Back to Journals » Cancer Management and Research » Volume 13
The Guiding Significance of the Number of Positive Sentinel Lymph Nodes in Frozen Section for Intraoperative Axillary Dissection in Early Breast Cancer
Authors Liang C , Li L , Zhu M , Hu J , Yu Y
Received 27 February 2021
Accepted for publication 18 May 2021
Published 17 June 2021 Volume 2021:13 Pages 4803—4810
DOI https://doi.org/10.2147/CMAR.S308796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Chenlu Liang, Liuyi Li, Meizhen Zhu, Jiejie Hu, Yang Yu
Department of Breast Tumor Surgery, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, Zhejiang, 310022, People’s Republic of China
Correspondence: Yang Yu
Department of Breast Tumor Surgery, Zhejiang Cancer Hospital, No. 1, Banshan Road, Hangzhou, 310022, Zhejiang Province, People’s Republic of China
Tel/Fax +86 571 8812 2011
Email [email protected]
Purpose: The results of large randomised trials have changed the treatment strategy of axillary lymph nodes. Axillary lymph node dissection (ALND) can be avoided in some patients with one to two sentinel lymph nodes (SLNs) metastasis based on final paraffin section (FPS) results which called into question the need for intraoperative frozen section (FS). This study aims to assess the guiding value of the number of positive SLN detected via FS for intraoperative ALND.
Patients and Methods: This study retrospectively analyzed data from 3303 patients with breast cancer who underwent SLN biopsy between 2015 and 2019. Combined with the FPS results, FS sensitivity, specificity, and false negative rate (FNR) were calculated and the difference in the number of positive SLNs between FS and FPS was analyzed.
Results: The overall FNR of FS was 23.21%, which was 76.47% in isolated tumor cells, 62.28% in micrometastasis, and 12.09% in macrometastatic disease. The size of SLN metastasis were significantly associated with a higher FNR (p< 0.001). The accuracy rate of the number of positive SLNs detected via FS was 92.62%. Human epidermal growth factor receptor 2 (HER2) (p< 0.03) and Ki67 (p< 0.02) were significant factors affecting the accuracy rate.
Conclusion: FS is a effective method for SLN biopsy, ALND can be avoided in patients with one or two positive SLNs detected via FS.
Keywords: breast neoplasms, sentinel lymph node, frozen section, false negative rate, axillary lymph node dissection
Introduction
Accurate axillary lymph node staging is an important basis for comprehensive treatment of breast cancer. Long-term results from randomized trials have documented that there are no significant differences between sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND) in patients with negative sentinel lymph nodes (SLNs).1–4 SLNB can accurately evaluate the axillary status and reduce complications caused by ALND. Recently, some researchers have tried to establish a less invasive predictive model based on tumor markers or imaging parameters to evaluate lymph node status in pre-operative stage. Okuno et al constructed a prediction model consisting of miR-98, tumor size, and lymphovascular invasion for SLN metastasis with high accuracy in Estrogen receptor (ER)- positive/Human epidermal growth factor receptor 2 (HER2)- negative breast cancer.5 Zhang et al verified that a multiparametric MRI-based radiomics nomogram incorporating the radiomics signature, and MRI-determined axillary lymph node burden had a favorable performance in predicting the SLN burden.6 Although these techniques have high accuracy, prospective clinical studies including a larger number of patients are needed to confirm detection efficiency of this techniques in clinical practice. SLNB is still the standard method for axillary lymph node staging in early-stage breast cancer with clinical negative axilla.
Final paraffin section (FPS) are the recommended gold standard for SLNB.7 However, a second surgery for ALND may be needed in patients with positive SLNs. Intraoperative frozen section (FS) is an effective alternative method for SLNB,8 which can reduce the risks associated with secondary surgery. When SLNs metastasis is detected via FS, ALND can be performed immediately during the operation.9 Therefore, FS is a routine procedure in most institutions.
