Back to Journals » Patient Preference and Adherence » Volume 14
The Impact of a Forced Non-Medical Switch of Inhaled Respiratory Medication Among Patients with Asthma or Chronic Obstructive Pulmonary Disease: A Patient Survey on Experience with Switch, Therapy Satisfaction, and Disease Control
Authors Gilbert I , Wada K, Burudpakdee C , Ghai C, Tan L
Received 13 December 2019
Accepted for publication 26 June 2020
Published 20 August 2020 Volume 2020:14 Pages 1463—1475
DOI https://doi.org/10.2147/PPA.S242215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Ileen Gilbert,1 Keiko Wada,2 Chakkarin Burudpakdee,2 Chirag Ghai,2 Laren Tan3
1AstraZeneca, Wilmington, DE, USA; 2IQVIA, Falls Church, VA, USA; 3Loma Linda University Health, Department of Medicine, Division of Pulmonary, Critical Care, Hyperbaric, Allergy and Sleep Medicine, Loma Linda, CA, USA
Correspondence: Ileen Gilbert Email [email protected]
Purpose: Budesonide/formoterol pressurized metered-dose inhaler (pMDI) was removed from a Medicare Part D formulary, and patients switched to fluticasone-based dry powder inhaler (DPI) therapies. This study describes the experience, satisfaction, and disease control among patients with asthma or chronic obstructive pulmonary disease (COPD) who switched due to removal from the formulary.
Patients and Methods: A patient survey was conducted among adults with asthma or COPD who used budesonide/formoterol pMDI for ≥ 3 months prior to the formulary block and the new medication for ≥ 3 weeks after switching, recruited by providers in a research panel. Survey comprised both validated instruments (PASAPQ, OEQ, ACQ-6, and CAT) and stand-alone questions. Patient characteristics, switch experience, device and treatment satisfaction, onset of effect, and disease control were compared between disease (asthma and COPD) and medication (once and twice daily) cohorts. Minimal significance for group differences: P≤ 0.05.
Results: Among 100 patients, 93% received communication from their doctor or nurse about the switch and 73% received training on using the new inhaler. Patients used their new treatment for an average of 7 months prior to completing the survey. Patient satisfaction with the new therapy was high (PASAPQ; mean overall satisfaction: 6.2 for asthma; 6.0 for COPD; P=0.338). However, asthma was not well controlled (ACQ-6) in 62% of patients with asthma, and 56% of patients with COPD reported high/very high impact of their illness on their lives (CAT). Sixty-eight percent and 70% of patients with asthma and COPD, respectively, required reliever medication (≥ 3 puffs) most days during the week prior to the survey. There were no significant differences in disease control (ACQ-6, CAT) between once-daily and twice-daily treatments (P> 0.05 for both asthma and COPD).
Conclusion: Even when reporting satisfaction with their new medication, objective measures showed substantial morbidity, regardless of DPI device or dosing regimen.
Keywords: asthma, pulmonary disease, chronic obstructive, Medicare Part D, dry powder inhalers, metered dose inhalers, surveys, questionnaires
Introduction
Non-medical medication switching and formulary blocks are becoming more common, and insurers have increasingly expanded their exclusion lists.1,2 There are few published reports regarding non-medical or non-consented medication switching and its impact; however, a small number of studies describe many unintended consequences of non-medical switches that potentially interfere with disease management and/or lead to adverse health outcomes in diseases such as gastroesophageal reflux disease, hypertension, type 2 diabetes, and rheumatoid arthritis.3,4 A 2016 US online survey was conducted with residents of Tennessee covered by private insurance (52%), public insurance (34%), and dual-public/private type (14%).5 The poll reported that 58% of respondents had their insurance company make changes to their health plan’s formulary that reduced coverage of their prescribed medication, that 95% of respondents experienced worsened symptoms when they were forced to switch medication, and 68% required multiple treatments before they found an alternative that worked.5 Furthermore, a quantitative study using information from a primary care database in the UK found that the likelihood of unsuccessful treatment following a non-medical switch was almost two times that of patients who remained on their current treatment.6
Studies in patients with asthma or chronic obstructive pulmonary disease (COPD) have shown such involuntary switches to be directly associated with reduced medication adherence and poor disease control, as demonstrated by increases in health care visits and oral corticosteroid and short-acting β2-agonist (SABA) dispensals.7,8 In asthma or COPD, switching inhalers or adding different types of devices to a patient’s regimen without consultation can lead to incorrect inhalation technique and/or decreased adherence to treatment; the latter is especially true if a patient has no involvement in the choice of device.9–11 Using different or multiple devices may also be associated with reduced disease control and quality of life, increased use of other treatments and health care services, and poorer treatment outcomes.12,13 All these factors can ultimately result in decreased efficacy of treatment and increased patient morbidity.6,14 Although non-medical switches are often for cost-related reasons, such strategies may actually be more expensive for both patients and insurance companies in the long term.11,15 Repeat visits for device training, the need for further switching, and an increased likelihood of worse disease control can all contribute to greater health care resource utilization.6,11 Non-medical inhaler switches also have a negative impact on the physician–patient relationship, due to the feeling of loss of personal control and reduced patient confidence in the physician.10,15
Budesonide/formoterol is a fixed-dose combination product containing an inhaled corticosteroid (ICS) and long-acting β2-agonist (LABA), administered as two puffs twice daily via a single pressurized metered-dose inhaler (pMDI). This combination is indicated for the treatment of asthma in patients aged ≥6 years and for maintenance treatment of airflow obstruction and reducing exacerbations in patients with COPD, including chronic bronchitis and/or emphysema.16 Budesonide/formoterol pMDI was a preferred product in a Medicare Part D formulary in 2016 but was removed on January 1, 2017, resulting in a forced non-medical switch to a dry powder inhaler (DPI) ICS/LABA therapy of fluticasone propionate/salmeterol (one puff twice daily [Diskus™]) or fluticasone furoate/vilanterol (one puff once daily [Ellipta™]).
