Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Impact of LEP rs7799039 Polymorphism and Obesity on the Severity of Coronavirus Disease-19
Authors Mohamed AA, Alrohaimi AH, Sayed Abdelgeliel A , Albogami S, Jafri I, Fayad E, Mohamed N, Nassar NA, Adaroas AS, Eldeeb HH, Abdel Halim A, Ramadan A , Elnagar RM, Abdelghafour RAM, Mohamed AY, Mahmoud MO, El-Kasses A, El-Sayed M, Mohammed MA, Alwaleed EA , Mousa S, Abdel Salam S, Abd el salam SM
Received 9 October 2022
Accepted for publication 22 December 2022
Published 21 February 2023 Volume 2023:16 Pages 515—522
DOI https://doi.org/10.2147/DMSO.S391869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Amal Ahmed Mohamed,1 Abdulmohsen H Alrohaimi,2 Asmaa Sayed Abdelgeliel,3 Sarah Albogami,4 Ibrahim Jafri,4 Eman Fayad,4 Nouran Mohamed,5 Nourelhuda Ahmed Nassar,6 Ahmad Sobhy Adaroas,6 Hala H Eldeeb,6 Ahmed Abdel Halim,7 Ahmed Ramadan,8 Rehab M Elnagar,9 Reem Ahmed Mohamed Abdelghafour,10 Amira Yones Mohamed,11 Maha O Mahmoud,12 Ahmed El-Kasses,13 Marwa El-Sayed,14 Mostafa Ahmed Mohammed,15 Eman A Alwaleed,3 Shrook Mousa,16 Sherief Abdel Salam,17 Soha M Abd el salam18
1Biochemistry and Molecular Biology Department, National Hepatology & Tropical Medicine Research Institute, Cairo, Egypt; 2Department of Pharmacy Practice, College of Pharmacy, Shaqra University, Shaqra, Saudi Arabia; 3Department of Botany and Microbiology, South Valley University, Qena, Egypt; 4Department of Biotechnology, College of Science, Taif University, Taif, Saudi Arabia; 5Faculty of Biotechnology, Misr University for Science and Technology, Giza, Egypt; 6Clinical Pathology Department, Elsahel Teaching Hospital, Cairo, Egypt; 7Tropical Medicine Department, National Hepatology & Tropical Medicine Research Institute, Cairo, Egypt; 8Endemic Medicine Department, Cairo University, Cairo, Egypt; 9Radiology Department, Faculty of Medicine, Tanta University, Tanta, Egypt; 10Internal Medicine Department, Damnohour Teaching Hospital, Cairo, Egypt; 11Internal Medicine Department, Mataria Teaching Hospital, Cairo, Egypt; 12Biochemistry Department, Faculty of Pharmacy, Egyptian Russian University, Cairo, Egypt; 13Radiology Department, Elsahel Teaching Hospital, Cairo, Egypt; 14Department of Microbiology and Immunology, Faculty of Medicine, South Valley University, Qena, Egypt; 15Department of Microbiology and Immunology, Faculty of Pharmacy, Al Azhar University, Assiut Branch, Assiut, Egypt; 16Internal Medicine Department, Cairo University, Cairo, Egypt; 17Department of Hepatogastroenterology and Infectious Diseases, Tanta University, Tanta, Egypt; 18Department of Medical Microbiology and Immunology, Faculty of Medicine, Suez University, Suez, Egypt
Correspondence: Sherief Abdel Salam, Department of Hepatogastroenterology and Infectious Diseases, Tanta University, Tanta, Egypt, Email [email protected]
Background and Aims: SARS-CoV-2 infection has been recorded in 230 countries to date. Obesity has a negative impact on one’s quality of life and is one of the main causes of mortality globally. Obesity affects the immune system, making the host more susceptible to infectious infections. Also, obesity commonly provokes the severity of respiratory diseases so the correlation of LEP rs7799039 Polymorphism in corpulent patients with COVID-19 infection was clearly investigated in the current study.
Methods: A total of 232 patients were recruited, 116 patients were obese with COVID-19 infection, and 116 patients were non obese COVID-19. Fasting blood glucose test (FBG), hemoglobin A1C (HbA1C), complete blood count (CBC), international normalized ratio (INR), urea, alanine transaminase (ALT), aspartate aminotransferase (AST), D dimer and C-reactive protein (CRP) were estimated. C.T. scan was performed for each patient, and C.T. severity score was calculated. Genotyping for the leptin rs7799039 SNPs was performed by TaqMan® (Applied Biosystems Step One TM Real-time PCR).