However, the results of some large randomized controlled clinical trials changed the standard approach to axillary surgery. The American College of Surgeons Oncology Group (ACOSOG) Z0011 study results showing that patients with one or two metastatic SLNs who are treated with breast-conserving surgery, whole breast irradiation, and adjuvant systemic treatment can be spared ALND.10 The International Breast Cancer Study Group (IBCSG) 23–01 study indicated that ALND should be avoided when only micrometastasis is observed in the SLN.11 The AMAROS study reported no additional benefit from ALND when compared to axillary radiotherapy in disease-free survival (DFS) (86.9% in the ALND group vs 82.7% in the radiotherapy group, P = 0.18) for patients with positive SLNs.12 The 15th St. Gallen International Breast Cancer Conference in 2017 recommended that patient with one or two metastatic SLNs and meet the enrollment criteria of the Z0011 study should not undergo ALND. ALND can be replaced with axillary radiotherapy in patients who had had mastectomy and one to two metastatic SLNs.13
However, because of the high false negative rate (FNR) in FS reportedly ranging from 6.7% to 43%,14–17 the accuracy of the number of metastatic SLNs has been controversial. There are no clinical guidelines recommending that ALND should be avoided in patients with positive SLNs detected via FS. Therefore, ALND is performed in one operation when positive SLNs are detected via FS, which may inevitably lead to adverse reactions, such as lymphedema, upper extremity sensory and motor deficits, and alteration in shoulder mobility.
The present study evaluated the accuracy of intraoperative FS and explored whether ALND can be avoided in patients with one to two metastatic SLNs detected via FS by comparing the difference in SLN status between FS and FPS results.
Patients and Methods
Patient Selection
Patients with breast cancer who underwent breast-conserving surgeries or mastectomies with SLNB at Zhejiang Cancer Hospital were retrospectively selected between January 2015 and December 2019. The inclusion criteria were as follows: 1) adult women with invasive breast cancer confirmed by histology; 2) tumor size ≤5 cm (cT1-2) and clinically negative axilla (cN0); and 3) patient did not receive any anti-tumor therapy before surgery. Collected data included age, tumor size, histological type, histological grade, hormone receptor status, HER2 status, Ki67 status, positive number of SLNs, and SLNB and ALND status.
Evaluation of SLN and ALND Strategy
SLNB was performed using a dye-guided method with or without radio-guided methods. Technetium-99m sulfur colloid (Xinkesida Pharmaceutical Technology Co., Ltd, Beijing, China) was injected subcutaneously at 1–3 sites either intradermally above the tumor or peritumorally one day before surgery. A total of 1–2 mL of methylene blue were injected in the same manner 10 min before surgery. Blue-stained and radioactive lymph nodes were considered to be SLNs.
SLNs were examined via FS during the operation. Each node was sliced into 5-μm-thick FS (3–6 levels) and interpreted by cytopathologists immediately after hematoxylin and eosin (H&E) staining. The FS results were then used as a basis for surgeons to determine the need for ALND. FS and remaining unfrozen tissue samples were fixed in formalin and embedded in paraffin that was then cut into 4 μm-thick serial sections at 250μm intervals. Serial sections were stained with H&E and used for a final pathological examination as permanent sections. Immunohistochemistry (IHC) analysis for cytokeratin was performed when suspicious metastases were observed in H&E staining.
Metastases were classified as either micrometastases (0.2–2 mm) or macrometastases (>2 mm) according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual.18 Isolated tumor cells (ITCs), which were defined as tumor foci measuring up to 0.2 mm, were regarded as a positive node. Patients with positive SLNs detected by FS underwent an ALND of level I, II, or higher in the same operative setting.
Histopathological Characteristics
A cut-off value of ≥1% for positively stained nuclei was used as the definition of ER- and progesterone receptor (PR)-positive disease.19 HER2 positivity was defined as a score of 3+ by IHC or as positive on fluorescence in situ hybridization (FISH).20 The histologic grade was determined according to the Scarff-Bloom-Richardson grading system.21 Ki67 was scored as the percentage of positive tumor cell nuclei by counting 500 cells.22 The Ki67 cut-off level for the classification was defined as 20%.23
Data and Statistical Analysis
The FPS results were used as the standard to calculate FNR, false positive rate (FPR), sensitivity, and accuracy of intraoperative FS for SLN. The biopsies were classified as false positive (FS+/FPS−), false negative (FS−/FPS+), true positive (FS+/FPS+) and true negative (FS−/FPS−). FNR for FS was defined as (false negative)/(true positive+false negative). FPR was defined as (false positive)/(true negative+false positive). Sensitivity was defined as (true positive)/(true positive+false negative). Accuracy was defined as (true positive+true negative)/all cases.
