Back to Journals » Clinical Interventions in Aging » Volume 16
The Impact of Frailty on Prognosis in Elderly Hemodialysis Patients: A Prospective Cohort Study
Authors Li Y , Zhang D , Ma Q, Diao Z, Liu S, Shi X
Received 16 July 2021
Accepted for publication 8 September 2021
Published 15 September 2021 Volume 2021:16 Pages 1659—1667
DOI https://doi.org/10.2147/CIA.S329665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Yuanyuan Li,1,* Dai Zhang,1,* Qing Ma,1 Zongli Diao,2 Sha Liu,2 Xiaotian Shi1
1Department of Geriatrics, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Nephrology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Ma
Department of Geriatrics, Beijing Friendship Hospital, Capital Medical University, No. 95, Yong’an Road, Xicheng District, Beijing, 100050, People’s Republic of China
Tel +86 10 63137641
Fax +86 10 63138730
Email [email protected]
Purpose: To explore the impact of frailty on adverse outcomes in elderly hemodialysis (HD) patients.
Patients and Methods: An observational and prospective cohort study was conducted in elderly patients (≥ 60) with HD, with an average 12-month follow-up. Fried frailty phenotype (FFP) was used to define frailty. Negative binomial regression was used to estimate the impact of frailty on the incidence of emergency visits, hospitalizations, acute cardiovascular events, and falls within a year. Cox regression analysis was used to assess the influence of frailty on all-cause mortality in elderly HD patients.
Results: Our study enrolled 150 elderly HD patients, and the prevalence of frailty was 34.7%. After adjustment, frailty was independently associated with increased all-cause mortality [hazard ratio (HR)=4.10, 95% CI: 1.09– 15.43, p=0.037] and emergency visits [incidence rate ratio (IRR)=2.78 95% CI: 1.70– 4.60, p< 0.001]. Gait speed was an independent risk factor for all-cause deaths (HR=5.56 95% CI: 1.41– 22.00, p=0.014), emergency visits (IRR=2.52 95% CI: 1.48– 4.33, p< 0.001), and hospitalizations (IRR=2.24, 95% CI: 1.19– 4.21, p=0.010) in elderly HD patients.
Conclusion: Frailty was an independent indicator of all-cause mortality and emergency visits in elderly patients with HD.
Keywords: elderly people, frailty, hemodialysis, mortality, emergency visits, hospitalizations
Introduction
Frailty is a complex age-related clinical condition characterized by the decline in physiological capacity of several organ systems, thereby increasing the susceptibility to corresponding stressors.1 Frailty increases the risk of side effects such as deterioration of the disease, disability, hospitalization and death.2 Elderly people with advanced age, comorbidities and polypharmacy are more susceptible to frailty, and chronic kidney disease (CKD) is one of these conditions. In most developed and developing countries, the average prevalence of CKD is 10–15%.3 The prevalence of CKD is as high as one-third in people over 60 years of age.3 The prevalence of frailty in CKD in the general population is about 14%.4 With the progress of medicine, the proportion of HD in the elderly population is increasing each year, and the prevalence of frailty is higher in elderly patients with hemodialysis (HD). McAdams DeMarco et al found that the prevalence of frailty in older HD patients was as high as 50.0%, and frailty increased the risk of hospitalization and mortality in HD patients.5 However, there are few studies on the effect of frailty on prognosis of elderly hemodialysis patients.
The goal of this study was to follow-up and observe the influence of frailty on the prognosis in elderly HD patients, including emergency visits, hospitalizations, acute cardiovascular events, falls and all-cause deaths.
Materials and Methods
Participants
This was an observational and prospective cohort study, which recruited maintenance HD patients at the Beijing Friendship Hospital, Capital Medical University between July 2019 and October 2019. The inclusion criteria were as follows: (1) age ≥60 years; (2) willing to give informed consent. The exclusion criteria were as follows: (1) unable to complete comprehensive geriatric assessment due to severe dementia or communication barriers; (2) severe metabolic wasting disease such as acute disease and advanced malignancy.
