Back to Journals » Medical Devices: Evidence and Research » Volume 13
The Impact of Age on the Outcomes of Minimally Invasive Lumbar Decompression for Lumbar Spinal Stenosis
Authors Mekhail NA, Costandi SJ, Armanyous S, Vallejo R, Poree LR , Brown LL , Golovac S , Deer TR
Received 27 February 2020
Accepted for publication 15 May 2020
Published 4 June 2020 Volume 2020:13 Pages 151—161
DOI https://doi.org/10.2147/MDER.S251556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Nagy A Mekhail,1 Shrif J Costandi,1 Sherif Armanyous,1 Ricardo Vallejo,2 Lawrence R Poree,3 Lora L Brown,4 Stanley Golovac,5 Timothy R Deer6
1Evidence-Based Pain Management Research, Cleveland Clinic, Cleveland, OH, USA; 2Millennium Pain Center, Bloomington, IL, USA; 3Pain Management Center, UCSF Health, San Francisco, CA, USA; 4TruWell Health, St. Petersburg, FL, USA; 5Florida Pain Institute, Merritt Island, FL, USA; 6Center for Pain Relief, Charleston, WV, USA
Correspondence: Nagy A Mekhail Email [email protected]
Background and Purpose: Minimally invasive lumbar decompression (mild®) is an effective long-term therapy for patients with symptomatic lumbar spinal stenosis (LSS) resulting primarily from hypertrophic ligamentum flavum (HLF). Most subjects in clinical studies of the mild procedure have been older adults (age≥ 65). While the incidence of LSS increases with age, a substantial number of adults (age< 65) also suffer from neurogenic claudication secondary to HLF. In this report, outcomes of mild were compared between adults and older adults.
Patients and Methods: All prospective studies of the mild procedure with a 1-year follow-up completed since the beginning of 2012 that allowed the inclusion of adult patients of all ages were reviewed. Outcomes of visual analog scale (VAS), Oswestry Disability Index (ODI), Pain Disability Index (PDI), Roland Morris Low Back Pain and Disability Questionnaire (RMQ), standing time and walking distance were compared for adults and older adults.
Results: Four studies met the inclusion criteria, resulting in an analysis of 49 adults and 160 older adults. Patients in both age groups experienced significant mean improvements in all but one outcome measure at 6- and 12-month follow-up. Differences between the two age groups in all scores at 6 and 12 months were not statistically significant.
Conclusion: Analysis of the four studies indicated that symptom improvements for adults and older adults were significant from baseline, and no statistically significant difference was observed between the two age groups. These results illustrate that mild can be an effective treatment for LSS due primarily to HLF, regardless of the adult patient age.
Keywords: mild, neurogenic claudication, ligamentum flavum, LSS
Introduction
Lumbar spinal stenosis (LSS) is a narrowing of the spinal canal, which may compress neural elements and impede their blood supply leading to ischemia of the cauda equina, manifesting as low back and leg pain. Since the size of the spinal canal decreases with the extension of the lumbar spine,1 this pain, commonly referred to as neurogenic claudication, worsens with standing or walking (lumbar spine extension), and is relieved with sitting or leaning forward (lumbar spine flexion). LSS generally occurs from a combination of degenerative changes including hypertrophic ligamentum flavum (HLF), facet arthropathy, and bulging disc, each of which may contribute to a decrease in spinal canal diameters.2 LSS is most commonly found in the elderly, and is one of the most frequent indications for spine surgery in patients older than 65 years.3 However, while the prevalence of symptomatic LSS has been measured at 12–14% in the 60–79 year age group, it also affects 2% of 40–49 year-olds and 6–7% of 50–59 year-olds.4,5 This suggests that estimated seven million adults in the US between the ages of 40 and 65 are currently experiencing symptomatic LSS (US Census Bureau, https://data.census.gov/cedsci/all?q=age&hidePreview=false&tid=ACSST1Y2018.S0101&t=Age%20and%20Sex). Further, nearly all of those affected (94–100%) will suffer from neurogenic claudication.2
