Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
The Frequency and Predictors of Unsuppressed HIV Viral Load Among People with HIV in Nyaruguru District, Rwanda
Authors Hakizayezu F, Biracyaza E , Niyompano H, Umubyeyi A
Received 24 May 2022
Accepted for publication 29 July 2022
Published 12 August 2022 Volume 2022:14 Pages 381—395
DOI https://doi.org/10.2147/HIV.S376053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
François Hakizayezu,1 Emmanuel Biracyaza,2 Hosee Niyompano,3 Aline Umubyeyi1
1Department of Epidemiology and Biostatistics, College of Medicine and Health Sciences, University of Rwanda, Kigali, Rwanda; 2Programme of Sociotherapy, Prison Fellowship Rwanda, Kigali, Rwanda; 3Department of Laboratory, Mibilizi District Hospital, Kigali, Rwanda
Correspondence: François Hakizayezu; Emmanuel Biracyaza, Tel +250 788681637 ; +250 785686886, Email [email protected]; [email protected]
Background: By the end of 2015, epidemiological studies approximated 37 million people living with HIV (PLHIV) and 46.3% of them were initiated to antiretroviral therapies. From the 90-90-90 strategy, by 2020 at global level, 90% of all people living with HIV were expected to suppress viral load (VL). Although VL suppression is an important indicator of treatment success in PLHIV, studies on this indicator remain scarce in Rwanda where the prevalence of HIV is 3% with 9% for non-suppression. This work, thus, determined the prevalence of VL non-suppression and its associated predictors among PLHIV.
Methods: A cross-sectional study was conducted among 637 PLHIV enrolled in healthcare services between 2016 and 2017 in Nyaruguru district. Socio-demographic, treatment, clinical, immunological and VL data were extracted from medical records. Bivariate and multivariate logistic regression analyses were performed to determine associated factors with VL suppression considering 95% confidence intervals and statistical significance of p< 0.005.
Results: More than half of participants were female (57.77%). The prevalence of unsuppressed HIV VL was 8.9% and 88.7% of respondents were satisfied with the service provided. Males were more likely to be unsuppressed HIV VL [aOR = 3.02; 95% CI (1.19– 7.64), p = 0.02] than females. Higher likelihoods of VL non-suppression were among those with history of clinical failure [aOR = 3.14; 95% CI (1.70– 14.03), p = 0.034] or history of treatment interruption [aOR = 8.29; 95% CI (2.60– 26.42) p = 0.002]. Those with a bad perception toward the whole life treatment were more likely to be unsuppressed [aOR = 4.32; 95% CI (1.98– 18.99), p = 0.049] than their counterparts.
Conclusion: Sex, treatment interruption, bad perception toward the whole life treatment, clinical failure and lack of confidentiality were the major predictors of being unsuppressed. More efforts on counseling HIV patients to improve their knowledge would drop levels of VL non-suppression, so improving the quality of service should be prioritized to increase suppression.
Keywords: unsuppressed, HIV/AIDS, adherence, antiretroviral, suppression
Background
At the end of 2015, 36.7 million of people globally lived with HIV and a majority of them were from low- and middle-income countries (LMICs). Among these cases, more than 2.1 million were new infected cases on an annual basis. In that period, the antiretroviral (ART) coverage was 46.3% among all HIV-infected people.1,2 The number of people accessing ART therapies increased with time as ART is one of the transmission prevention measures and around 18.2 million HIV-infected people were on ART in 2016 worldwide. The number of people who accessed ART treatment in 2010 and 2015 (7.5 million and 15.8 million, respectively) kept increasing; however, people living with HIV with poor adherence to ART therapies remain a public health burden, mainly in Sub-Saharan Africa (SSA) which accounts for more than 70% for non-suppression which decimates the quality of life of those who are affected.2–5 Efforts were combined to decrease new infections and increase quality of life for PLHIV through suppression of VL. Despite those measures such as “test and treat”, sensitization on male circumcision, condom use and the increased access to ART therapies among PLHIV, the VL monitoring on a regular basis for PLHIV is essential for early reaction to non-suppressing among people with HIV.2,3,6 To address this concern, early strategies to achieve an undetectable or suppressed VL are substantial as important ways to prevent HIV transmission and increase quality of life for PLHIV.7,8
Therefore, monitoring treatment success remains the gold standard for viral load testing and control.9 Further, unsuppressed viral load predicts that either drugs are not taken correctly or there is a resistance somewhere.6 The target 90-90-90 set by the Joint United Nations Program on HIV/AIDS in 2014 says that by 2020, 90% of people PLHIV will be aware of their status, 90% of them will access treatment and 90% of them will have HIV suppressed viral load. Moreover, the viral load monitoring coverage in 2014 was only 50%. This low coverage was due to the test cost, the availability of trained staff and limited sites due to the complexity of the testing procedures. This gap in VL monitoring was challenging clinicians in decision making on what to do for HIV-infected people when on follow-up.9,10 Earlier studies documented