Clinical Evaluation and Laboratory Tests
Trained doctors conducted personal interviews to collect participants’ information, including age, gender, height, dry weight, body mass index (BMI), smoking status, and medical history [such as hypertension, diabetes (DM), coronary heart disease (CHD) and types of end stage renal disease (ESRD)]. Baseline laboratory tests were performed on mid-week pre-dialysis blood samples using the common laboratory protocols of the hospital. To evaluate the dialysis efficiency, we calculated the urea reduction rate (URR) based on measurement of pre- and post-dialysis blood urea nitrogen (BUN).
Comprehensive Geriatric Assessment
Fried frailty phenotype (FFP) scale was used to identify frailty. The five components of FFP scale were measured as follows: Shrinking (Unexpected weight reduction of 4.5 kg in the last twelve months); exhaustion (self-reported); low activity; weakness; and slow gait speed. The cutoff values corresponded to the original standard and each tested component was scored. The final FFP score was calculated as the sum of the individual component scores (range 0–5), and categorized as non-frail (0–2), and frail (3–5). Moreover, malnutrition is one of the important geriatric syndromes, and elderly HD patients are susceptible to malnutrition owing to dialysis-induced nutrient losses, metabolic acidosis, poor appetite etc.6 Malnutrition increases the risk of mortality7 and readmission.8 As an important confounding factor, the nutrition status was assessed by mini-nutrition assessment short form (MNA-SF) in this study. MNA-SF score was classified as follows: normal nutrition (>11 points), malnutrition risk (7–11 points) and malnutrition (<7 points).9
Follow-Up
The follow-up duration ended in August 2020. All emergency visits, hospitalizations, acute cardiovascular events (including acute coronary syndrome, acute heart failure and sudden cardiac death), falls and deaths due to any causes were recorded, which included the first events and subsequent events. The primary outcome of this study was all-cause mortality.
Statistical Analysis
The data was represented as number (percentage) for categorical variables, the mean ± standard deviation for normal distribution continuous variables, and as the median [interquartile range (IQR)] for skewed distribution continuous variables. The chi-square test or the Fisher’s exact test was used to compare the categorical variables. Continuous variables were compared by the Student’s t-test or the Mann–Whitney U-test. Log rank test and Cox proportional hazards model was used to analyze the relationship between frailty status and all-cause mortality. The associations between frailty status and the incidence of emergency visits, hospital admissions, acute cardiovascular events, and falls were analyzed using negative binomial regression. All models were adjusted for variables, including age, gender, albumin (<40.0 g/L), URR(<70%). For models of all-cause mortality, emergency visits, and hospitalizations, we also adjusted for MNA-SF (≤11), medical history of DM and CHD. For acute cardiovascular events, we further adjusted for medical history of DM and CHD, low-density lipoprotein, and smoking status. For model of fall, we also adjusted for MNA-SF(≤11). All statistical analyses were conducted with the version R 3.6.3 (R-project of the statistical calculation, Vienna, Austria). A p-value <0.05 was considered as statistical significance.
Results
Baseline Characteristics of Participants
The study included 150 patients (51.3% women), with a median age of 69 years (IQR 64–75 years). The most common etiology of ESRD was chronic glomerulonephritis (n=44, 29.3%), followed by diabetic nephropathy (n=27, 18%), hypertensive renal disease (n=23, 15.3%), chronic interstitial nephritis (n=22, 14.7%), polycystic kidney (n=15, 10%) and others (n=19, 12.7%). (The etiology of ESRD between the two groups was shown as Supplementary Table 1).
Figure 1 shows the prevalence of frailty. According to the FFP, 65.3% of the participants were categorized as non-frail and 34.7% as frail.
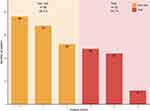 |
Figure 1 Prevalence of frailty in elderly hemodialysis patients according to the FFP. |
Demographic characteristics and laboratory test results are presented in Table 1. Frail patients were significantly older, had lower albumin level and MNA-SF score, and higher prevalence of DM and CHD. However, frail and non-frail groups were similar in gender and URR.