Treatment options for LSS begin with conservative management, which has the potential to reduce pain using analgesics, to reduce inflammation through rest and use of NSAID medications or epidural steroid injections, and to improve core muscle strength using physical therapy and extension exercises. These treatments do not address the exact pathology and often fail to provide long-term relief. A percutaneous decompression method (mild®; Vertos Medical, Aliso Viejo, CA, USA) offers a minimally invasive alternative for LSS patients. Optimal candidates for mild present with neurogenic claudication and narrowing of the spinal canal due to HLF, as confirmed on lumbar MRI scans. In addition, MR scans commonly show multiple other spinal pathology/comorbidities including foraminal stenosis, facet hypertrophy, and lateral recess stenosis. The mild procedure uses a percutaneous dorsal approach to preferentially resect small pieces of lamina and debulk thickened ligamentum flavum to increase space in the spinal canal without impacting the integrity of the bony spines. This procedure has previously been described.6
The mild procedure is well established as a viable long-term option for patients with moderate-to-severe LSS. Numerous studies to investigate the long-term effectiveness of the mild therapy have been performed, and all studies reported statistically significant and clinically meaningful improvement in function and reduction in pain.7–12 A recent report of a 2-year follow-up of 99 mild patients enrolled in the ENCORE randomized controlled clinical trial described clinically meaningful and statistically significant improvement of all outcome measures through 6-month, 1-year, and 2-year follow-up.11 A cross-disciplinary consensus group using the US Preventive Services Task Force (USPSTF) criteria for evidence level and degree of recommendation performed a systematic review of all published studies of the mild procedure, which resulted in a strong consensus for the highest recommendation of the procedure for the treatment of LSS secondary to HLF.13 The present study analysis retrospectively compared clinical outcomes of the mild procedure in two age groups: adults (age<65) and older adults (age≥65).
Patients and Methods
An analysis was undertaken to compare the 1-year outcomes of adults versus older adults. This analysis included all mild patients in prospective, IRB-approved studies with a 1-year follow-up completed since the beginning of 2012. A comprehensive literature search performed in PubMed and Cochrane CENTRAL databases included all current relevant publications through July 2019. The following keywords were used: “mild + Lumbar Spinal Stenosis,” “mild + LSS,” “mild + neurogenic claudication,” and “mild + ligamentum flavum.” The search was limited to clinical references published in English. The rationale for omission included reports of cost-effectiveness, general reviews, study designs, and letters to the editor. In order to conduct this analysis, it was also a requirement that the studies allowed the inclusion of adult patients of all ages, thereby enabling an age group comparison between adults and older adults. Selected studies are summarized in Table 1.
 |
Table 1 Summary of Included Studies |
Outcome Measures
Each study recorded patient-reported outcomes as primary and/or secondary endpoints. The visual analog scale (VAS) measures back and leg pain using a numeric scale, with zero representing no pain and 10 indicating the worst pain. Each study recorded VAS scores at baseline and at 6- and 12-month follow-up visits. The scores from each of the four studies were stratified into two groups: adults and older adults. VAS scores at 6- and 12-month follow-up were compared to baseline scores in each age group to determine the magnitude of improvement and its statistical significance following the mild procedure.
The Oswestry Disability Index (ODI) evaluates the degree of disability to perform the activities of daily living due to pain. ODI is based on a 100-point scale, with lower scores indicating better health status. Brown, Deer and the MiDAS III study recorded ODI scores at baseline and at 6- and 12-month follow-up. The ODI scores from each of these three studies were stratified into the same two groups based on patient age and analyzed to provide for comparison of results within and between the age groups.