The objective of the current study was to conduct a patient survey to understand the experience, satisfaction, and disease control among patients diagnosed with asthma or COPD who switched to a fluticasone-based DPI from budesonide/formoterol pMDI due to its removal from the formulary.
Patients and Methods
Patients
Patients aged ≥18 years and with a physician-confirmed diagnosis of asthma or COPD were recruited into the study by their physicians. Patients were required to have their asthma or COPD diagnosis prior to January 1, 2017 and to be insured on January 1, 2017 by the Medicare Part D plan that implemented the formulary block. Patients were also required to have used budesonide/formoterol pMDI for ≥3 months prior to the switch date, after which they were switched by their health care provider (HCP) to DPI ICS/LABA due to the formulary block and used it for ≥3 weeks. Patients were also required to have used a short-acting bronchodilator (albuterol, levalbuterol, or ipratropium bromide) within 3 months prior to screening. Patients were ineligible for participation if the treatment switch occurred for reasons other than the formulary block in January 2017, if they had a confirmed diagnosis of both asthma and COPD, or if they were unable to complete a 30-minute survey. Prior to recruitment, this study received an IRB exemption from the New England Independent Review Board (NEIRB) under the category of research involving the use of a survey.
Study Design
Assessments
In this patient survey study, patients completed a paper survey comprising validated instruments as well as stand-alone questions during a physician office visit (Table 1). The survey consisted of patient demographics, their experience with the switch (quality of HCP communication concerning the switch and device training received for the new therapy); their satisfaction with and perceptions of the new inhalation device, dosing schedule, and therapeutic efficacy of the new medication; and the degree of their obstructive lung disease control. The surveys had 39 and 42 questions for patients with asthma and COPD, respectively. The number of patients required for this study was determined based on the feasibility of recruitment and preliminary precision calculations. A minimum sample size of 50 patients in each patient cohort was targeted.
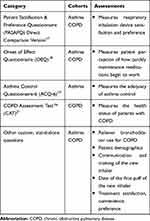 |
Table 1 Patient Survey Components |
The Patient Satisfaction and Preference Questionnaire (PASAPQ) Direct Comparison Version17 was used to assess device satisfaction among patients with asthma or COPD (Table 1). The PASAPQ is a patient-reported measure of respiratory inhalation device satisfaction and preference and has been validated in both patients with asthma and patients with COPD.17 It contains 14 satisfaction items grouped into Performance and Convenience domains and provides a Total PASAPQ score, ranging from 0 (least) to 100 (most), with a change of 10 considered the minimally important difference. Domain scores were calculated by summing items within each domain then transformed to a scale of 0 to 100 points. Performance domain score is based on seven items (overall feeling of inhaling, inhaled dose goes to lungs, amount of medication left, works reliably, ease of inhaling a dose, using the inhaler, and speed medicine comes out). The convenience domain score is based on six items (instructions for use, size of inhaler, durability of inhaler, ease of cleaning inhaler, ease of holding during use, and convenience of carrying). The remaining item was a stand-alone question that provided overall satisfaction, which ranged from 1 (very dissatisfied) to 7 (very satisfied).
The Onset of Effect Questionnaire (OEQ),18 also used in patients with either asthma or COPD (although to date it has only been validated in patients with asthma), is a self-administered instrument that assesses patient perception of how quickly a maintenance medication begins to work (Table 1). It is a five-item instrument evaluating whether patients feel their medication working right away and assessing satisfaction with how quickly they feel their medication begins to work.