Logistic regression models were fitted to estimate the OR to assess the effect of the size of SLNs metastases on FNR and sensitivity. Chi-Square test (and Fisher’s exact test, if necessary) was employed to compare the difference of clinicopathological characteristics between SLNs metastasis coincidence group and non-coincidence group. A 2-tailed p value <0.05 was considered statistically significant. All analyses were performed using SPSS 22 version (IBM Corporation, Armonk, NY, USA)
Results
Patient Characteristics
A total of 3303 cases meeting the inclusion criteria were enrolled in the study. Patient clinicopathological characteristics are presented in Table 1. The average patient age at the time of diagnosis was 51.57 years old (standard deviation, 10.89). The mean number of SLNs was 3.55 (standard deviation, 2.56). Invasive ductal carcinoma was the predominant histological type (90.49%). The tumor histological grades were as follows: 176 (5.33%) were grade I, 1710 (51.77%) were grade II, 850 (25.73%) were grade III, and the remaining 567 (17.17%) were unclear. The main pathological tumor stage was T1 (64.54%), of which T1c (42.29%) accounted for the largest proportion. More than half of all cases were hormone receptor-positive (71.48%) and HER2-negative (70.03%). Ki67 show a high expression in 2040 (61.76%) patients.
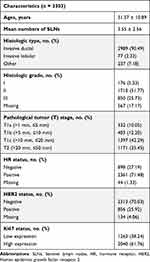 |
Table 1 Clinicopathological Characteristics of Patients |
Diagnostic Performance of FS for SLNs
Positive SLNs were found in 810 (24.52%) FPS specimens. FS was positive in 622 and negative in 188 specimens. FNR was 23.21% (Table 2). Of the 2493 cases with negative SLNs in FPS, eight cases showed metastasis in FS. FPR was 0.32%. In addition, FS sensitivity was 76.79% (622 of 810 cases) and accuracy was 94.07% (3107 of 3303 cases) in this study.
 |
Table 2 Accuracy of SLNs Detection via Frozen Section |
The FNRs for FS detection of macrometastasis, micrometastasis, and ITCs were 12.09%, 62.28%, and 76.47%, respectively, while sensitivity values were 87.91%, 37.72%, and 23.53%, respectively. The smaller the SLN metastases, the higher the odds of a false negative diagnosis (p<0.001, Table 3).
 |
Table 3 FNR and Sensitivity of Frozen Sections in SLNs by Size of SLNs Metastases (n = 810) |
Comparison of SLN Metastasis Number Between FS and FPS
Of the 3303 cases, the number of metastatic SLNs in FS examinations were reported in 420 cases (Table 4). A total of 363 cases with SLNs macrometastasis were found in FS, of which 330 cases had one to two metastases, and 33 cases had more than two metastases. Among the 330 cases with one or two macrometastatic SLNs, 30 cases were diagnosed with more than two macrometastases, 9 cases were micrometastatic, and 1 case was ITCs in FPS. One of the 33 cases with more than two macrometastatic SLNs in FS was diagnosed with one or two macrometastases in FPS. A total of 57 of 430 cases were diagnosed with micrometastases and ITCs via FS, 43 cases were micrometastatic, 14 cases had ITCs, and the number of positive SLNs was all less than or equal to two. After classifying the number of positive SLNs with ≤2 and >2, the positive number of SLNs detected via FS in 31 cases changed in FPS results, while 389 cases did not change. The accuracy rate was 92.62%. Further comparison of clinicopathological characteristics between SLNs metastasis coincidence group and non-coincidence group showed that HER2 (p<0.03) and Ki67 (p<0.02) were significant factors affecting the accuracy rate (Table 5).