 |
Table 1 Baseline Characteristics of the Included Participants by FFP |
Impact of Frailty on All-Cause Mortality
A total of 147 patients completed the follow-up and the average follow-up duration was 12 months. Three patients were lost to follow-up. One person received kidney transplantation and two persons lost contact after they transferred to other HD centers. A total of 15 patients died, of which 11 cases of death were in the frail group and four cases in the non-frail group. The unadjusted Kaplan-Meier curve showed a significant reduction of survival probability in the frail patients (Figure 2). After adjusting for age, gender, albumin, medical history of DM, CHD, MNA-SF and URR, the Cox regression showed that the risk of all-cause mortality in the frail group (hazard ratio [HR] = 4.10, 95% CI: 1.09–15.43, p=0.037) was significantly higher than the non-frail group (Table 2). After adjustment, slow gait speed (HR=5.56 95% CI: 1.41–22.00, p=0.014) was associated with increased all-cause mortality in older patients with HD (Table 2).
 |
Table 2 The Impact of Frailty and Its Components on Adverse Outcomes(Adjusted) |
 |
Figure 2 Kaplan–Meier survival curves by frailty status. |
Impact of Frailty on Emergency Visits
The numbers of emergency visits for the frail and non-frail groups were 114 and 94 cases, respectively. The results of the negative binomial regression are shown in Table 2. After adjusting to age, gender, albumin, medical history of DM, CHD, MNA-SF and URR, the emergency visits incidence among frail patients increased obviously (IRR=2.78 95% CI: 1.70–4.60, p<0.001). Slow gait speed (IRR=2.52 95% CI: 1.48–4.33, p<0.001), exhaustion (IRR=2.07, 95% CI: 1.34–3.23, p=0.001), and low physical activity (IRR=1.92, 95% CI: 1.22–3.04, p=0.004) were associated with increased incidence of emergency visits.
Impact of Frailty on Hospitalization
The hospitalization events in this study included the hospitalization in the inpatient or emergency department lasting for more than 24 h, but hospitalization for renal transplantation was not included. The numbers of hospitalization in the frail and non-frail groups were 45 and 37 cases, respectively. Binomial negative regression model was used to compare the incidence rate ratio of hospitalization. After adjusting for age, gender, albumin, MNA-SF, medical history of CHD, DM, and URR, there was no significant difference (IRR=1.70, 95% CI: 0.92–3.12, p=0.085) (Table 2). Table 2 shows that slow gait speed (IRR=2.24, 95% CI: 1.19–4.21, p=0.010) and low physical activity (IRR=1.90, 95% CI: 1.12–3.24, p=0.017) were independently associated with hospitalizations.
Impact of Frailty on Acute Cardiovascular Events
HD patients are susceptible to acute cardiovascular events. The number of acute cardiovascular events in the frail and non-frail groups was 19 and 14, respectively. The results of negative binomial regression are presented in Table 2. After adjusting for age, gender, albumin, medical history of DM, CHD, low-density lipoprotein cholesterol, smoking status and URR, frailty was not an independent risk factor for acute cardiovascular disease in elderly HD patients (IRR=2.14, 95% CI: 0.67–7.07, p=0.190) (Table 2). However, exhaustion was associated with acute cardiovascular events in elderly HD patients (IRR=4.98, 95% CI: 1.57–18.03, p=0.005) (Table 2).
Impact of Frailty on Fall
A fall was defined as an unexpected event in which the participants come to rest on the ground, floor, or lower level, but not caused by an internal event (such as syncope or stroke). All falls meeting the above criteria were included.10 In this study, the number of falls in the frail and non-frail groups was 17 and 9, respectively. Using a negative binomial regression model to compare the incidence of falls in the frail and non-frail groups, after adjusting for age, gender, albumin, MNA-SF and URR, there was no difference between the two groups (IRR=1.28, 95% CI: 0.37–4.29, p=0.656) (Table 2).
The unadjusted result concerning the impact of frailty and its components on adverse outcomes are shown as Supplementary Table 2.
Discussion
This study showed that frailty was common in elderly HD patients. Frailty was an independent risk factor for the incidence of emergency visits and all-cause mortality. Slow gait was independently associated with increased all-cause mortality, emergency visits and hospitalizations in elderly HD patients. Low physical activity was related with emergency visits and hospitalizations in elderly HD patients.