The 6-item Asthma Control Questionnaire (ACQ-6)19 is a self-administered questionnaire specific to patients with asthma that measures the adequacy of, and change in, asthma control that occurs either spontaneously or as a result of treatment (Table 1). This instrument has a multi-dimensional construct assessing symptoms (five items) and reliever bronchodilator use (one item). Patients are asked to respond on a 7-point scale (0 = no impairment, 6 = maximum impairment for symptoms and reliever use). Patients with an ACQ-6 score ≤0.75 are considered to have adequately controlled asthma, and a score ≥1.5 indicates asthma that is not well controlled.20
The COPD Assessment Test™ (CAT)21 is a validated, self-administered questionnaire with eight items that measures the health status of patients with COPD (Table 1). It is a unidimensional instrument that evaluates the impact of COPD symptoms (cough, sputum, dyspnea, and chest tightness) on patients’ daily lives. The CAT has a scoring range of 0 to 40; each item is scored 0 to 5. A CAT score <10, 10 to 20, >20 to 30, and >30 denotes an overall low, medium, high and very high impact, respectively, of COPD on a patient’s life.
Additional stand-alone questions assessed patient demographics; reliever bronchodilator use for COPD; communication and training on the new inhaler; date of the first puff of the new inhaler; and medication satisfaction, convenience, and preference (Table 1).
Statistical Analyses
Results of all analyses were provided as descriptive statistics. For categorical measures, findings were reported as the frequency (number of cases [N]) and percentage (%) for each cohort. For continuous variables, findings were reported as the mean, standard deviation (SD), and median. When necessary, continuous variables were categorized into intervals, with the distribution of patients (N, %) for each interval provided. Missing values were considered as a separate category.
All patient-reported outcome data were scored according to the instrument developers’ recommendations. Item-level responses were reported with the distribution of patients (N, %) for each of the response options.
Pair-wise comparisons were made between the asthma and COPD cohorts as well as between the fluticasone propionate/salmeterol twice-daily or fluticasone furoate/vilanterol once-daily cohorts, using parametric t-test (mean) and nonparametric Wilcoxon rank-sum test (median) for continuous variables and chi-squared test for categorical variables. A P value of <0.05 was considered statistically significant for all analyses.
Results
Patients
A total of 100 patients with a physician-confirmed diagnosis of asthma or COPD completed the survey. Patient demographics and clinical characteristics are described in Table 2. Percentages of patients were approximately evenly divided between those with asthma who experienced a forced non-medical switch to fluticasone propionate/salmeterol one puff twice daily (26%) or fluticasone furoate/vilanterol one puff once daily (24%), and those with COPD who were switched to fluticasone propionate/salmeterol via DPI twice daily (27%) or fluticasone furoate/vilanterol once daily (23%). Dosage strengths for the new DPI ICS/LABA to which patients switched are shown in Table 2; pre-switch budesonide/formoterol pMDI dosage strengths were not available. There were no significant differences in demographic or disease characteristics between groups (asthma vs COPD or once- vs twice-daily DPI; all P>0.05), although there were more Hispanic/Latino patients in the asthma group vs the COPD group (26% vs 6%; P=0.006), and more asthma patients used the ProAir rescue inhaler (38%) vs COPD patients (14%; P=0.006). Patients with asthma were treated with a new DPI ICS/LABA for a mean (SD) of 6.9 (2.5; range, 1.2–10.5) months and patients with COPD for 7.0 (2.4; range, 1.6–11.2) months prior to completing the survey.
 |
Table 2 Patient Characteristics |
Patient Switch Experience
Almost all patients received communication from their HCP about the switch from budesonide/formoterol pMDI to a DPI ICS/LABA and training for the new device. In total, 93% of patients learned about the switch from their HCP (physician or nurse practitioner), and 73% of patients reported that their HCPs demonstrated how to use the new inhaler and/or watched them using the new inhaler (Table 3). There were no significant differences between patients with asthma or COPD or between those switched to the twice-daily vs once-daily ICS/LABA formulations in the proportions of patients who received communication about, or received training for, their new device (all P>0.05; data not shown).
 |
Table 3 Patients’ Responses on Communication and Training of the New Therapy |
Treatment Satisfaction
Treatment satisfaction, as assessed by scores on the PASAPQ, is shown in Figure 1. No significant differences in patient-reported satisfaction with their new device were detected between patients with asthma and patients with COPD (mean total PASAPQ score, 82.7 vs 77.6; P=0.105) or between those receiving twice-daily and once-daily treatment (mean total PASAPQ score, 79.4 vs 81.0; P=0.611). There was no significant difference in the percentage of respondents stating that they somewhat/strongly agreed that they were satisfied with how quickly they could feel their controller medication begins to work between patients with asthma or COPD (OEQ; 80% vs 78%; P=0.806) or between patients receiving twice-daily treatment with fluticasone propionate (83%) or once-daily treatment with fluticasone furoate (74%; P=0.295; Figure 2).