 |
Table 4 Comparison of SLNs Number Between Frozen Section and Final Paraffin Section (n = 420) |
 |
Table 5 Comparison of Clinicopathological Characteristics Between SLNs Metastasis Coincidence Group and Non-Coincidence Group |
Non-SLN Metastasis Rate
The rate of non-SLN metastasis was also evaluated in patients with different numbers of positive SLNs in FS (Table 6). The positive rate of non-SLN in patients with macrometastasis was 33.88%, of which patients with 1–2 and >2 macrometastatic SLNs via FS accounted for 30.61% and 66.67%, respectively. The positive rate of non-SLN in patients with micrometastasis and ITCs was 9.30% and 7.14%, respectively. Only 1 of 14 patients diagnosed with ITCs by FS had additional metastasis in lymph nodes removed via ALND.
 |
Table 6 Rate of Non-Sentinel Lymph Node Metastasis in Different Sizes and Numbers of Metastatic SLNs (n= 420) |
Discussion
Intraoperative FS allows patients with positive SLNs to undergo ALND in the same operative setting. However, results from some large clinical studies, such as ACOSOG Z0011, AMAROS, and IBCSG 23–01, had changed the treatment strategy for patients with positive SLNs and called into question the need for intraoperative FS of SLNs. How to avoid unnecessary ALND while retaining the advantages of FS is still worth exploring. There are currently no clinical studies evaluating whether patients with positive SLNs detected by FS can avoid ALND during surgery. Therefore, the present retrospective study was designed to further explore the guiding significance of the number of positive SLNs detected by FS for surgeons in the management of axillary lymph nodes during surgery.
Compared with the Z0011 study, the proportion of tumor stage in this study was similar to that of the Z0011 study, while the proportion of histological grade I and mean age was lower, and the proportion of invasive ductal carcinoma was higher, which may be related to ethnic differences. The average number of SLNs removed in this study was 3.55±2.56, which was higher than that reported in previous studies.24 This is because the SLNB was performed using a dye-guided method with radio-guided methods in most of patients in our institution.25 The hormone receptor status and HER2 status in this study were similar to those reported in previous studie.26
In this study, total sensitivity and FNR values for FS were 76.79% and 23.21%, respectively. Further analysis showed that the size of SLN metastasis was the key factor affecting false negative outcomes and FS sensitivity (p<0.001, Table 3). FS sensitivity values for SLN macrometastasis, micrometastasis, and ITCs were 87.91%, 37.72%, and 23.53%, respectively. FNRs were 12.09%, 62.28%, and 76.47%, respectively. This is consistent with results from previous studies.15,27–29
In this study, 420 cases of FS recorded the number of SLN metastases, which have not been routinely recorded in FS reports (Table 4). In patients with 1–2 positive SLNs detected by FS, 92.25% (357 of 387) of patients had no more than two positive SLNs in FPS. According to Z0011 and AMAROS trial results, ALND can be avoided or replaced by axillary radiotherapy in these patients.10,12 Only 9.09% (30 of 330) of cases with 1–2 SLN macrometastases were confirmed to have more than two positive SLNs in FPS. In all patients with SLN micrometastasis and ITCs detected by FS, the number of SLNs metastasis was ≤2 in FPS. Furthermore, one patient with more than two macrometastatic SLNs in FS was confirmed to have only two metastatic SLNs in FPS. After re-examination by a pathologist, an enlarged lymph node was cut into two parts by a surgeon during the operation, resulting in an increase in the number of positive SLNs in FS.
After classifying the number of positive SLNs with ≤2 and >2, the results of FS and FPS were not consistent in 31 patients. We further analyzed the clinicopathological characteristics of these 31 patients and found that HER2 status (p<0.03) and Ki67 (p<0.02) were significant factors affecting the accuracy rate of the number of positive SLNs (Table 5). Fanizzi et al23 found that the prognostic factors HER2 and Ki67 can help to improve the predictive performance of the CancerMath (CM) model for SLN positive. These results suggest that the HER2 positive and the high expression of Ki67 may be associated with SLN metastasis. It seems to be a good research spot to establish a prediction model based on the number of SLNs detected via FS and combined with HER2 and ki67 to predict the number of SLN metastasis in FPS.