In addition to VAS, Mekhail recorded patient-reported outcome scores from the Pain Disability Index (PDI) and the Roland Morris Low Back Pain and Disability Questionnaire (RMQ), as well as standing time and walking distance at baseline and at 6- and 12-month follow-up. The PDI is a validated, self-reported questionnaire that measures the degree to which neurogenic claudication pain interferes with the patient’s ability to perform seven activities of daily living.14–16 Patients rate their level of pain disability for each activity from 0 to 10, with lower scores signifying less disability; overall PDI scores range from 0 to 70. RMQ is a validated, self-assessment tool that measures functional disability on a 24-point scale, with lower scores indicating less severe symptoms.17 Standing time is a measure of how long a patient can stand unassisted before being limited by neurogenic claudication. Walking distance is a measure in feet of how far a patient can walk unassisted before being limited by neurogenic claudication. Both of these measures were patient self-reported. However, data were verified in clinic with a member of the research team who was not involved in the study. Patients chosen at random were allowed to walk or stand in the clinic until they felt the symptoms of neurogenic claudication in order to verify the self-reported data.
Statistical Methods
Data from all studies were pooled prior to analysis. A paired t-test was used to determine if improvements in scores between baseline and 6 months as well as between baseline and 12 months for all participants were significant. In addition, a post hoc analysis using an unpaired t-test was used to assess whether the mean score for each of the outcomes between each age group was significantly different at baseline, 6, and 12 months. P-values were adjusted for false discovery rate. Hypothesis testing was performed at a 5% level of significance (P = 0.05). Analyses were performed using R Package (version 3.5.2; R Studio, Boston, MA, USA).
Descriptive analysis was performed using mean ± standard deviation (SD) for continuous variables and count (percent) for categorical variables. Linear mixed modelling (LMM) was used to assess whether the change in various outcomes with time was significantly different between adult and older adult participants, ie statistically significant interaction between age and time for each of the outcomes. Results are based on Type III sum of squares ANOVA with P-values computed using Satterthwaite approximation to account for differences in sample variances. F values, which represent the ratio between the variance explained by group means and the mean of the within-group variances, were also calculated.
Pearson’s correlation was used to assess the association between the percent change in VAS score (at 6 and 12 months) and age (as a continuous variable), as the VAS score is the single outcome common to all four studies. Simple linear regression was also used to assess whether age was a significant predictor of percent change in VAS scores at 6 and 12 months.
Results
Included Studies
The keyword search of the literature and clinical study database identified 149 publications. Of these, 122 were not reports of the mild procedure and were excluded. Of the remaining 27 articles, 22 were excluded because they were review articles, reports of cost-effectiveness, letters to the editor, a description of study design, or did not include a 1-year follow-up completed since 2012. Five publications met these initial criteria (Staats,11 Benyamin,18 Brown,7 Deer,8 and Mekhail10). The papers by Staats and Benyamin were eliminated because they were both reports of the ENCORE study which included patients aged 65 and older only (Figure 1).
 |
Figure 1 Flow diagram of study identification, exclusion and inclusion in the analysis. |
The remaining three studies were included together with the previously unreported IRB-approved MiDAS III study. Descriptions of each of the four studies are presented in Table 1. Each of these studies utilized similar patient selection criteria allowing for pooling of data, and all studies also reported a 1-year follow-up.
The studies by Brown,7 Deer8 and Mekhail10 have each been previously reported. The MiDAS III study, previously unreported, was a two-arm observational study comparing the treatment of symptomatic LSS using the mild system to epidural steroid injections (ESIs) for symptom relief. Patient reluctance to forego the potential long-term relief from the mild treatment, by being selected for the ESI arm, prevented the planned randomization of the two study arms of this study involving 22 centers; once converted to an observational study, 122 patients chose the mild procedure while 16 patients opted for ESI. Inclusion criteria common to all studies were diagnosis of symptomatic LSS with neurogenic claudication due to HLF via preoperative MRI or CT scan at the appropriate spinal level(s). In all four studies, patients with mild to moderate comorbid conditions commonly associated with spinal stenosis, such as foraminal stenosis, lateral recess stenosis, facet hypertrophy, minor spondylolisthesis, and/or disk protrusion were included. In addition, each patient failed prior conservative therapy and was able to walk only a short distance or stand for a few minutes unaided before being limited by pain. All studies used the mild system with the same general procedure,6 were prospective, and used similar selection criteria.