Brigitte et al found that the prevalence of frailty in the elderly community-dwelling Europeans living in 10 countries was 17.0% (15.3–18.7%).11 The prevalence of frailty in older HD patients is obviously higher. The loss of appetite and lower food intake result in protein energy waste and sarcopenia;12–14 the physical activity and muscle strength show a downward trend;15,16 and concentrations of pro-inflammatory cytokines, including IL-6 and TNF-α are increased,17 which could lead to frailty in elderly HD patients.13 Due to the heterogeneity of the research population and frailty assessment tools, the prevalence of frailty in HD patients was found to vary from 30% to 77%.18–21 Therefore, comparing the prevalence of frailty using different frailty assessment methods remains challenging. Although the best method for frailty assessment in HD patients has not been determined, researchers prefer to use the FFP to assess frailty-related outcomes,13 because it uses an objective measurement method including handgrip and gait speed, which is more helpful for comparison between studies. In this study, the FFP was used to evaluate elderly HD patients. The prevalence of frailty was 34.7%, which was consistent with other studies.22,23
Recent studies by McAdams-DeMarco,5 Bao,24 Lee,22 and Garcia-Canton25 confirmed that frailty could independently increase the risk of all-cause mortality in HD patients (HR: 1.57–2.60), which is consistent with our findings. However, our study focused on the elderly people and the value of HR (4.10) was higher, suggesting that the risk of frailty was more pronounced in the elderly HD patients. Moreover, repeated emergency visits increase the medical burden of the elderly and reflect an increased susceptibility to stressors. However, most older adults are not equipped with primary healthcare at home to prevent complications, leading to repeated visits to the emergency department. The present study found that frailty was obviously related to the incidence of emergency visits, which was consistent with the study by Garcia-Canton.25 When the elderly HD patients visit the emergency department repeatedly, it uses medical resources, which may interfere with the care of other patients who need emergency assistance. Moreover, it may increase the risk of iatrogenic injury for elderly HD patients. Therefore, the harm related to frailty should be considered and primary healthcare should be gradually improved to reduce mortality and emergency visits.
This study further explored the influence of five frailty manifestations on outcomes in elderly HD patients. We found that reduced gait speed was an independent risk factor for all-cause mortality, emergency visits and hospitalizations. According to previous reports, gait speed can predict survival rate, hospitalization, dementia, falls and disability in elderly community-dwelling residents.26 Studies by Johansen23 and Kutner27 also confirmed the independent predictive effect of slow gait on mortality in elderly HD patients. However, there is insufficient evidence on the correlation between gait speed and clinical prognosis, especially emergency visits, among elderly HD patients. As one of the important factors of frailty, gait speed is affected by many factors, especially in elderly HD patients. They walk slowly because of lower extremity pain or numbness, fractures, knee and hip replacements, and fatigue, as well as sarcopenia.27 Measurement of gait speed requires only a stopwatch, which is convenient and objective. It can be used as a simple screening test for physical function and long-term prognosis in elderly HD patients. Moreover, gait speed can be improved. Coordination and balance, independent of force, are the determinants of the gait speed and are less concerning in the HD patients. They can also be improved by increasing physical activity.23,28,29 According to reports by Takahiro Shimoda30 and Ryota Matsuzawa,31 low physical activity was associated with mortality in HD patients. Our study found that low physical activity was also associated with frequency of emergency visits and hospitalizations. HD patients have relatively less physical activity due to the 4-hour dialysis time limit and post-dialysis exhaustion.31 But according to the 7-year prospective cohort study by Takahiro Shimoda,30 walking at least 4000 steps on non-dialysis days can reduce mortality. Therefore, we can encourage elderly HD patients to exercise on non-dialysis days to increase their physical activity and gait speed.
The decline of renal function independent of age in HD patients is the main driving force of cardiovascular aging.32 If the kidney does not excrete water and excrements (uremic toxins), the heart and vasculature will be exposed to the toxins and accelerate aging.32 The end result is known as uremic cardiomyopathy, vascular calcification, calciphylaxis or calcified uremic arterial lesions.32 Moreover, a study by Kelly enrolled 1016 patients with human immunodeficiency virus infection, and demonstrated that frailty was associated with acute cardiovascular events (IRR=3.83, 95% CI: 1.59–9.23).33 Therefore our study used the negative binomial regression model to explore whether frailty increased the risk of cardiovascular events in elderly patients with HD. However, the result was negative. The impact of frailty on acute cardiovascular diseases in the elderly HD population needs further study to explore. If frailty does increase the risk of cardiovascular disease, early intervention of frailty would greatly benefit elderly HD patients.
