During Pre- And Post-Interventions |
The difference in total cost of carbapenems between the two groups was indicated by a net cost-reduction in carbapenem use during the intervention period, which was $5691.7 (Figure 4), and the pharmaceutical interventions had significantly lower mean total cost of carbapenems ($13,828.8 vs $8137.1, p=0.004). Compared with the pre-intervention group, the length of hospital stay in the post-intervention group was decreased significantly (9.3±1.5 vs. 15.9±2.2, P=0.014). There was a significant reduction in 30-day mortality in the post-intervention group (9.46% vs 17.86%, P=0.013) while there were no differences found in the 30-day readmission (20.19% vs. 20.66%, p=0.99), as is detailed in Table 6.
 |
Figure 2 Time trends of DDDs per 100 occupied bed-days of carbapenems during pre- and post-interventions (vertical line). Abbreviation: DDD, defined daily doses. |
 |
Figure 3 Time trends of DOTs per 100 occupied bed-days of carbapenems during pre- and post-interventions (vertical line). Abbreviation: DOTs, days of therapy. |
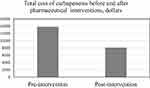 |
Figure 4 Total cost of carbapenems before and after pharmaceutical interventions. |
 |
Table 6 Outcomes Before And After Pharmaceutical Interventions |
Discussion
This study assessed the impact of pharmaceutical interventions on the rational use of carbapenems in a Chinese hospital, and the results indicate that prescriptions for pathogen-directed therapy were improved significantly in the post-intervention group. Furthermore, DDDs and DOTs decreased significantly and the total cost of carbapenems was reduced by $5691.7 with the pharmaceutical interventions.
It was observed from this study that the physicians in our hospital were more likely to accept pharmaceutical interventions, especially the dose optimization and optimization of frequency based on pharmacokinetics and dynamics. This may be attributed to the educative activities which were carried out by clinical pharmacists based on local and international guidelines. However, approximately 17.21% of the pharmaceutical interventions were rejected by the physicians. The main reason for rejection is discontinuing antibiotics; the most possible reason for rejection may be that some of the patients developed complications, in this situation, physicians may opt to be cautious.
The DDD is a valuable tool for assessing the overall quality of prescribed antimicrobials.10,11 The results of our study are in line with other programs; in the study by Ruiz-Ramos J12 implementing an antimicrobial intervention reduces the consumption of antimicrobials with a net benefit of €71,738 in the short term, and in the long term, the maintenance of the intervention involves an additional cost to the system of €107,569. Hersh et al13 assessed the impact of an antimicrobial stewardship program intervention and showed an average monthly decline in days of therapy/1000 patient-days of 5.7%, for the select subset of antibiotics, the average monthly decline was 8.2% in freestanding children’s hospitals. A main difference in relation to previous studies is that our pharmaceutical interventions not only focused on the rational use of drugs, but also on the clinical outcomes of patients. Our study indicated that the DDDs per 100 occupied bed-days decreased significantly from 281.96 to 174.28 (p=0.038) after pharmaceutical interventions, which demonstrated successful restriction of the use of carbapenems. And there was a significant reduction in 30-day mortality in the pharmaceutical intervention group 9.46% vs 17.86%, P=0.013), which is an encouraging and important finding, as acceptance of pharmaceutical interventions for carbapenem discontinuation or de-escalation in this study did not adversely influence patient safety but in fact could safely reduce carbapenem use.
Considering the large population base in China, unreasonable antibiotic use may lead to the escalation in prescribing care cost in China. Therefore, it is necessary to demonstrate cost-effective interventions and to improve cost savings in antimicrobial use in Chinese hospitals. Our results showed that the substantial cost of carbapenems and the economic burden of the patients in this study were significantly decreased by pharmaceutical interventions. In addition, there was a decrease in both length of hospital stay and duration of therapy after pharmaceutical interventions, but no significant difference was found in the overall 30-day readmission between the two groups. Regarding the readmission rate of patients, our findings are similar to most studies which have demonstrated little to no impact on 30-day readmission.14,15 This may be because of the large number of factors that may affect clinical response and 30-day readmission, such as patient care and health insurance.
To the best of our knowledge, little is known about the impact of pharmaceutical interventions on the rational use of carbapenems in China. This study has shown that pharmaceutical interventions were able to provide better support for the rational use of carbapenems. For every patient, the risk–benefit profile of antibiotics should be carefully considered. For the physicians, their prescriptions of antibiotics should be carefully evaluated as well as for all other drugs. Furthermore, every indication of drugs should be patient based rather than only guideline based.16,17 This study highlights that pharmaceutical interventions can have a significant impact on the rational use of carbapenems.