a lower prevalence of HIV viral suppression and the prevalence differs between countries. A multi-country study conducted by Médecins Sans Frontièrs evidenced that 30% of people for whom treatment failure was suspected, had an elevated viral load, which means that 70% might have been unnecessarily switched to the second-line regimes if no VL test was done to confirm the treatment failure.6,11 The rate of non-suppression of VL was different depending on the country, e.g. 15% in South Africa, 9.6% in Nigeria,12 16% in Swaziland, 20% in Ethiopia,13 29% in Uganda, 23.2% in Cambodia, 14.9% in Zimbabwe, and 27% in Los Angeles. Although VL testing has been important in following-up PLHIV, the above highlighted challenges have been identified worldwide in this strategy to the extent that it becomes difficult to determine early whether a person is suppressing or not, where challenges are still being faced.14–16
The quality of service is among elements that contribute to a good treatment adherence mainly for patients on long-term treatment or whole life treatment. Indicators of the quality of service include confidentiality and short waiting time. It has been seen that the adherence to ART can depend on how a client is satisfied with the service delivery in relation to healthcare providers with professional guiding principles including keeping patients’ personal information confidential and a good treatment outcome. A previous study in Vietnam conveyed that only 42.4% of patients on ART treatment were satisfied with the quality of service. In the same study, only few patients (18.8%) were satisfied with the treatment outcome.17 But in some key aspect of services, this was slightly different; 16% of HIV patients reported to lack privacy when discussing with clinicians about their illness. As far as confidentiality is concerned, the majority of patients (96%) reported to be comfortable with the mechanisms involved in their information keeping in terms of confidentiality and secrecy.18
Preceding studies stated that PLHIV have almost normal life expectancy when suppressing their VL.19 Studies in Vietnam stated that 93% of patients with HIV/AIDS were suppressing and the predictors were sustainability and uninterrupted ARV treatment during the first year of treatments.20 Later studies in South Africa indicated that 85% of patients were suppressing after six months of treatment and the major factors were providing of counseling, appropriate healthcare services, good adherence to ART therapies and psychosocial support.21 Besides, the important factors of lack of suppression were substance use (e.g. alcohol and tobacco) in first month of initiating ART therapies, pregnancies, mental health outcomes (e.g. depression), lack of psychosocial support, side effects of ART therapies, and prior ART exposure.20–22
The main target of initiating ART treatment is to improve someone’s immune system and suppress HIV VL. Although the importance of utilizing effectively ART therapies was documented, factors leading to low suppression of HIV VL are the barriers leading to poor quality of life of the patients with HIV. Socio-demographic characteristics, prolonged intake of alcohol in the first month of treatment initiation, pregnancies, mental health disorders, substance abuse, weak social support, depressive symptomatology, side effects of medications, prior ART exposure, stress, patients’ bad perception towards the illness and treatment, low baseline CD4, ARV regimen, previous treatment failures, long period on ART therapies, drug resistance, drug toxicity, weak social support, sexually transmissible infections (STIs), awareness of viral suppression, comorbidities, poor adherence to treatment, patient relationship with their clinicians and poor diet were documented as the major factors.23,24 Further, all of these factors associated with unsuppressed VL can easily lead to poor adherence, thus, unsuppressed VL.20,25 Indeed, the factors such as low community-based supports mainly in young people or adolescents, co-infections, poor nutrition, social stigma, mismanagement of opportunistic infection, poor quality of healthcare service in term of waiting time, service delivery and irregularity in adherence are important factors that increase the risk to experience non-suppression.26,27
The prevalence of HIV in Rwanda is around 3% across the country and females (3.7%) are at a higher risk for HIV than males (2.2%). While 83% of Rwandan people live in rural zones, HIV is more prevalent in urban (7.1%) compared with rural settings (2.3%).28–30 The VL suppression rate among people living with HIV in Rwanda has been projected to be 9%.26,27 While unsuppressed HIV VL refers to having more than 200 copies of HIV nucleic acids per milliliter of blood after at least one year of treatment,31 only 91% of people with HIV experienced VL suppression. This indicated that 9% of PLHIV have unsuppressed HIV VL. Despite the efforts of the government of Rwanda to manage HIV and its impact on well-being of PLHIV and their caregivers or family members, unsuppressed HIV VL remains a public health burden in Rwanda. There are few studies conducted on the prevalence of non-suppression of VL and its associated predictors in Rwanda. This study, therefore, determined the prevalence of unsuppressed HIV VL and its associated predictors among PLHIV in Nyaruguru District.