Based on the stand-alone medication satisfaction questions, a greater percentage of patients with asthma (66%) than patients with COPD (38%) reported they were “extremely satisfied” or “very satisfied” with the ability of the new DPI to prevent or treat their condition (P=0.005; Figure 3). When comparing the twice-daily and once-daily regimens, there were no significant differences in terms of responses of extremely/very satisfied regarding the ability of their new treatment to manage their condition (fluticasone propionate/salmeterol, 49% vs fluticasone furoate/vilanterol, 55%).
Significantly more patients with asthma (60%) than patients with COPD (32%) reported they were “extremely satisfied” or “very satisfied” with how often they used the new DPI (P=0.005), and there were no significant differences between the twice-daily and once-daily regimen groups in terms of responses of extremely/very satisfied with the frequency of dosing (fluticasone propionate/salmeterol, 47% vs fluticasone furoate/vilanterol, 45%; P>0.05; Figure 3).
Disease Control and Symptom Management
The majority of patients with either asthma or COPD reported disease that was not well controlled (as measured by validated control and symptom impact scores or use of reliever medications) after switching (Figure 4 and Table 4). Sixty-two percent of patients with asthma had an ACQ-6 score19 ≥1.5 (ie, “not well controlled”). Fifty-six percent of patients with COPD had a CAT score21 >20 (ie, “high impact” or “very high impact” of COPD on patients’ daily life). No significant differences were detected between patients treated with twice-daily or once-daily therapy in terms of ACQ-6 or CAT scores (P>0.05 for both ACQ-6 and CAT scores). Sixty-eight percent of patients with asthma reported using their reliever bronchodilator an average of ≥3 puffs most days during the week prior to the survey. Among patients with COPD, 70% reported using their bronchodilator ≥3 puffs most days during the week before the survey. Reliever medication usage did not differ significantly between once-daily and twice-daily dosing with the new DPI for patients with asthma (P=0.680) or COPD (P=0.481).
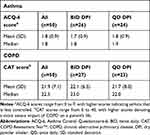 |
Table 4 Disease Control After Switching to New DPI |
Discussion
As insurers continue to expand their medication exclusion lists, health care providers are increasingly switching their patients from one therapy to another for non-medical reasons. As non-medical switches have the potential to interfere with management of chronic diseases,7 it is important to understand their impact on patients. When patients received good communication about a Medicare Part D formulary block and training on the device technique of their switched-to inhaler, patients using a new ICS/LABA for approximately 7 months reported satisfaction with their medication. However, objective measures of disease morbidity showed their obstructive lung disease was not well controlled and patients reported daily symptoms. Patients who switched from budesonide/formoterol pMDI to twice-daily DPI ICS/LABA were as equally not well controlled as those on a once-daily DPI, although they reported being as satisfied with the efficacy and dosing regimen of their new therapy.
Non-medical switching in patients with asthma or COPD is often associated with negative effects on clinical outcomes. A switch to a different inhaler device can impact patient adherence and preference, dosing accuracy, and clinical results. Most of the Medicare Part D patients in our study with asthma or COPD who were required to switch to a new ICS/LABA for non-medical reasons received communication about their medication switch and training on a new inhaler technique. They reported overall satisfaction with their new medication, regardless of dosing frequency or type of DPI. A prior large (N=42,553) US-based study of pharmacy claims (from which these patients were a small subset) exploring a non-medical switch from budesonide/formoterol to DPI ICS/LABA showed that 54% of patients with asthma and/or COPD attempted to fill a prescription for the excluded medication after the formulary block, potentially suggesting poor communication of the change in treatment.22 Of these patients, 15% did not receive any long-term medication to control their disease in the year following the forced non-medical switch. Even among those patients who did not attempt to fill a budesonide/formoterol prescription, 32% did not fill a prescription that included a controller medication.22 This finding highlights a gap in treatment that could have an impact on clinical outcomes. Indeed, it has been shown that many patients with asthma hospitalizations have a very low rate of prescription fills of ICS or ICS/LABA fixed-dose combination therapy.23 Furthermore, the results from this study are consistent with those of a systematic review, which identified negative clinical and economic patient impacts associated with non-medical switches across a diverse array of non-inhaled medication types.4
In the current study, more than 70% of patients received training on how to use their new inhaler. Recruiting physicians were aware of the survey contents and appeared to take care of their patients well by providing adequate communication of the switch and training their patients on the new device. However, in real-life clinical practice, education and assessment occur less often than this. In one study of 205 pulmonologists, although 70% stated that they discussed device use, only 43% felt knowledgeable about teaching patients how to use their devices.24 Additionally, for many patients, training is offered only at the time of the initial prescription, with no follow-up training or re-evaluation to assess the maintenance of correct technique.25,26 In a survey of 513 HCPs, 88% stated that they had provided device training to their patients with COPD, but fewer than one-half checked the patient’s ability to use the device themselves.27 Poor inhaler technique can result in highly variable doses and potentially no medication delivery.28 This issue is of particular concern for elderly patients who may have difficulty reading or understanding written instructions for using their new device7 and therefore is highly relevant to our study, in which most patients were aged ≥65 years.