In recent years, some researchers have called into question the need for intraoperative SLNB assessment. Bishop et al suggested that patients who meet the Z0011 clinical criteria had a low probability of finding more than two positive SLNs with at least one macrometastasis during final pathology.30 Intraoperative FS for SLN should not be routinely performed for all breast cancer patients.31,32 However, omitting intraoperative FS will increase the number of subsequent operations for ALND.31 In the present study, patients with more than two positive SLNs identified via FS can undergo ALND during the same surgical procedure, which reduces the second operation rate by 7.62% (32 of 420). However, if the FS is omitted, 14.76% (62 of 420) of patients with more than two macrometastatic SLNs identified during final pathology need a second operation.
In addition, non-SLN metastasis status for different SLN metastasis numbers and sizes detected via FS were explored (Table 6). The total rate of non-SLN metastasis in patients with positive SLNs detected via FS was 30.48% (128 of 420), which was lower than the 33% reported by the AMAROS study (positive SLN defined as macrometastasis, micrometastasis, and ITCs). This can be explained by the fact that SLNs with only ITCs were no longer regarded as SLNs-positive in the AMAROS study after a protocol amendment.12 Excluding patients with more than two positive SLNs, the non-SLN metastasis rate in the remaining patients was 27.39% (106 of 387), which was similar to the 27.3% reported in the ACOSOG Z0011 study (positive SLN defined as macrometastasis, micrometastasis, and ITCs).10 The total non-SLN metastasis rate in patients with SLN micrometastasis and ITCs detected by FS was 8.77% (5 of 57), which was lower than the 13% reported in the IBCSG 23–01 study (positive SLN defined as micrometastasis and ITCs).11 The non-SLN metastasis rate in patients with positive SLNs in FS in this study was similar to that reported in previous studies. This result further confirmed the feasibility of avoiding ALND in patients with one or two SLNs containing metastases identified by FS. Remarkably, in patients with more than two positive SLNs in FS, the rate of non-SLN metastasis was as high as 66.67% (22 of 33). For these patients, ALND should be performed immediately during the operation.
In conclusion, these findings indicate that FS is a safe and effective method for SLNB. More than half of the false negative cases are due to micrometastasis and ITCs and a second operation for ALND is not necessary. The accuracy rate of the number of positive SLNs detected via FS was 92.62%. ALND can be avoided or replaced by axillary radiotherapy in patients with one or two positive SLNs detected via FS during the operation.
Ethical Approval/Informed Consent
This study was approved by the institutional Ethics Committee of Zhejiang Cancer Hospital (IRB-2018-137). The study was conducted in accordance with the ethical principles laid down in the Declaration of Helsinki. We declare that the data was anonymized and maintained with confidentiality. Written informed consent for the use of patient information in this study was obtained from all patients at the time of admission as a routine practice at zhejiang cancer hospital.
Acknowledgments
This research was funded by project of Natural And Science Foundation of Zhejiang Province, No. LY19H160005.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised Phase 3 trial. Lancet Oncol. 2010;11:927–933. doi:10.1016/S1470-2045(10)70207-2
2. Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95(3):279–293. doi:10.1007/s10549-005-9025-7
3. Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600. doi:10.1097/SLA.0b013e3181c0e92a
4. Zavagno G, De Salvo GL, Scalco G, et al. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247(2):207–213. doi:10.1097/SLA.0b013e31812e6a73
5. Okuno J, Miyake T, Sota Y, et al. Development of prediction model including MicroRNA expression for sentinel lymph node metastasis in ER-positive and HER2-negative breast cancer. Ann Surg Oncol. 2020;28(10):310–319. doi:10.1245/s10434-020-08735-9
6. Zhang X, Yang Z, Cui W, et al. Preoperative prediction of axillary sentinel lymph node burden with multiparametric MRI-based radiomics nomogram in early-stage breast cancer. Eur Radiol. 2021:1–16. doi:10.1007/s00330-020-07674-z
7. Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32(13):1365–1383. doi:10.1200/JCO.2013.54.1177
8. Wong J, Yong WS, Thike AA, et al. False negative rate for intraoperative sentinel lymph node frozen section in patients with breast cancer: a retrospective analysis of patients in a single Asian institution. J Clin Pathol. 2015;68(7):536–540. doi:10.1136/jclinpath-2014-202799
9. Akay CL, Albarracin C, Torstenson T, et al. Factors impacting the accuracy of intraoperative evaluation of sentinel lymph nodes in breast cancer. Breast J. 2018;24:28–34. doi:10.1111/tbj.12829
10. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis. JAMA. 2011;305(6):569–575. doi:10.1001/jama.2011.90
11. Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi:10.1016/S1470-2045(13)70035-4
12. Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981–22023 AMAROS trial. Ann Surg Oncol. 2010;17:1854–1861. doi:10.1245/s10434-010-0945-z
13. Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol. 2017;28:1700–1712. doi:10.1093/annonc/mdx308
14. Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999;91:368–373. doi:10.1093/jnci/91.4.368
15. Weiser MR, Montgomery LL, Susnik B, et al. Is routine intraoperative frozen-section examination of sentinel lymph nodes in breast cancer worthwhile? Ann Surg Oncol. 2000;7(9):651–655. doi:10.1007/s10434-000-0651-3
16. Rahusen FD, Pijpers R, Van Diest PJ, et al. The implementation of the sentinel node biopsy as a routine procedure for patients with breast cancer. Surgery. 2000;128(1):6–12. doi:10.1067/msy.2000.107229
17. Canavese G, Gipponi M, Catturich A, et al. Sentinel lymph node mapping opens a new perspective in the surgical management of early-stage breast cancer: a combined approach with vital blue dye lymphatic mapping and radioguided surgery. Semin Surg Oncol. 1998;15(4):272–277. doi:10.1002/(SICI)1098-2388(199812)15:4<272::AID-SSU17>3.0.CO;2-I
18. Edge SB, Byrd DR, Compton CC, eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010.
19. Hammond ME, Hayes DF, Wolff AC, et al. American society of clinical oncology/college of American pathologists Guideline Recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–197. doi:10.1200/JOP.777003
20. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline update. Arch Pathol Lab Med. 2014;138(2):241–256. doi:10.5858/arpa.2013-0953-SA
21. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi:10.1111/j.1365-2559.1991.tb00229.x
22. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–1664. doi:10.1093/jnci/djr393
23. Fanizzi A, Pomarico D, Paradiso A, et al. Predicting of sentinel lymph node status in breast cancer patients with clinically negative nodes: a Validation Study. Cancers. 2021;13(2):352. doi:10.3390/cancers13020352
24. Chung A, Yu J, Stempel M, et al. Is the “10% rule” equally valid for all subsets of sentinel-node-positive breast cancer patients? Ann Surg Oncol. 2008;15(10):2728–2733. doi:10.1245/s10434-008-0050-8
25. Wong SL, Edwards MJ, Chao C, et al. Sentinellymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg. 2001;192:684–689. doi:10.1016/S1072-7515(01)00858-4
26. Nadia H, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):2504–2511. doi:10.1093/jnci/dju055
27. Liu LC, Lang JE, Lu Y, et al. Intraoperative frozen section analysis of sentinel lymph nodes in breast cancer patients: a meta-analysis and single-institution experience. Cancer. 2011;117:250–258. doi:10.1002/cncr.25606
28. Wada N, Imoto S, Hasebe T, et al. Evaluation of intraoperative frozen section diagnosis of sentinel lymph nodes in breast cancer. Jpn J Clin Oncol. 2004;34:113–117. doi:10.1093/jjco/hyh023
29. Jensen AJ, Naik AM, Pommier RF, et al. Factors influencing accuracy of axillary sentinel lymph node frozen section for breast cancer. Am J Surg. 2010;199(5):629–635. doi:10.1016/j.amjsurg.2010.01.017
30. Bishop JA, Sun J, Ajkay N, Sanders MAG. Decline in frozen section diagnosis for axillary sentinel lymph nodes as a result of the American College of Surgeons Oncology Ggroup Z0011 trial. Arch Pathol Lab Med. 2016;140:830–835. doi:10.5858/arpa.2015-0296-OA
31. Yoon KH, Park S, Kim HS, et al. Is the frozen section examination for sentinel lymph node necessary in early breast cancer patients? Ann Surg Treat Res. 2019;97:49–57. doi:10.4174/astr.2019.97.2.49
32. Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364(5):412–421. doi:10.1056/NEJMoa1008108
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