Methods
Study Design
This retrospective hospital-based cross-sectional study design was conducted at Nyaruguru District Hospital using the medical records of 637 PLHIV under ART treatments.
Study Settings
The study took place in Nyaruguru district. This district is one of 30 districts of Rwanda, located in the Southern province and counting 323,624 inhabitants. It is a district bordering Burundi. Further, Nyaruguru district borders Huye, Gisagara and Nyamagabe districts. Nyaruguru is composed by 14 sectors where you find 16 health centers and 22 health posts. Nyaruguru district is irrigated by some of Rwanda main rivers such as Akanyaru and Mwogo. Four sectors of this district are bordering Nyungwe National Park. The whole zone of Nyaruguru district is served by Munini District Hospital that has 72 beds with full functional minimum district package. ART therapy is provided in all Nyaruguru health facilities.
Study Population
The study population was 2325 people living with HIV in Nyaruguru district, under antiretroviral therapy who were alive during our study period and who were willing to participate voluntarily in this study. The study included the participants with HIV who were aged 18 years or above. It also included the respondents who had at least one VL result in the period between July 2017 and at the time of conducting this study. The participants who received ART therapies at the health facilities in the period from July 2016 to June 2017 were exhaustively included in the research. People with lack of at least one VL result in period of July 2017 up to the period of data collection were excluded . This research also excluded the people who were not on ART therapies in the period of July 2016 to June 2017.
Sampling Method and Study Sample
A sample size was computed using the formula of Cochran (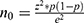 ) based on the recent unsuppressed HIV VL in Rwanda of 9% that was documented in 2018. The parameters from this formula are “
) based on the recent unsuppressed HIV VL in Rwanda of 9% that was documented in 2018. The parameters from this formula are “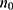 ” stands for the minimal sample size, “z” for the confidence intervals (this equals to 95% or 1.96), “p” for the updated proportion of depression (p = 0.09) and “e” stands for the margin error (e = 0.05). Using this formula (
” stands for the minimal sample size, “z” for the confidence intervals (this equals to 95% or 1.96), “p” for the updated proportion of depression (p = 0.09) and “e” stands for the margin error (e = 0.05). Using this formula (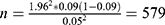 ), a minimum sample size of 579 was found. Then, we added 10% for missing data from medical records and thus the study enrolled 637 medical records.
), a minimum sample size of 579 was found. Then, we added 10% for missing data from medical records and thus the study enrolled 637 medical records.
Study Variables
Outcome variable was unsuppressed HIV viral load. Thus, primary outcome was virological suppression, defined as having ≤ 1000 copies of viral RNA/mL of blood plasma. This classification was provided by the National guideline for treatment and control of HIV/AIDS in 2018. Independent variables comprised socio-demographic and clinical variables. Socio-demographic variables were of age, sex, marital status, occupation, religion, Ubudehe category, residence sector, residence cell, health insurance, level of education and family attitude. Clinical variables included duration of antiretroviral therapy, HIV clinical stage, immunological failure, adherence on treatment, clinical failure, opportunistic disease, physical status, sexual transmission infection, mental health status, pregnancy status, side effects of medications, prior ART exposure and disability. Environmental and behavior factors were variables such as tobacco use, alcohol use, lifestyle, sexual behavior, social stigma, depressive symptomatology, family discrimination, social isolation, drugs use, geographic accessibility and diet.