In the studies by McAdams-DeMarco,5 Bao,24 Lee,22 Garcia-Canton,25 and Cynthia Delgado,19 frailty was independently associated with hospitalization and fall. Although our results were negative during the one-year follow-up observation, we will extend the follow-up duration to observe the effect of frailty on hospitalizations and falls.
The main strengths of this study were that a relatively comprehensive set of clinical outcomes were observed in elderly HD patients and the effects of frailty components on prognosis in elderly HD patients were further analyzed. Although no association was found between frailty and acute cardiovascular events in elderly HD patients in this study, it deserves further study.
Limitations
The main limitations of this study were that it was a single center study, with limited number of patients and short follow-up time, which lowers the universality of results. Although we controlled multivariate analyses for important data connected with the outcomes, some unmeasured confounders could contribute to residual confounding. In particular, information related to hemodialysis vintage was not available in this study.
Conclusion
This study showed that frailty was associated with the increased incidence of emergency visits and all-cause mortality.
Abbreviations
HD, hemodialysis; FFP, Fried frailty phenotype; HR, hazard ratio; IRR, incidence rate ratio; DM, diabetes; CHD, coronary heart disease; CPD, chronic pulmonary disease; CKD, chronic kidney diseases; ESRD, end stage renal disease; URR, urea reduction rate; BUN, blood urea nitrogen; MNA-SF, Mini-nutritional assessment-short form; IQR, interquartile range; WBC, white blood cells; TC, total cholesterol; TG, triglycerides; TS, transferrin saturation; PTH, parathyroid hormone.
Data Sharing Statement
In order to protect the confidentiality of participants, the data are not publicly available. The data supporting the results of this study can be provided at the request of the corresponding author.
Ethics Approval and Informed Consent
The research protocol was in compliance with the Helsinki Declaration and approved by the Clinical Research Ethics Committee of the Beijing Friendship Hospital, Capital Medical University (Project number: 2019-P2-170-01). Before participating in the study, all patients signed the written informed consent form.
Acknowledgments
The authors thank all patients, researchers and staff who were involved in the study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding
The research reported in this publication was funded by the Beijing Municipal Health Commission (Process No. 19-7).
Disclosure
The authors report that there are no conflicts of interest in this work.
References
1. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi:10.1016/S0140-6736(19)31785-4
2. Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(12):
3. Kooman JP, van der Sande FM, Leunissen KML. Kidney disease and aging: a reciprocal relation. Exp Gerontol. 2017;87(Pt B):156–159. doi:10.1016/j.exger.2016.02.003
4. Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi:10.1053/j.ajkd.2012.05.017
5. McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi:10.1111/jgs.12266
6. Sahathevan S, Khor B-H, Ng H-M, et al. Understanding development of malnutrition in hemodialysis patients: a narrative review. Nutrients. 2020;12(10):3147. doi:10.3390/nu12103147
7. Takahashi H, Inoue K, Shimizu K, et al. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J Renal Nutr. 2017;27(3):201–206. doi:10.1053/j.jrn.2016.12.005
8. Lim SL, Ong KCB, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345–350. doi:10.1016/j.clnu.2011.11.001
9. Rubenstein L, Harker J, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol a Biol Sci Med Sci. 2001;56(6):M366–M372. doi:10.1093/gerona/56.6.m366
10. Lamb SE, Jorstad-Stein EC, Hauer K, Becker C; Prevention of Falls Network E, Outcomes Consensus G. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53(9):1618–1622. doi:10.1111/j.1532-5415.2005.53455.x