Continuity of device type may also play a role in clinical outcomes. A primary care database study in the UK showed that patients with COPD using different devices (eg, pMDIs and DPIs) were more likely to experience exacerbations and had a greater use of reliever medication than those whose medications were delivered using the same type of device.29 Using the same database, it has also been reported that using a similar type of device for both maintenance and reliever therapy in patients with asthma was associated with better asthma control and a reduced frequency of exacerbations compared to using different types of devices.30
Another key issue in ensuring medication delivery is the requirement for a certain level of peak inspiratory flow rate (PIFR) with a specific device. The two most common types of inhaler devices are pMDIs, which deliver the drug in aerosol form to the lungs and are very similar to the patient’s reliever inhaler,31,32 and DPIs, which deliver the drug to the lungs in the form of dry powder. In DPIs, an optimal PIFR of >60 L/min is required in order to disaggregate and adequately disperse the drug particles into an aerosol for inhalation, whereas PIFRs <30 L/min are considered insufficient to generate any effect.33 Factors affecting PIFRs include increasing age, short stature, and low forced vital capacity in patients with COPD.33,34 In addition, lung hyperinflation may contribute to reduced inspiratory muscle strength.35 It has been estimated that suboptimal PIFR is present in up to 30% of elderly patients with severe COPD.33,34 In one study, suboptimal PIFR was significantly associated with a higher rate of COPD readmissions within 90 days of discharge following an acute exacerbation and a shorter time period until COPD readmittance.36 In the outpatient setting, it is not a common or well-accepted practice to check PIFR in patients with obstructive lung diseases and, as a result, some patients who are switched over to a DPI by their clinicians may be unable to effectively use their inhaler.
Of interest, there were no differences between DPIs for patient satisfaction with the onset of effect; patient reports regarding onset of effect were not noticeably different between those taking salmeterol and those taking vilanterol, the two LABAs. Some studies have shown that onset of clinically meaningful lung function improvement is faster for fluticasone furoate/vilanterol than fluticasone propionate/salmeterol both in patients with COPD37 and in patients with asthma,38 whereas one head-to-head study in patients with asthma showed no important differences for time to onset of bronchodilator effect.39
In the present study, even when patients reported an optimal experience with a non-medical switch, objective measures showed substantial morbidity—poor asthma control, high impact of COPD on daily life, and the need for frequent reliever medication. These morbidities were similar in the once-daily and twice-daily dosing groups. The prior large US-based pharmacy claim study also found that, whether patients filled a once-daily or twice-daily DPI ICS/LABA, no differences were observed in adherence (using surrogate measures of pharmacy claims showing proportion of days covered) or persistence with either the twice-daily fluticasone propionate/salmeterol or once-daily fluticasone furoate/vilanterol formulation. In addition, no differences were observed in the use of an acute medication indicating loss of control or an exacerbation (oral corticosteroids for patients with asthma and oral corticosteroids and/or antibiotics for patients with COPD).22 In short, the results suggest there were no significant differences in disease control (ACQ-6 and CAT) between patients treated with the once-daily and twice-daily treatments (P>0.05 for both asthma and COPD).