Data Collection
Data collection was performed by the data clerks and ART therapy providers using close-ended questions in a developed questionnaire. Data were collected using a structured data-abstraction tool customized from Federal Ministry of ARV-therapy patient-intake forms, follow-up charts, and registers. A retrospective review of routinely collected HIV information and viral load test data for patients from March 1 to 20, 2019 was done. Four supervisors and a principal investigator monitored all activity on a daily basis throughout. Data collection included basic patient information, such as socio-demographic characteristics of ARV-therapy patients, clinical and treatment characteristics, laboratory results, information, and comorbidities: STIs, TB infection, and medically diagnosed in communicable disease. All patient files were labeled with numeric numbers and by using Excel, these were a random selection of numbers. After the random selection of numbers, files with selected numbers were picked up to be reviewed for data collection and patients to which files were selected, were interviewed to gain any additional information which could not be found in the file.
Statistical methods
Data were checked, coded, and entered into EpiData Entry Client 3.1 and then cleaned for statistical analyses. Both descriptive and analytical analyses were performed using STATA software version 13.0. In descriptive analyses, statistical parameters such as mean, standard deviation, proportion and frequency were utilized. Univariate analysis was used to describe the respondents and determine the prevalence of non-suppression and quality of adherence to treatments. For analytical analyses, bivariate logistic regression analyses were employed to find out the associated predictors of non-suppression of HIV VL. Then, multivariate logistic regression models were performed based on odds ratios (ORs) to determine associated predictors of unsuppressed HIV VL. Hence, 95% for the confidence intervals, significance levels of p<0.05 and odds ratio were used to identify significant variables and factors associated with unsuppressed HIV VL. Based on the adjusted odds ratio, all significant predictors with unsuppressed HIV VL were stated as significant confounders of unsuppression.
Ethics
This study was approved by the committee of the Institutional Review Board of the College of Medicine and Health Sciences at the University of Rwanda with the reference number (CMHS/IRB/289/2019). All methods of this study were applied in accordance with the standards and regulations of the Declaration of Helsinki.37 The confidentiality in relation to patient information, data management and storage was secured. As the present study used secondary data from medical records, consent forms were not sought from the patients but the hospital provided the authorization to conduct this study.
Results
Socio-Demographic Characteristics of the Study Participants
Nearly two out of three study participants were female (57.77%). Further, the majority was between 38–47 years (36.26%), 71.59% were married, 55.57% had no formal education, 93.72% had health insurance, 44.43% were in Ubudehe category 3, and 86.1% of study participants had family members who were aware of the serologic status (Table 1).
 |
Table 1 Description of Socio-Demographic Characteristics (n = 637) |
Prevalence of Unsuppressed VIH Viral Load and Clinical History
Our result showed that 8.95% of PLHIV were unsuppressed HIV VL while 91.05% were suppressed. Only 55.26% of PLHIV experienced more than 10 years under ART treatment, 83.83% for good adherence, 90.42% for not having interrupted their treatment, 94.03% for having no history of clinical failure, 78.81% for those who received 1 tablet per day, 72.21% for those with no history of having opportunistic diseases, and 89.95% for not having sexual transmitted disease in their history. A majority (95.92%) of the PLHIV indicated that they had no history of mental disease. More than half (54.89%) of females living with HIV experienced no pregnancy during the treatment period while 99.69% of the PLHIV were not exposed to ART before becoming HIV positive (Table 2).
 |
Table 2 Prevalence of Unsuppressed HIV Viral Load and Clinical History |
Behavioral Changes and Satisfaction for the Patients
Among the study participants, 57.14% were consuming vegetables 1–3 days in a week while 62.64% of participants were drinking milk for 1– 3 days in a week, 98.74% had no physical disability, 57.46% were taking 30–60 minutes to reach the health facility, 56.04% were alcohol users, 70.64% had no history of having more than one sexual partner, 78.18% had a good perception towards an incurable disease, 88.7% of participants were satisfied with the service delivery while 86.03% and 83.83% were satisfied with the clinician confidentiality and had a positive attitude toward a whole life treatment, respectively (Table 3).
 |
Table 3 Behavior and Patient Satisfaction |
Relationship Between Socio-Demographic, Behavior and Environment Factors and Viral Non-Suppression
At the end of the analysis of socio-demographic, behavior and environment factors, variables were seen to be probably associated with unsuppressed HIV viral load such as age (p<0.001), gender (p = 0.013), level of study (p =<0.002), awareness of family member on the participant HIV status (p<0.001), history of having more than one sexual partner (p = 0.029), client perception towards incurable illness (p<0.001), client perception towards whole life treatment (p<0.001), level of satisfaction on the service delivery (p = <0.005), level of satisfaction on confidentiality across the whole process of treatment (p =<0.016). The findings after bivariate analysis of socio-demographic, behavior and environmental factors are shown in Table 4.