Descriptive Statistics
The analysis included 209 participants (58.4% females, and 41.6% males). Adults represented 23.4% (n = 49) of the study sample while older adults represented 76.6% (n = 160). (See Table 2).
 |
Table 2 Descriptive Statistics for Included Studies |
Within-Group Improvements at Follow-Up
The mean score for all outcomes for adults was significantly different (P ≤ 0.05) at both 6- and 12-month follow-up visits compared to baseline, with the exception of RMQ at 12 months which showed improvement but did not reach the statistical significance of P ≤ 0.05 (Figure 2). Similarly, the mean score for all outcomes for older adults was significantly different (P ≤ 0.05) at both 6- and 12-month follow-up visits compared to baseline (Figure 3).
 |
Figure 2 Change in outcomes with time within the adult group for (A) VAS, (B) ODI, (C) PDI, (D) Standing time, (E) RMQ, and (F) Walking distance. |
 |
Figure 3 Change in outcomes with time within the older adult group for (A) VAS, (B) ODI, (C) PDI, (D) Standing time, (E) RMQ, and (F) Walking distance. |
Between-Group Comparison of Improvements at Follow-Up: Adults versus Older Adults
Results from the linear mixed model analysis (ANOVA) showed that the interaction between age and time was not statistically significant at the 5% level for any of the outcomes. The changes in VAS, ODI, PDI and RMQ scores, standing time and walking distance were not significantly different between adult and older adult participants as indicated by the Fint-values and P-values shown in Table 3. Figure 4 shows the results for the post hoc pairwise comparisons between groups at each of the time points. There was no statistically significant difference in the mean scores between both groups and any of the time points.
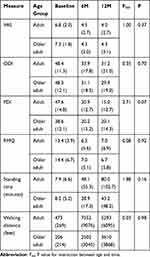 |
Table 3 Descriptive Analysis and LMM Interaction Results |
 |
Figure 4 Change in outcomes across adult and older adult groups for (A) VAS, (B) ODI, (C) PDI, (D) Standing time, (E) RMQ, and (F) Walking distance. |
Correlation Between Age and Percent Change in VAS
Pearson’s correlation between age and percent change in VAS at 6-month follow-up was not statistically significant (R = 0.07, P = 0.33), indicating that age is not significantly associated with the percent change in VAS at 6 months. Similarly, the percent change in VAS at 12 months was not significantly associated with age (R = −0.0031, P = 0.97). The results for both follow-up time periods are shown in Figure 5.
 |
Figure 5 Correlation between age and % change in VAS at (A) 6 months and (B) 12 months. |
The analysis found that the slope coefficients were not significantly different from 0 at 6-month follow-up (β = 0.9, P = 0.62) and at 12-month follow-up (β = −0.06, P = 0.97), indicating that the percent reduction in pain does not appear to be related to participant age (Table 4).
 |
Table 4 VAS Linear Regression Analysis: Percent Reduction in Pain Does Not Change with Age |
Complications
No major device- or procedure-related complications or serious adverse events were reported in any of the four studies included in the analysis, precluding comparative analysis between age groups.
Discussion
This comparison of 49 adults to 160 older adults, each treated with the mild procedure, determined that at 6- and 12-month follow-up, both groups experienced statistically significant mean improvement of all outcome measures (P ≤ 0.05) compared to baseline, with the one exception being an improvement in RMQ for the adult group at 12 months. Although RMQ showed marked improvement, it did not reach statistical significance at P value ≤0.05 which may be due to the small number of subjects. There were no statistically significant differences between the two age groups at 6- and 12-month follow-up for any of the outcome measures.