Results: Regarding LEP polymorphism, all individuals of non-obese groups significantly had the homozygous allele GG (100%), whereas only 56% of obese groups had GG alleles (P = 0.001). The severity scores significantly (P = 0.001) varied regarding LEP polymorphism regarding Rs7799039, where the largest proportion of those with Grade IV had the homozygous allele AA (57.1%).
Conclusion: There was a correlation between the leptin gene allelic discrimination and COVID-19 CT brutality in obese patients. The A allele was considered a risk factor for severity in COVID-19 patients while the G allele contributes to decreasing that risk.
Keywords: COVID-19, leptin, gene polymorphism, immunity, obesity
Introduction
A new coronavirus disease (COVID-19) was discovered in Wuhan, Hubei Province, China, in December 2019.1,2 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-instigated coronavirus disease 2019 (COVID-19) has diffused, resulting in a considerable number of cases and deaths.3
SARS-CoV-2 instances have been recorded in 230 countries to date.1 A vast number of diverse hazard factors are concomitant with disease harshness, as respiratory dysfunctions, heart disease, hypertension, older age, diabetes, hypertension, and most recently obesity.4 Obesity commonly provokes the severity of respiratory diseases.5 A rise in studies of clinical reports designate that obesity is a hazard factor for COVID-19 severity.6
According to a 100 million health study (2019), the prevalence of obesity in Egypt has climbed in adults to almost 40%, up from 36% in the 2017 STEP SMART survey.7 Obese people with COVID-19 have a higher risk of severe disease, hospitalization, and mortality, according to several studies.1,8
Leptin (LEP) is a peptide that is mostly released in white adipose tissue and operates by interacting to the leptin receptor (LEPR). It was identified in 1994.9 LEP’s key actions are appetite suppression, increased energy expenditure, and lipid metabolism management, all of which help to control obesity as well as its potential as a blockbuster weight loss treatment sparked interest.10
Other neuroprotective properties of LEP include increased endothelial cell oxidative stress, stimulation of vascular smooth muscle cell development, induction of arterial vascular wall damage, stimulation of reactive oxygen species generation, and stimulation of the renin-angiotensin–aldosterone system.11
Changes in adipokines (such as decreased adiponectin and increased leptin), insulin resistance, higher blood glucose, and chronic low-grade inflammation have all been linked to obesity.12 Signaling of Leptin is precarious in the retort of T lymphocytes to inflammatory effectors by upregulating cellular glycolysis and boosting the construction of effector cytokines like IFN- and TNF- during infection.11,12
These metabolic factors control immune cell metabolism, which impacts how well SARS-CoV-2 viruses work.13 Additionally, a higher Body Mass Index (BMI) is linked to a higher frequency of the anti-inflammatory CD4 T cell subsets Th2 and T regulatory cells. Increased anti-inflammatory cells may impede the ability to control viral spread by inhibiting the inflammatory responses required to control viral spread. Following infection, Regulatory T cells (Tregs) predominantly resolve immune cell-mediated inflammation.14
The concentration of pro-inflammatory cells, such as macrophages, dendritic cells, cytotoxic T cells, and Th1 cells, in the adipose tissue of obese people causes an auxiliary discrepancy in immune cell subsets, causative to the expansion of insulin resistance and chronic inflammation.15
Enlarged serum inflammatory cytokines, such as C-reactive protein (CRP), IL-6, type I, and type III interferons, were seen in these pro-inflammatory immune cells and hypertrophic adipocytes.16 Changes in systemic immune cell populations, as well as their accrual in adipose tissue, have been identified as important mediators of COVID-19 severity in obese people.17–25
Surveys on the influenza A virus suggest that persons with obesity may have enhanced viral detaching of SARS CoV 2. Obese people were found to be more infectious than thin patients. Several causes have contributed because of their impaired immune response, symptomatic obese people shed the influenza A virus 42% longer than slender patients.18 Furthermore, BMI has a favorable relationship with viral consignment in exhaled breath. This indicates that obese patients exhale a higher viral load, therefore increasing the probability to infect others.19 As SARS-CoV-2 has a similarity to influenza virus strain, it seems likely that these findings can be also applied to obese patients infected with SARS-CoV-2.20–31 So, the aim of this current study was to evaluate if obesity can be considered an important risk factor for disease severity or not and also to compare between allelic discrimination of the LEP rs7799039 Polymorphism in obese COVID-19 patients against non-obese COVID-19 patients.