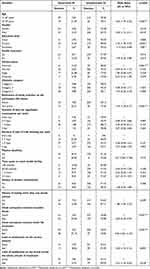 |
Table 4 Bivariate Analysis of Socio-Demographic, Behavior and Environment Factors |
Bivariate Analysis for the Associations Between Non-Suppression and Clinical Characteristics
The analysis of clinical characteristics has shown some variables significantly associated with unsuppressed HIV viral load such as: HIV status of the participant sexual partner (p = 0.007), ARV treatment duration of 1–5 years (p = 0.047), history of clinical failure (p<0.001), history of treatment interruption since ARV initiation (p<0.001), fair quality of adherence (p<0.001), poor quality of adherence (p<0.001), taking 2 or 3 ARV tablets per day (p<0.001), history of opportunistic disease since the HIV positivity (p<0.001) and history of depression since HIV positivity (p<0.001) (Table 5).
 |
Table 5 Bivariate Analysis of Clinical Characteristics of the Study Participants |
Multivariate Logistic Regression Models for the Predictors of Unsuppression Among PLHIV
Our results indicated that males were more likely to have unsuppression [aOR = 3.02, 95% CI (1.19–7.64), p = 0.02] than females. Participants who experienced treatment interruptions were more prone to be unsuppressed [aOR = 8.29, 95% CI (2.60–26.42), p = 0.002] than their counterparts. Those with no good perceptions toward the confidentiality across the treatment processes had greater odds to be unsuppressed [aOR = 1.11; 95% CI (0.82–1.40), p = 0.006] than their counterparts. Respondents with negative perception toward the whole life treatment [aOR = 4.32, 95% CI (1.98–18.99), p = 0.049], history of treatment interruption [aOR = 8.29, 95% CI (2.60–26.42), p = 0.002], history of clinical failure [aOR = 3.14; 95% CI (1.70–14.03), p = 0.034], negative perception toward the confidentiality across the treatment process [aOR = 1.11, 95% CI (0.02–0.53), p = 0.006] were more likely to be unsuppressed that their counterparts (Table 6).
 |
Table 6 Multivariate Logistic Regression Models for the Factors of Viral Load Suppression |
Discussion
The purpose of this study was to determine the prevalence of unsuppressed HIV VL and the factors associated with uncontrolled viral load among people living with HIV in the Nyaruguru district of Rwanda. Our results revealed that the majority (57.77%) of the participants were females. This increased number of females is expected since in Rwanda the prevalence of HIV is higher in females than in males. According to this, it is expected to have so many females living with HIV than males among HIV-infected people on follow up in any health facility.32 This is comparable to previous studies that reported 39.8% were males while 60.2% were females,12,33 although Basavaprabhu et al found 69% of males and 31% of females in their study.24
In this study, the prevalence of unsuppressed HIV viral load among our participants was 8.95%. This is comparable to the findings of Rangarajan et al that reported 7% for unsuppressed HIV VL in PLHIV.20 But our findings are slightly different from the findings of Limmade et al that documented 20% for not suppressing HIV VL.21 This good suppression prevalence is due to extensive efforts of the Rwandan Ministry of Health and Rwanda Biomedical Center in following up closely people living with HIV and by implementing the principle of “test and treat” where people are tested and put on ARVs directly after being identified as HIV positive.32
We measured the patient satisfaction toward the service delivery, where we found that 88.7% were positively satisfied with the quality of the service. The satisfaction rate is good due to daily sensitization on good service delivery which is included in all levels of public and private sectors to meet the customer’s need. In agreement with preceding studies, our findings revealed that males were more likely to be unsuppressed PLHIV when compared with females. Hence, in our study males were almost five times more likely to have unsuppressed HIV VL than females. These findings corroborate with findings from previous studies.35 Due to their lifestyle, such as alcohol consumption, sexually active, movement into or outside the country for economic issues, low level of using health services, etc, males are more likely to interrupt the treatment and finally have poor adherence which can lead them to have higher rates of unsuppressed HIV VL compared with females. These results are relevant to previous studies.34
Regarding the consumption of medication, patients with interrupted treatment were almost eight times more likely to have unsuppressed HIV compared with those who are having all prescribed doses without interruption. These findings are relevant to preceding studies conducted in SSA countries such as Kenya which reported that patients interrupting treatment were almost three times more likely to have unsuppressed HIV VL compared with their counterparts.36 Regarding attitudes towards whole life treatments, negative perception to the whole life treatment was significantly associated with unsuppressed HIV VL. This finding fitted with prior studies that found that a bad perception toward the whole life treatment was significantly associated with non-suppressed viral load in adolescents. As with other drugs, ARV have a limited duration in someone’s body. Any drug interruption decreases the synergic capacity of ARV then the virus can perform its metabolism so that new viruses are produced.