Specifically, for the most commonly used measures, the mild procedure achieved statistically significant mean improvements at 12 months in VAS and ODI of 2.8 and 17.2, respectively, compared to baseline for adult patients. The older adult group also experienced statistically significant mean improvements at 12 months in VAS and ODI measures compared to baseline, with improvements of 3.0 and 18.4, respectively. Importantly, these improvements for VAS and ODI in both groups were also clinically meaningful in that they exceeded the validated clinical minimally important-change thresholds of a ≥10 point improvement in ODI and a ≥2 point improvement in VAS.19–23 The regression analysis showed that while pain was reduced in most patients following percutaneous decompression, there was little or no relationship between the amount of improvement and age, especially at 12-month follow-up. The results between the two groups at 12 months were not statistically significantly different. It is also notable that mean standing time increased by over 10 times for patients in the adult group, and mean walking distance increased by over 11 times from less than 500 feet to over a mile. This change in walking distance represents an important improvement in the quality of life for these younger patients.
While LSS can be congenital, the acquired form resulting from a degenerative spine is considered to be the most common and is most likely due to aging. Loss of disc height from degeneration of the intervertebral disc can shift axial stress toward the posterior elements of the spine, including the facet joints and ligamentum flavum, which may lead to arthritic changes of the facet joints as well as deformation and thickening of the ligamentum flavum and resulting narrowing of the spinal canal.5,24 In a report by Hansson et al, the ligamentum flavum was found to contribute up to 85% of load-induced narrowing of the spinal canal, confirming hypertrophic ligamentum flavum as the problem causing compression of intraspinal blood supply and nerve roots.25 The compression of blood vessels, nerve roots and associated tissues in central canal stenosis is thought to induce painful neurogenic claudication.26–28
It is widely accepted that the most important risk factor for the onset of LSS is age;29 however, an estimated seven million people in the US between the ages of 40 and 65 suffer from symptomatic LSS and neurogenic claudication. The results of the current analysis of percutaneous decompression studies mirror those seen with investigations of the effectiveness of open surgical decompression in different age groups. Hansraj et al studied 77 patients with an average age of 65 years who underwent laminectomy with partial facetectomy for LSS and completed a self-reported questionnaire (Zurich Claudication Questionnaire). The authors found that adult patients had a greater improvement in function and a greater reduction in severity scores than older adults, although satisfaction was similar in both groups, and that comorbidities did not influence the outcome.30 Hee and Wong treated 68 patients with ages ranging from 60 to 82 years with either laminectomy alone (84%) or together with posterolateral arthrodesis (16%), and by use of a patient-reported questionnaire found that surgical outcomes were not influenced by age or comorbidities.31 Athiviraham et al treated 88 patients with an average age of 66 years with either decompression alone (56%) or decompression and posterolateral fusion (44%). Using the Roland-Morris questionnaire, the authors found no relationship between outcomes and patient age.32,33 While each of these studies utilized different outcome measures, they are consistent in their findings that patient age does not appear to influence procedure outcomes.
The results of this analysis may encourage practitioners to use percutaneous decompression for adult patients to provide the same improvements in pain and function that have been demonstrated for older adult patients. Equally important, since percutaneous decompression has been demonstrated as a cost-effective procedure for appropriate older adult patients when compared to continuation of conservative treatment and repeated serial ESIs, or to proceeding to laminectomy surgery,34 the cost savings of this procedure is expected to be at least as great for adult patients faced with the same treatment options. Since this is a one-time procedure in the vast majority of patients, percutaneous decompression has the potential to amplify these cost savings and provide adult patients with the benefits of this procedure over an even longer period of time compared with older adults.
Limitations
Limitations of this study include the relatively small number of patients treated with the mild procedure and the smaller number of patients in the two age groups subject to comparison, which allows for evaluation of differences between groups but is underpowered to provide predictive conclusions. One of the studies was randomized while the other three were not; however, the performance of the mild procedure appears consistent across all four studies. Only one measurement outcome, the VAS scoring system, was common to all four of the studies, but each measurement outcome was analyzed independently of the others to allow for valid comparisons of each measure between age groups. The MiDAS III study is not published. It was designed as a randomized study comparing the mild procedure to a series of epidural steroid injections as the control arm. However, poor recruitment was encountered as patients were reluctant to forego the potential long-term relief from the mild treatment and be enrolled in the ESI arm to receive a treatment that they had already failed. Therefore, a decision was made to continue prospective observational data collection in this study involving 22 centers. Additional studies comparing mild outcomes of adults versus older adults would be of value to support these findings.