Decreased medication adherence can occur after non-medical switching,9 which may contribute to poorer clinical outcomes.7 Real-life studies have shown that reduced adherence to ICS therapy is associated with an increased frequency of emergency department visits and requirement for oral corticosteroids compared to persistence with therapy,40 and that continuous ICS use has a preventive effect on asthma exacerbations.41,42 In particular, patients’ acceptance of the new device can influence adherence, which is impacted by their involvement with the choice, as well as other factors such as its appearance and ease of use.9 Shared decision-making can also enhance adherence to ICS therapy (with or without LABA).43 In the study presented here, there was no difference in patient satisfaction between once- and twice-daily dosing; a similar study exploring non-medical switching from budesonide/formoterol pMDI to DPI ICS/LABA has also shown no differences between once- and twice-daily dosing frequency on patient adherence following the switch (proportion of days covered and persistence using pharmacy claims).22 This finding is perhaps of interest, as in the published literature it has been reported that adherence to ICS therapy is greater when administered once daily vs twice daily (an estimated 20% increase).44 However, no studies have been published to date that compare ICS/LABA therapies in which patients may be able to feel the LABA component working as it bronchodilates with each use, and in which they were asked to rate when they could begin to feel an ICS/LABA begin to work. One study of budesonide/formoterol pMDI in patients with asthma reported that significantly more patients felt their study medication working right away compared to patients receiving budesonide and placebo (P≤0.004).45 Similar results (P≤0.001) were observed for patient satisfaction with how quickly patients receiving budesonide/formoterol felt their medication begins to work.
Patient surveys are an effective and convenient means of obtaining data. They are inexpensive and cost-effective, especially if self-administered, and are versatile in that different questions can address different aspects of patient experience and behaviors related to a topic. Surveys are generalizable, allowing a collection of responses from a large number of people; reliable when standardized (the same questions phrased in exactly the same way for all participants); conveniently analyzed and quantified by statistical software; and it is easy to compare previous and future research findings from other surveys. Nonetheless, there are some disadvantages, such as closed-ended questions that may restrict the information provided; risk of recall bias as participants may be forgetful or not consider the entirety of the recall period; and possible selection bias as a result of patient attrition and results representing only patients who remained and completed the survey. Researcher bias may also affect survey design or data interpretation.
Limitations of the current study include having a small sample size, potentially leading to selection bias. More specifically, physicians may have chosen patients who were informed of the study and who had received education regarding their new device. These patients therefore received the best communication regarding the forced switch, which could potentially influence satisfaction. Also, we did not capture patients who discontinued budesonide/formoterol pMDI and did not start fluticasone propionate/salmeterol or fluticasone furoate/vilanterol, for whom the impact of the formulary block would be expected to be very different from the surveyed population. In the large pharmacy claim study of a non-medical switch from budesonide/formoterol pMDI, for example, 23% of patients discontinued controller therapy altogether after the formulary block.22 In that study, patients aged ≥12 years with asthma and/or COPD received budesonide/formoterol as their last ICS/LABA in 2016. Patients were followed through 2017 to observe ICS/LABA switches, changes in controller medications, and use of acute medications and evaluated as to whether they switched off therapy due to a formulary block.22
Information collected here only relates to patient responses at the time of the survey, which took place an average of 7 months after the medication switch. Information about care setting at the time of survey completion (eg, inpatient, outpatient) or patients’ disease control, severity, or treatment adherence before the switch were not available; future studies comparing therapy satisfaction and disease control from before and after a switch are warranted. Furthermore, the inability to compare therapy satisfaction and disease control data from before patients switched medications is a limitation of this study. Participating physicians were aware of the survey contents, which may have influenced how they cared for, or communicated with, patients in the study. Survey results, in particular those concerning communication, suggested that participating physicians cared for their patients closely. Lastly, patients may have had ample time to become familiar with and adjust to the new treatment. Future studies of non-medical switches may benefit from less time between the medication change and survey administration to address any variables that may occur during the transition between medications.
Conclusions
In conclusion, patients with Medicare Part D using a new ICS/LABA for an average of 7 months following a non-medical switch received adequate communication about the switch and received training on inhaler technique with the new device. Despite patient-reported satisfaction with their new medication, objective measures showed that the majority of patients with asthma exhibited poor control, and patients with COPD found that their disease had a high or very high impact on their daily life. The use of reliever medication by both patient groups was also high. The current study shows that greater attention to actual disease control and symptom severity is needed in order to obtain a realistic assessment of patient disease morbidity. Simply asking about patient satisfaction with treatment efficacy and convenience may lead to an under-estimation of this problem.
Abbreviations
ACQ, Asthma Control Questionnaire; CAT, COPD Assessment Test™; COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; HCP, health care provider; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; N, number of cases; OEQ, Onset of Effect Questionnaire; PASAPQ, Patient Satisfaction and Preference Questionnaire; PIFR, peak inspiratory flow rate; pMDI, pressurized metered-dose inhaler; SABA, short-acting β2-agonist; SD, standard deviation; UK, United Kingdom; US, United States.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Informed Consent
In August 2017, prior to patient recruitment, this study received an IRB exemption from the New England Independent Review Board (NEIRB) under the category of research involving the use of a survey.