Our results revealed that a poor quality of service such as regarding confidentiality was significantly associated with unsuppressed HIV VL. Thus, our result proved that PLHIV with poor quality of services were more likely to be unsuppressed while PLHIV with bad perception toward keeping of confidentiality were more likely to have unsuppressed HIV VL when compared with those with a good perception toward secrecy regarding the illness. When the patient does not believe in the clinician in terms of secret keeping and confidentiality, the adherence becomes poor and the motivation of attending the health facility is not good and all of these can even lead to periodic treatment interruption. This finding corroborates with preceding research which established that confidentiality and disease secrecy are key in quality of service where lack of confidentiality increased the risk to be unsuppressed HIV VL.25 In cases of poor quality of service mainly when confidentiality is not respected, some patients will have poor adherence characterized by irregular ART intake and as said above, this can lead to poor treatment outcome.
This study is not without limitations. First, we reviewed records of patients with viral load test results, which may have resulted in underestimation of the actual proportion of patients on ARV therapy with unsuppressed viral loads. In addition, such adherence as on-time drug pickups, pill counts, and other factors that could affect adherence, such as alcohol consumption, mental health status, and psychosocial factors, (depression and stigma) were not included in the medical records. Despite these limitations, this study has some strengths. First, the results are novel because a number of additional factors, such as the number of days of vegetable and milk consumption, were explored in the study. Second, this study has implications for nutritional and dietary modifications for improving the health outcomes in this population.
Conclusion
Our study found that the prevalence of viral non-suppression among the people with HIV was 8.95%. Given factors identified as being associated with unsuppressed HIV VL such as male gender, treatment interruption, whole life treatment, confidentiality of health care provider and clinical failure, improving adherence and quality of service will help in having suppressed VL, and patients’ continuous education is needed to sensitize on adherence so that they will avoid drugs interruption which was highlighted as a cause of unsuppressed VL. Psychological support is also needed to be involved in patient follow up given that lack of confidentiality and negative perception toward the whole life treatment were found to be associated with unsuppressed VL. Based on the findings from this study, the Rwanda Ministry of Health through the Rwanda Biomedical Center should reinforce the regular monitoring of peripheral health facilities and try to do a regular analysis of data from those health facilities for early tracking of some factors associated with unsuppressed HIV VL such as irregular adherence and treatment failure. Further, the District Hospital supervision and HIV mentor team should intervene early at all levels of the catchment area in cases of irregular adherence and treatment interruption as well as any other suspected factor. Lastly, health care providers should increase their capacity in terms of confidentiality management and good service delivery. Information, Education and Communication (IEC) from all health care providers to people living with HIV, should focus on how to live with incurable disease and whole life treatment.
Data Sharing Statement
All relevant data are included in this manuscript. Data may be shared upon reasonable request to the corresponding author.
Ethics approval
The ethical approval was obtained from the Institutional Review Board, the College of Medicine and Health Sciences (IRB/CMHS) at the University of Rwanda with the reference number (CMHS/IRB/289/2019). Confidentiality and privacy of the participants were kept. All data were anonymously collected and kept. All methods were conducted in accordance with the standards and regulations of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Authors of the current study assert that they received no financial support for the research, authorship and/or publication of this study.
Disclosure
The authors declare no conflicts of interests with respect to this work, the research, authorship and/or publication of this work.