Conclusion
The relationship between the onset of LSS and age has been the subject of much research and is well documented. The analysis of four studies using the mild percutaneous approach to LSS decompression indicated that symptom improvements for adult mild patients were statistically significant, and were not statistically different from the improvements for older adults. These results illustrate that percutaneous decompression is an effective treatment for symptomatic LSS due primarily to hypertrophic ligamentum flavum, regardless of patient age. Therefore, the mild procedure presents a viable option for not only older adults but also for the estimated seven million patients in the US below the age of 65 who suffer from symptomatic LSS and may not want to undergo open spine surgery. Earlier usage of mild may also decrease healthcare resource utilization of less beneficial and more costly options like ESIs.34 Since mild in the vast majority of patients is a one-time procedure, the results of this analysis may encourage practitioners to utilize the mild procedure in the younger adult age group, thereby allowing these patients to enjoy the benefits of this procedure for a larger number of years.
Acknowledgments
The authors thank Britt Norton of Medavise LLC for manuscript assistance.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Nagy A Mekhail reports personal fees from Abbott, Boston Scientific, Sollis Therapeutics, Relievant Medsystems, Saluda Medical, Nevro, Vertos Medical, and Nuvectra, grants from Mallinckrodt, Mesoblast, Halyard, Neuros Medical, outside the submitted work. Dr Timothy R Deer reports grants, personal fees from and is a consultant and research investigator including stock option for Vertos Medical and Vertiflex, during the conduct of the study. He also reports personal fees from Abbott, Flowonix, Axonics, SpineThera, Saluda Medical, Mainstay Medical, Nalu, Cornerloc, Ethos, SPR Therapeutics, Stimgenics, SI Bone, Nevro, Medtronic as consultant, advisory board member, and/or research investigator, outside the submitted work. In addition, Dr Timothy R Deer has a patent pending with Abbott. The authors report no other conflicts of interest in this work.
References
1. Schönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: an experimental study in vitro. J Orthop Res. 1989;7:115–121. doi:10.1002/jor.1100070116
2. Levy RM, Deer TR. Systematic safety review and meta-analysis of procedural experience using percutaneous access to treat symptomatic lumbar spinal stenosis. Pain Med. 2012;13:1554–1561. doi:10.1111/j.1526-4637.2012.01504.x
3. Ishimoto Y, Yoshimura N, Muraki S, et al. Associations between radiographic lumbar spinal stenosis and clinical symptoms in the general population: the Wakayama Spine Study. Osteoarthritis Cartilage. 2013;21:783–788. doi:10.1016/j.joca.2013.02.656
4. Ishimoto Y, Yoshimura N, Muraki S, et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Osteoarthritis Cartilage. 2012;20:1103–1108. doi:10.1016/j.joca.2012.06.018
5. Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9:545–550. doi:10.1016/j.spinee.2009.03.005
6. Deer TR, Kapural L. New image-guided ultra-minimally invasive lumbar decompression method: the mild procedure. Pain Physician. 2010;13:35–41.