Acknowledgments
Medical writing support was provided by Allison Michaelis, PhD, of MedErgy (Yardley, PA, USA), which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Wilmington, DE, USA).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Ileen Gilbert is an employee of AstraZeneca. Keiko Wada, Chakkarin Burudpakdee, and Chirag Ghai are employees of IQVIA, which was contracted by AstraZeneca to conduct this research study. Laren Tan is an advisor for AstraZeneca, consultant for Boston Scientific, and speaker for Boehringer Ingelheim, Sanofi Genzyme, and Regeneron. Dr Tan also reports personal fees from AstraZeneca, during the conduct of the study; personal fees from Boehringer Ingelheim, AstraZeneca, Sanofi Genzyme, Regeneron, GlaxoSmithKline, Novartis, and Boston Scientific, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Seven takeaways from the new 2017 CVS health and express scripts formulary exclusion lists, August 3, 2016. Available from: https://www.drugchannels.net/2016/08/seven-takeaways-from-new-2017-cvs.html.
2. Kodjak A. Will your prescription meds be covered next year? Better check! Available from: http://www.npr.org/sections/health-shots/2016/08/15/489790412/will-your-prescription-meds-be-covered-next-year-better-check.
3. Chambers JD, Rane PB, Neumann PJ. The impact of formulary drug exclusion policies on patients and healthcare costs. Am J Manag Care. 2016;22(8):524–531.
4. Nguyen E, Weeda ER, Sobieraj DM, Bookhart BK, Piech CT, Coleman CI. Impact of non-medical switching on clinical and economic outcomes, resource utilization and medication-taking behavior: a systematic literature review. Curr Med Res Opin. 2016;32(7):1281–1290. doi:10.1185/03007995.2016.1170673
5. Tennessee Patient Stability Coalition, Global Healthy Living Foundation. Tennessee patient sentiment toward non-medical drug switching; February 2016. Available from: https://creakyjoints.org/wp-content/uploads/2018/10/GHLF-Tennessee_Patient_Sentiment.pdf.
6. Thomas M, Price D, Chrystyn H, Lloyd A, Williams AE, von Ziegenweidt J. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1. doi:10.1186/1471-2466-9-1
7. Bjornsdottir US, Gizurarson S, Sabale U. Potential negative consequences of non-consented switch of inhaled medications and devices in asthma patients. Int J Clin Pract. 2013;67(9):904–910.
8. Bjornsdottir US, Sigurethardottir ST, Jonsson JS, et al. Impact of changes to reimbursement of fixed combinations of inhaled corticosteroids and long-acting β2-agonists in obstructive lung diseases: a population-based, observational study. Int J Clin Pract. 2014;68(7):812–819. doi:10.1111/ijcp.12473
9. Chrystyn H. Do patients show the same level of adherence with all dry powder inhalers? Int J Clin Pract. 2005;59(suppl 149):19–25. doi:10.1111/j.1368-504X.2005.00723.x
10. Doyle S, Lloyd A, Williams A, et al. What happens to patients who have their asthma device switched without their consent? Prim Care Respir J. 2010;19(2):131–139. doi:10.4104/pcrj.2010.00009
11. Braido F, Lavorini F, Blasi F, Baiardini I, Canonica GW. Switching treatments in COPD: implications for costs and treatment adherence. Int J Chron Obstruct Pulmon Dis. 2015;10:2601–2608. doi:10.2147/COPD.S79635
12. Klijn LS, Hiligsmann M, Evers S, Roman-Rodriguez M, Molen T, Van Boven J. Effectiveness and success factors of educational inhaler technique interventions in asthma & COPD patients: a systematic review. NPJ Prim Care Respir Med. 2017;27:24. doi:10.1038/s41533-017-0022-1
13. Miravitlles M, Montero-Caballero J, Richard F, et al. A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD. Int Chron Obstruct Pulmon Dis. 2016;11:407–415. doi:10.2147/COPD.S91118
14. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. doi:10.1016/j.rmed.2011.01.005
15. Ballinger R, Friedemann C, Golics CJ, Lloyd A, Doyle S. Impact of non-consented switch and subsequent switch in asthma medication: qualitative study of patient perspective in the UK. Value Health. 2014;17(7):
16. SYMBICORT® (budesonide and formoterol fumarate dihydrate) [package insert]. Dunkerque, France: AstraZeneca Dunkerque Production; December, 2017
17. Kozma CM, Slaton TL, Monz BU, Hodder R, Reese PR. Development and validation of a patient satisfaction and preference questionnaire for inhalation devices. Treat Respir Med. 2005;4(1):41–52. doi:10.2165/00151829-200504010-00005
18. Leidy NK, Mathias SD, Parasuraman BM, Patrick DL, Pathak D. Development and validation of an onset of effect questionnaire for patients with asthma. Allergy Asthma Proc. 2008;29(6):590–599. doi:10.2500/aap.2008.29.3164
19. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
20. Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the asthma control questionnaire. Respir Med. 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012
21. Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD assessment test (CAT) scores. BMC Pulm Med. 2011;11:42. doi:10.1186/1471-2466-11-42
22. Devane K, Gilbert I, Davis J, Fox K Disruption in care after a forced formulary switch in inhaled respiratory medications: evaluating the effects of a national Medicare Part D payer’s NDC block on budesonide/formoterol fumarate dihydrate.