Methods
Two hundred and thirty-two confirmed COVID-19 patients by PCR were divided into two groups, group I: 116 obese COVID-19, and group II included 116 non obese COVID-19 patients. The study was ethically approved from the institutional ethical committee of the National Hepatology & Tropical Medicine Research Institute, Cairo, Egypt. The study complies with the declaration of Helsinki. Informed consent was acquired from all the studied patients.
Assessment of the Patients
Anthropometric Measurements
The BMI was computed by multiplying the body weight (kg) by the height2 (m2). The clinicopathological data of the patients were recorded, including age and gender. Several laboratory tests were carried out including complete blood count, liver function tests, Hb A1C, FBS, PPBS, D-dimer, ferritin and C reactive protein (CRP).
Blood Sample
10 mL venous blood samples were collected from each of the patients for several investigations. For biochemical and enzyme-linked immunosorbent assay (ELISA) analysis (serum leptin) in sterile coagulant-free tubes 3 mL of venous blood was collected from each volunteer while 5 mL of blood was put in Ethylenediaminetetraacetic acid (EDTA) tubes for DNA extraction and gene polymorphism for leptin gene polymorphism and last two mL were collected in EDTA tube for complete blood count (CBC).
Biochemical Analysis
Wet chemistry Bachman machine was used to analyze liver enzymes such as Serum Glutamate Oxalate Transaminase (SGOT) IU/L and Serum Glutamate Pyruvate Transaminase (SGPT) IU/L.; total, direct bilirubin, albumin (g/dl), Fasting Blood Glucose (FBG) mg/dl, Lipid profile included triglycerides mg/dl, High-density lipoprotein-cholesterol (HDL-c), cholesterol mg/dl, low-density lipoprotein mg/dl –cholesterol (LDL-c) mg/dl. Complete blood count (CBC) was done for each participant by Beckman coulter while Thermostat Pt Analyzer was used to determine the concentration of INR for each participant. To determine serum leptin levels, after coagulation by centrifugation, serum was instantly collected and stored at −80° until used by Elisa (Sandwich) DRG® Leptin ELISA (EIA-2395), Inc., USA.
Genotyping Testing
In accordance with the manufacturer’s recommendations, the genotyping and DNA were retrieved using (QIAGEN GmbH, Hilden, Germany) QIA amp® DNA Blood Mini Kit (Schur, Bjerke et al 2001).
The Nano Drop® (ND-1000) Spectrophotometer was used to determine the concentration of extracted DNA (Nano Drop Technologies Inc., Washington, USA).
The 260/280 nm absorbance ratio of isolated DNA was 1.7–1.9.
Genotyping for the Leptin rs7799039 SNPs Polymorphism
On chromosome 7; 128128730 on GRCh38, catalogue number: 4351379. The allelic discrimination was done by a real-time polymerase chain reaction using TaqMan® allelic discrimination test software and an Applied Biosystems Step One TM Real-Time PCR system Thermal Cycling Block (Singapore).
Statistical Analysis
The data was loaded into the computer and assessed with IBM SPSS software version 24.0. The normalized mean values were also used, the significance of the acquired results was determined at a 5% level of significance. ANOVA test: for normally quantitative variables, to compare amid more than two investigated groups, Spearman coefficient; to correlate between two abnormally quantitative variables, Chi-square test; for categorical variables, to differentiate different groups, Student’s t-tests; for normally quantitative variables, to differentiate amid II studied groups, ANOVA test; for normally quantitative variables, to compare among more than II investigated groups, Spearman coefficient used.