11. Santos-Eggimann B, Cuénoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol a Biol Sci Med Sci. 2009;64(6):675–681. doi:10.1093/gerona/glp012
12. Kim J, Kalantar-Zadeh K, Kopple J. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24(3):337–351. doi:10.1681/asn.2012010047
13. Nixon A, Bampouras T, Pendleton N, Woywodt A, Mitra S, Dhaygude A. Frailty and chronic kidney disease: current evidence and continuing uncertainties. Clin Kidney J. 2018;11(2):236–245. doi:10.1093/ckj/sfx134
14. Carrero J, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Renal Nutr. 2013;23(2):77–90. doi:10.1053/j.jrn.2013.01.001
15. Johansen K, Chertow G, Ng A, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57(6):2564–2570. doi:10.1046/j.1523-1755.2000.00116.x
16. Johansen K, Chertow G, Kutner N, Dalrymple L, Grimes B, Kaysen G. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78(11):1164–1170. doi:10.1038/ki.2010.312
17. Carrero J, Stenvinkel P. Inflammation in end-stage renal disease–what have we learned in 10 years? Semin Dial. 2010;23(5):498–509. doi:10.1111/j.1525-139X.2010.00784.x
18. Takeuchi H, Uchida HA, Kakio Y, et al. The prevalence of frailty and its associated factors in Japanese hemodialysis patients. Aging Dis. 2018;9(2):192–207. doi:10.14336/AD.2017.0429
19. Delgado C, Shieh S, Grimes B, et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol. 2015;42(2):134–140. doi:10.1159/000439000
20. Johansen KL, Dalrymple LS, Delgado C, et al. Association between body composition and frailty among prevalent hemodialysis patients: a US renal data system special study. J Am Soc Nephrol. 2014;25(2):381–389. doi:10.1681/asn.2013040431
21. Delgado C, Doyle JW, Johansen KL. Association of frailty with body composition among patients on hemodialysis. J Renal Nutr. 2013;23(5):356–362. doi:10.1053/j.jrn.2013.02.010
22. Lee SY, Yang DH, Hwang E, et al. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr. 2017;27(2):106–112. doi:10.1053/j.jrn.2016.11.003
23. Johansen KL, Delgado C, Kaysen GA, et al. Frailty among patients receiving hemodialysis: evolution of components and associations with mortality. J Gerontol A Biol Sci Med Sci. 2019;74(3):380–386. doi:10.1093/gerona/gly206
24. Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi:10.1001/archinternmed.2012.3020
25. Garcia-Canton C, Rodenas A, Lopez-Aperador C, et al. Frailty in hemodialysis and prediction of poor short-term outcome: mortality, hospitalization and visits to hospital emergency services. Ren Fail. 2019;41(1):567–575. doi:10.1080/0886022x.2019.1628061
26. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi:10.1007/s12603-009-0246-z
27. Kutner NG, Zhang R, Huang Y, Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US renal data system special study. Am J Kidney Dis. 2015;66(2):297–304. doi:10.1053/j.ajkd.2015.01.024
28. Shin S, Chung HR, Fitschen PJ, et al. Postural control in hemodialysis patients. Gait Posture. 2014;39(2):723–727. doi:10.1016/j.gaitpost.2013.10.006
29. Magnard J, Hristea D, Lefrancois G, Testa A, Paris A, Deschamps T. Implicit postural control strategies in older hemodialysis patients: an objective hallmark feature for clinical balance assessment. Gait Posture. 2014;40(4):723–726. doi:10.1016/j.gaitpost.2014.07.009
30. Shimoda T, Matsuzawa R, Yoneki K, et al. Changes in physical activity and risk of all-cause mortality in patients on maintence hemodialysis: a retrospective cohort study. BMC Nephrol. 2017;18(1):154. doi:10.1186/s12882-017-0569-7
31. Matsuzawa R, Roshanravan B, Shimoda T, et al. Physical activity dose for hemodialysis patients: where to begin? Results from a prospective cohort study. J Renal Nutr. 2018;28(1):45–53. doi:10.1053/j.jrn.2017.07.004
32. Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388(10041):276–284. doi:10.1016/s0140-6736(16)30508-6
33. Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty is an independent risk factor for mortality, cardiovascular disease, bone disease, and diabetes among aging adults with human immunodeficiency virus. Clin Infect Dis. 2019;69(8):1370–1376. doi:10.1093/cid/ciy1101
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