7. Brown LL. A double-blind, randomized, prospective study of epidural steroid injection vs. the mild® procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract. 2012;12:333–341. doi:10.1111/j.1533-2500.2011.00518.x
8. Deer TR, Kim CK, Bowman RG
9. Mekhail N, Vallejo R, Coleman MH, Benyamin RM. Long-term results of percutaneous lumbar decompression mild(®) for spinal stenosis. Pain Pract. 2012;12:184–193. doi:10.1111/j.1533-2500.2011.00481.x
10. Mekhail N, Costandi S, Abraham B, Samuel SW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract. 2012;12:417–425. doi:10.1111/j.1533-2500.2012.00565.x
11. Staats PS, Chafin TB, Golovac S, et al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg Anesth Pain Med. 2018;43:789–794. doi:10.1097/AAP.0000000000000868
12. Chopko BW. Long-term results of percutaneous lumbar decompression for LSS: two-year outcomes. Clin J Pain. 2013;29:939–943. doi:10.1097/AJP.0b013e31827fb803
13. Deer TR, Grider JS, Pope JE, et al. The MIST guidelines: the lumbar spinal stenosis consensus group guidelines for minimally invasive spine treatment. Pain Pract. 2019;19:250–274. doi:10.1111/papr.12744
14. Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59:974. doi:10.2466/pms.1984.59.3.974
15. Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The pain disability index: psychometric and validity data. Arch Phys Med Rehab. 1987;68:438–441.
16. Tait RC, Chibnall NT, Krause S. The pain disability index: psychometric properties. Pain. 1990;40:171–182. doi:10.1016/0304-3959(90)90068-O
17. Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi:10.1097/00007632-198303000-00004
18. Benyamin RM, Staats PS, MiDAS Encore I. mild® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE randomized controlled trial. Pain Physician. 2016;19:229–242.
19. Hägg O, Fritzell P, Nordwall A; Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi:10.1007/s00586-002-0464-0
20. Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain towards international consensus regarding minimal important change. Spine. 2008;33(1):90–94. doi:10.1097/BRS.0b013e31815e3a10
21. Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334. doi:10.1097/01.brs.0000164099.92112.29
22. Farrar JT, Young JP
23. Salaffi F, Stancati A, Silverstri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283–291. doi:10.1016/j.ejpain.2003.09.004
24. Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med. 2008;358:818–825. doi:10.1056/NEJMcp0708097
25. Hansson T, Suzuki N, Hebelka H, Gaulitz A. The narrowing of the lumbar spinal canal during loaded MRI: the effects of the disc and ligamentum flavum. Eur Spine J. 2009;18:679–686. doi:10.1007/s00586-009-0919-7
26. Lin J-H, Chiang Y-H, Chen -C-C. Lumbar radiculopathy and its neurobiological basis. World J Anesthesiol. 2014;3:162–173. doi:10.5313/wja.v3.i2.162
27. Kobayashi S. Pathophysiology, diagnosis and treatment of intermittent claudication in patients with lumbar canal stenosis. World J Orthop. 2014;5:134–145. doi:10.5312/wjo.v5.i2.134
28. Arbit E, Pannullo S. Lumbar stenosis: a clinical review. Clin Orthop Relat Res. 2001;384:137–143. doi:10.1097/00003086-200103000-00016
29. Bagley C, MacAllister M, Dosselman L, Moreno J, Aoun S, El Ahmadieh T. Current concepts and recent advances in understanding and managing lumbar spine stenosis. F1000Res. 2019;8:137. doi:10.12688/f1000research.16082.1
30. Hansraj KK, Cammisa FP
31. Hee HT, Wong HK. The long-term results of surgical treatment for spinal stenosis in the elderly. Singapore Med J. 2003;44:175–180.
32. Athiviraham A, Yen D. Is spinal stenosis better treated surgically or nonsurgically? Clin Orthop Relat Res. 2007;458:90–93. doi:10.1097/BLO.0b013e31803799a9
33. Athiviraham A, Wali ZA, Yen D. Predictive factors influencing clinical outcome with operative management of lumbar spinal stenosis. Spine J. 2011;11:613–617. doi:10.1016/j.spinee.2011.03.008
34. Udeh BL, Costandi S, Dalton JE, Ghosh R, Yousef H, Mekhail N. The 2-year cost-effectiveness of 3 options to treat lumbar spinal stenosis patients. Pain Pract. 2015;15:107–116. doi:10.1111/papr.12160
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