Results
There was no significant difference in the two groups regarding age and gender (P = 0.12, 0.17, respectively). Table 1 shows a significant difference in mean of BMI values among obese and non-obese groups (P = 0.001).
 |
Table 1 Comparison Between 2 Studied Groups Regarding Demographic Data |
The laboratory tests performed for the two groups are shown in Table 2. The two groups under study significantly varied regarding the estimated mean values of ferritin (P = 0.001), D-dimer (P = 0.001), CRP (P = 0.001), erythrocyte sedimentation rate (ESR) one hour (P = 0.001), and ESR at two hours (P = 0.001). Obese patients significantly tended to have higher mean levels of the previously estimated factors virtual to the non-obese group. The blood glucose variables, including HbA1c, FBS, and PPBS displayed significant variations among 2 groups (P = 0.001 for each estimated variable). Obese patients significantly displayed higher levels of HbA1c, FBS, and PPBS compared to non-obese patients. Also, the two groups showed significant variations regarding GPT, GOT, LDH, and INR, where Obese patients had significantly higher mean levels of GPT (P = 0.001), GOT (P = 0.001), LDH (P = 0.045), and INR (P = 0.001) compared to non-obese patients.
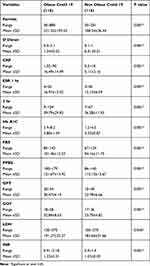 |
Table 2 Comparison Between 2 Studied Groups Regarding Laboratory Findings |
The two groups showed significant differences in the mean level of leptin (P = 0.0001), where the mean level of leptin was significantly higher among obese patients in comparison to non-obese patients, Table 3.
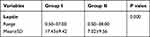 |
Table 3 Comparison Between the Studied Groups Regarding Serum Leptin ELISA Test |
The detection of LEP polymorphism at rs7799039 showed that all non-obese patients (100%) had the homozygous alleles GG, whereas 65 (56%) of obese groups had the same alleles, followed by 36 (31%) having GA alleles, and 15 (12.9%) having AA alleles; a significant difference was distinguished between the two groups regarding LEP polymorphism distribution (P = 0.001), Figure 1.
 |
Figure 1 Comparison between the two studied groups regarding rs7799039. |
Regarding LEP gene polymorphism, it was found that grad IV of severity which was considered the highest was significantly associated with AA homozygous allele, whereas other less severity grades were more common among those carrying GG alleles (P = 0.001), Table 4. Also, the higher grade of severity was significantly associated with morbid obesity, whereas patients with normal weight significantly with low grade of severity, and those with overweight and obese patients significantly had lower grades of severity compared to those with morbid obesity (P = 0.001), Table 4.
 |
Table 4 The Comparison Between Different CT Severity Score Regarding Leptin Gene rs7799039 and BMI Category |
Discussion
Obesity has been linked to the consequences of viral infections in the past, both with the influenza virus and with prior coronaviruses that caused widespread illnesses (SARS, Middle East Respiratory Syndrome (MERS)). Because SARS-CoV-2 (determining COVID-19), SARS-CoV (80%), and MERS-CoV (50%) have greater genetic similarities, this connection is crucial when assessing data.26–39 Obesity has been linked to an increased risk of COVID-19 severe course in several ways. Obesity has been linked to underlying conditions such type 2 diabetes, hypertension, thrombogenic risk, and lung disease. All these problems make it harder to deal with COVID-19.27–33
Obesity is amongst the most prominent factors that increases the mortality risk of SARS-CoV-2 patients tenfold.28 The current study investigations indicated that obese patients with COVID-19 significantly showed worse laboratory tests compared to non-obese patients with COVID-19 such as D-dimmer, ferritin, HbA1c, liver enzymes, LDH, and INR which is in agreement with previous studies. The current analysis approves that obesity is allied with an increased hazard of hospitalization in patients with COVID-19. This could be concluded through significant association between higher grade of COVID-19 severity that requires hospitalization which means increased cost effectiveness. The increase in the severity score was correlated to the increase with the BMI of COVID-19 patients. These results agreed with Lighter et al,6 who reported that obesity is allied with an improved threat of hospitalization in patients with COVID-19. Fascinatingly, the reminder of obesity with hospitalization was superior in younger.6
The link between obesity and the hazard of hospitalisation is supported by reports of an increase in mortality and ICU admission in obese hospitalized patients with COVID-19.29–36 The higher severity of COVID-19 in obese people could be due to a variety of factors. Excess weight has been linked to poorer COVID-19 outcomes in a few illnesses (eg, diabetes, cardiovascular diseases (CVDs), and respiratory disease).30,31 Even after correcting for those factors, the link between obesity and hospitalisation remains statistically significant, implying alternative mechanisms.