23. Belhassen M, Langlois C, Laforest L, et al. Level of asthma controller therapy before admission to the hospital. J Allergy Clin Immunol Pract. 2016;4(5):877–883. doi:10.1016/j.jaip.2016.06.012
24. Braman SS, Carlin BW, Hanania NA, et al. Results of a pulmonologist survey regarding knowledge and practices with inhalation devices for COPD. Respir Care. 2018;63(7):840–848. doi:10.4187/respcare.05717
25. Chrystyn H, van der Palen J, Sharma R, et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. NPJ Prim Care Respir Med. 2017;27(1):22. doi:10.1038/s41533-017-0016-z
26. Dhand R, Mahler DA, Carlin BW, et al. Results of a patient survey regarding COPD knowledge, treatment experiences, and practices with inhalation devices. Respir Care. 2018;63(7):833–839. doi:10.4187/respcare.05715
27. Hanania NA, Braman S, Adams SG, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5(2):111–123. doi:10.15326/jcopdf.5.2.2017.0168
28. Newman S. Improving inhaler technique, adherence to therapy and the precision of dosing: major challenges for pulmonary drug delivery. Expert Opin Drug Deliv. 2014;11(3):365–378. doi:10.1517/17425247.2014.873402
29. Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2017;12:59–71. doi:10.2147/COPD.S117196
30. Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184–191. doi:10.4168/aair.2012.4.4.184
31. Chrystyn H, Price DB, Molimard M, et al. Comparison of serious inhaler technique errors made by device-naïve patients using three different dry powder inhalers: a randomised, crossover, open-label study. BMC Pulm Med. 2016;16:12. doi:10.1186/s12890-016-0169-5
32. Norderud Lærum B, Telg G, Stratelis G. Need of education for dry powder inhaler storage and retention – a patient-reported survey. Multidiscip Respir Med. 2016;11(1):21. doi:10.1186/s40248-016-0057-0
33. Janssens W, VandenBrande P, Hardeman E, et al. Inspiratory flow rates at different levels of resistance in elderly COPD patients. Eur Respir J. 2008;31(1):78–83. doi:10.1183/09031936.00024807
34. Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–179. doi:10.1089/jamp.2012.0987
35. Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–1107. doi:10.1513/AnnalsATS.201702-156PS
36. Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal inspiratory flow rates are associated with chronic obstructive pulmonary disease and all-cause readmissions. Ann Am Thorac Soc. 2017;14(8):1305–1311. doi:10.1513/AnnalsATS.201611-903OC
37. Dransfield MT, Feldman G, Korenblat P, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108(8):1171–1179. doi:10.1016/j.rmed.2014.05.008
38. Lotvall J, Bateman ED, Busse WW, et al. Comparison of vilanterol, a novel long-acting beta2 agonist, with placebo and a salmeterol reference arm in asthma uncontrolled by inhaled corticosteroids. J Negat Results Biomed. 2014;13(1):9. doi:10.1186/1477-5751-13-9
39. Woodcock A, Bleecker ER, Lotvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest. 2013;144(4):1222–1229. doi:10.1378/chest.13-0178
40. Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. doi:10.1016/j.jaci.2004.09.028
41. Corrao G, Arfe A, Nicotra F, et al. Persistence with inhaled corticosteroids reduces the risk of exacerbation among adults with asthma: a real-world investigation. Respirology. 2016;21(6):1034–1040. doi:10.1111/resp.12791
42. Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185–1191 e1182. doi:10.1016/j.jaci.2011.09.011
43. Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–577. doi:10.1164/rccm.200906-0907OC
44. Wells KE, Peterson EL, Ahmedani BK, Williams LK. Real-world effects of once vs greater daily inhaled corticosteroid dosing on medication adherence. Ann Allergy Asthma Immunol. 2013;111(3):216–220. doi:10.1016/j.anai.2013.06.008
45. Kaiser H, Parasuraman B, Boggs R, Miller CJ, Leidy NK, O’Dowd L. Onset of effect of budesonide and formoterol administered via one pressurized metered-dose inhaler in patients with asthma previously treated with inhaled corticosteroids. Ann Allergy Asthma Immunol. 2008;101(3):295–303. doi:10.1016/S1081-1206(10)60495-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