While the present study has a number of methodological strengths, it has several limitations. First, this study utilized data from a single center and relative lower numbers of samples and a short-term period, therefore, the characteristics of this study may limit the generalizability of results. Furthermore, the DDD is widely used as a standardized measure of antibiotic consumption comparison across hospitals, but it potentially underestimates usage where reduced dosage is required,18 such as renal impairment. Future study with a larger sample size and a more rigorous design needs to be confirmed. In spite of the limitations, this study has identified significant improvement in the rational use of carbapenems in a Chinese hospital.
Conclusion
This study provides important evidence from a pre–post intervention study that pharmaceutical interventions were associated with the appropriateness of carbapenem prescribing overall. In addition, it was associated with a marked reduction of DDDs, DOTs, length of day and the cost of carbapenems. It is highly probable that the beneficial intervention can be promoted in other clinical settings.
Acknowledgments
We would like to acknowledge Dr Bo Yang (research question development, study selection) and Mrs Ting Xia (data analysis, interpretation of results) for their involvement. This study was supported by National Natural Science Foundation of China (81774270) and the new medical talent training plan of Zhejiang Province in 2017, "111" talent training project of Tongde Hospital of Zhejiang Province (2D01703). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chotiprasitsakul D, Han JH, Cosgrove SE, et al. Comparing the outcomes of adults with enterobacteriaceae bacteremia receiving short-course versus prolonged-course antibiotic therapy in a multicenter, propensity score-matched cohort. Clin Infect Dis. 2018;66(2):172–177. doi:10.1093/cid/cix767
2. Ten threats to global health in 2019. Available from: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019.
3. Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–iii78.
4. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. doi:10.1128/AAC.01882-17
5. Tängdén T, Giske CG. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J Intern Med. 2015;277(5):501–512. doi:10.1111/joim.2015.277.issue-5
6. Cao B, Zhao CJ, Yin YD, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis. 2010;51(2):189–194. doi:10.1086/653535
7. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63(5):e61–e111.
8. WHO collaborating centre for drug statistics methodology ATC/DDD index. Available from: http://www.whocc.no/.
9. Garau J, Bassetti M. Role of pharmacists in antimicrobial stewardship programmes. Int J Clin Pharm. 2018;40(5):948–952. doi:10.1007/s11096-018-0675-z
10. Fay LN, Wolf LM, Brandt KL, et al. Pharmacist-led antimicrobial stewardship program in an urgent care setting. Am J Health Syst Pharm. 2019;76(3):175–181. doi:10.1093/ajhp/zxy023
11. Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clin Interv Aging. 2018;13:657–667. doi:10.2147/CIA.S133640
12. Ruiz-Ramos J, Frasquet J, Roma E, et al. Cost-effectiveness analysis of implementing an antimicrobial stewardship program in critical care units. J Med Econ. 2017;20(6):652–659. doi:10.1080/13696998.2017.1311903
13. Hersh AL, De Lurgio SA, Thurm C, et al. Antimicrobial stewardship programs in freestanding children’s hospitals. Pediatrics. 2015;135(1):33–39. doi:10.1542/peds.2014-2579
14. Molayi A, Kirk A, Markley J, et al. Description of a restriction program for gram-positive antimicrobial agents at an academic medical center. Am J Infect Control. 2018;46(2):232–234. doi:10.1016/j.ajic.2017.08.038
15. Nagel JL, Huang AM, Kunapuli A, et al. Impact of antimicrobial stewardship intervention on coagulase-negative Staphylococcus blood cultures in conjunction with rapid diagnostic testing. J Clin Microbiol. 2014;52(8):2849–2854. doi:10.1128/JCM.00682-14
16. Timbrook TT, Caffrey AR, Ovalle A, et al. Assessments of opportunities to improve antibiotic prescribing in an emergency department: a period prevalence survey. Infect Dis Ther. 2017;6(4):497–505. doi:10.1007/s40121-017-0175-9
17. Ma X, Xie J, Yang Y, et al. Antimicrobial stewardship of Chinese ministry of health reduces multidrug-resistant organism isolates in critically ill patients: a pre-post study from a single center. BMC Infect Dis. 2016;16(1):704–713. doi:10.1186/s12879-016-2051-8
18. Silverberg SL, Zannella VE, Countryman D, et al. A review of antimicrobial stewardship training in medical education. Int J Med Educ. 2017;8:353–374. doi:10.5116/ijme.59ba.2d47
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