Obstructive sleep apnea was not one of the pathophysiological mechanisms that could attribute to the obesity-related risk of severe COVID-19 infection in the current investigation. The current research does not allow for the differentiation of possible pathogenic pathways. It was established that a higher BMI is a negative prognostic factor in COVID-19 patients, and obese patients, as obesity is a major risk factor for hospitalization in COVID-19 patients.31
There was no result has been published previously related to association between LEP gene polymorphism at rs7799039 and the severity grades of COVID-19.; therefore, we present the first data on this subject. The only limitation of the study may be limited number of patients. So, studies on larger number of patients are recommended to further validate these findings. A significant connotation was perceived between the highest grade of boundary of COVID-19 (grade IV) and the homozygous allele AA. On the other hand, the lower grades from I to III were more probe to be experienced by carriers of GG allele. Hence, we can conclude that the homozygous allele AA can be predictor for higher severity of COVID-19 and therefore poor prognosis. By combining this finding with the previous finding regarding the interconnection among BMI and cruelty of COVID-19, we can conclude that COVID-19 patients who are carriers of AA allele and per morbid obesity are more prone to experience severe COVID-19 infection and experience poorer prognosis. Therefore, we recommend establishing analysis for LEP gene polymorphism at rs7799039 among patients with BMI categories including overweight, obese, and morbid obese to provide them the necessary care and to avoid further complications.
Conclusion
Obesity is interrelated to severe COVID-19 and poor prognosis and assist as a prognosticator of deprived outcomes amid COVID-19 patients. Furthermore, the homozygous allele AA of LEP polymorphism at rs7799039 is another predictor of higher severity of COVID-19 and poor prognosis. The combination between AA allele and obesity can be additive predictors for superfluous severe COVID-19 and poorer outcomes.
Consent to Participate
Informed consensus was acquired from all studied subject.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mohamed AA, Mohamed N, Mohamoud S, et al. SARS-CoV-2: the path of prevention and control. Infect Disord Drug Targets. 2020;20:1–5. doi:10.2174/1871526520666200520112848
2. Mohamed AA, Mohamed N, Abd-Elsalam S, et al. COVID-19 in pediatrics: a diagnostic challenge. Curr Pediatr Rev. 2021;17:225–228. doi:10.2174/1573396317666210329153515
3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of f coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
4. Alberca RW, Oliveira LM, Branco ACCC, Pereira NZ, Sato MN. Obesity as a risk factor for COVID-19: an overview. Crit Rev Food Sci Nutr. 2020:1–15. PMID: 32539446. doi:10.1080/10408398.2020.1775546
5. Gao F, Zheng KI, Wang XB, et al. obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43(7):e72–e74. PMID: 32409499. doi:10.2337/dc20-0682
6. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi:10.1093/cid/ciaa415
7. Aboulghate M, Elaghoury A, Elebrashy I, et al. The burden of obesity in Egypt. Front Public Health. 2021;9:718978. doi:10.3389/fpubh.2021.718978
8. Mohammad S, Aziz R, Al Mahri S, et al. Obesity and COVID-19: what makes obese host so vulnerable? Immun Ageing. 2021;18(1):1. doi:10.1186/s12979-020-00212-x
9. Munzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64(1):13–23.
10. Haidong W, Wang C, Han W, et al. Association of leptin and leptin receptor polymorphisms with coronary artery disease in a North Chinese Han population. Rev Soc Bras Med Trop. 2020;53:e20190388.
11. Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117(25)):3238–3249. doi:10.1161/CIRCULATIONAHA.107.741645
12. Singla P, Bardoloi A, Parkash A. Metabolic effects of obesity: a review. World J Diabetes. 2010;1:76–88. doi:10.4239/wjd.v1.i3.76
13. Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, Maciver NJ. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192(1):136–144. doi:10.4049/jimmunol.1301158
14. van der Weerd K, Dik WA, Schrijver B, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61(2):401–408. doi:10.2337/db11-1065
15. McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127(1):5–13. doi:10.1172/JCI88876
16. McLaughlin T, Liu LF, Lamendola C, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637–2643. doi:10.1161/ATVBAHA.114.304636
17. Ryan PM, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28(7):1191–1194. doi:10.1002/oby.22843
18. Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218(9):1378–1382. doi:10.1093/infdis/jiy370
19. Grantham M, Pantelic J, Bueno de mesquite PJ, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci U S A. 2018;115(5):1081–1086. doi:10.1073/pnas.1716561115
20. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57(6):759–764. doi:10.1007/s00592-020-01522-8
21. Zolfaghari Emameh R, Falak R, Bahreini E. Application of system biology to explore the association of neprilysin, Angiotensin-Converting Enzyme 2 (ACE2), and Carbonic Anhydrase (CA) in Pathogenesis of SARS-CoV-2. Biol Proced Online. 2020;22:11. doi:10.1186/s12575-020-00124-6
22. Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi:10.1111/all.14657
23. Buscemi S, Corleo D, Randazzo C. Risk factors for COVID-19: diabetes, hypertension, and obesity. Adv Exp Med Biol. 2021;1353:115–129.
24. Zolfaghari Emameh R, Nosrati H, Eftekhari M, et al. Expansion of single cell transcriptomics data of SARS-CoV infection in human bronchial epithelial cells to COVID-19. Biol Proced Online. 2020;22:16. doi:10.1186/s12575-020-00127-3
25. Khorramdelazad H, Kazemi MH, Najafi A, et al. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of co-infection. Microb Pathog. 2021;152:104554. doi:10.1016/j.micpath.2020.104554
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736–E741. doi:10.1152/ajpendo.00124.2020
27. Sattar N, Mcinnes IB, Mcmurray JJV. obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. immune activation, and cytokine amplification in coronavirus disease 2019? Obesity; 28(7):1191–1194. doi:10.1161/CIRCULATIONAHA.120.047659
28. Goumenou M, Sarigiannis D, Tsatsakis A, et al. COVID 19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (review). Mol Med Rep. 2020;22:20–32. doi:10.3892/mmr.2020.11079
29. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York city. N Engl J Med. 2020;382:2372e4. doi:10.1056/NEJMc2010419
30. Palaiodimos L, Chamorro-Pareja N, Karamanis D, et al. Diabetes is associated with increased risk for inhospital mortality in patients with COVID-19: a systematic review and meta-analysis comprising 18,506 patients. Hormones. 2020;20:305–314.
31. Memtsoudis SG, Ivascu NS, Pryor KO, Goldstein PA. Obesity as a risk factor for poor outcome in COVID-19-induced lung injury: the potential role of undiagnosed obstructive sleep apnoea. Br J Anaesth. 2020;125(2):e262–e263. doi:10.1016/j.bja.2020.04.078
32. Sheervalilou R, Shirvaliloo M, Sargazi S, et al. Application of nanobiotechnology for early diagnosis of SARS-CoV-2 infection in the COVID-19 pandemic. Appl Microbiol Biotechnol. 2021;105(7):2615–2624. doi:10.1007/s00253-021-11197-y
33. Abdelmoemen G, Khodeir SA, Zaki AN, et al. Overexpression of hepassocin in diabetic patients with nonalcoholic fatty liver disease may facilitate increased hepatic lipid accumulation. Endocr Metab Immune Disord Drug Targets. 2019;19(2):185–188. doi:10.2174/1871530318666180716100543
34. COVIDSurg Collaborative; GlobalSurg Collaborative. SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia. 2022;77(1):28–39.
35. Sheervalilou R, Shirvaliloo M, Sargazi S, et al. Convalescent blood: current perspective on the efficacy of a legacy approach in COVID-19 treatment. Blood Purif. 2022;51(1):1–14. doi:10.1159/000513164
36. Abd-Elsalam S, Salama M, Soliman S, et al. Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial. Am J Trop Med Hyg. 2021;106(3):886–890. doi:10.4269/ajtmh.21-0606
37. Heidari Nia M, Rokni M, Mirinejad S, et al. Association of polymorphisms in tumor necrosis factors with SARS-CoV-2 infection and mortality rate: a case-control study and in silico analyses. J Med Virol. 2022;94(4):1502–1512. doi:10.1002/jmv.27477
38. Sargazi S, Sheervalilou R, Rokni M, et al. The role of autophagy in controlling SARS-CoV-2 infection: an overview on virophagy-mediated molecular drug targets. Cell Biol Int. 2021;45:1599–1612. doi:10.1002/cbin.11609
39. Rokni M, Heidari Nia M, Sarhadi M, et al. Association of TMPRSS2 gene polymorphisms with COVID-19 severity and mortality: a case-control study with computational analyses. Appl Biochem Biotechnol. 2022;194(8):3507–3526. doi:10.1007/s12010-022-03885-w
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