References
1. Joseph Davey D, Abrahams Z, Feinberg M, et al. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29:603–610. doi:10.1177/0956462417748859
2. World Health Organization. Global health sector strategy on HIV 2016–2021: towards ending AIDS. Available from: https://apps.who.int/iris/bitstream/handle/10665/246178/WHO-HIV-2016.05-eng.pdf.
3. Chipeta J, Schouten E, Aberle-grasse J. HIV prevalence and associated; 2009:225–241. Available from: https://dhsprogram.com/pubs/pdf/FR175/12Chapter12.pdf.
4. Cherutich C, Kurth A, Musyoki H, Kilonzo N, Maina W. HIV Self-testing in Sub-Saharan Africa: Strategies to Enhance and Measure Linkage to Care. Retrovirology. 2014;6:23. doi:10.4137/RRT.S12952
5. Williams BG, Korenromp EL, Gouws E, Dye C. The rate of decline of CD4 T-cells in people infected with HIV. arXiv preprint arXiv:0908.1556. 2009;4:3–4.
6. UNAIDS. The need for routine viral load testing: questions and answers; 2016:12.
7. Jackson-gibson M, Ezema AU, Orero W, et al. Facilitators and barriers to HIV pre- exposure prophylaxis (PrEP) uptake through a community-based intervention strategy among adolescent girls and young women in Seme Sub-County, Kisumu, Kenya. BMC Public Health;2021. 1–13. doi:10.1186/s12889-020-10013-y
8. Uganda Ministry of Health. Consolidated guidelines for Prevention and treatment of HIV. Kampala; 2016.
9. Minchella PA, Chipungu G, Kim AA, et al. Specimen origin, type and testing laboratory are linked to longer turnaround times for HIV viral load testing in Malawi. PLoS ONE. 2017;12(2):e0173009. doi:10.1371/journal.pone.0173009
10. Lehrer RI. Editorial Response: questions and answers about defensins. Clin Infect Dis. 1997;25(5):1141–1142. doi:10.1086/516082
11. Viral HIV, Monitoring L. Viral load toolkit an implementer ’ s guide to introducing; 2020.
12. Abdullah S, Ibrahim O, Okeji A, et al. Viral suppression among HIV-positive patients on antiretroviral therapy in northwestern Nigeria: an eleven-year review of tertiary care centre records, January 2009-December 2019. BMC Infect Dis. 2021;21:1031. doi:10.1186/s12879-021-06722-3
13. Waju B, Dube L, Ahmed M, Assefa SS. Unsuppressed viral load level in public health facilities: nonvirological predictors among adult antiretroviral therapy users in southwestern Ethiopia. HIV/AIDS - Res Palliat Care. 2021;13:513–526. doi:10.2147/HIV.S304653
14. Lecher SL, Fonjungo P, Ellenberger D, et al. HIV viral load monitoring among patients receiving antiretroviral therapy - eight Sub-Saharan Africa Countries, 2013–2018. MMWR Morb Mortal Wkly Rep. 2021;70(21):775–778. doi:10.15585/mmwr.mm7021a2
15. Stevens WS, Marshall TM. Challenges in implementing HIV load testing in South Africa. J Infect Dis. 2010;201(s1):S78–84. doi:10.1086/650383
16. Drain PK, Dorward J, Bender A, et al. Point-of-care HIV viral load testing: an essential tool for a sustainable global HIV/AIDS response. Clin Microbiol Rev. 2019;32(3):e00097–e18. doi:10.1128/CMR.00097-18
17. Tran BX, Phuong N, Nguyen T. Patient satisfaction with HIV/AIDS care and treatment in the patient satisfaction with HIV/AIDS care and treatment in the decentralization of services delivery in Vietnam; 2012.
18. Chimbindi N, Bärnighausen T, Newell M. Patient satisfaction with HIV and TB treatment in a public programme in rural KwaZulu-Natal: evidence from patient-exit interviews. BMC Health Serv Res. 2014;14. doi:10.1186/1472-6963-14-32
19. Katz IT, Maughan-Brown B. Improved life expectancy of people living with HIV: who is left behind? Lancet HIV. 2017;4(8):e324–e326. doi:10.1016/S2352-3018(17)30086-3
20. Rangarajan S, Colby DJ, Giang LT, et al. Factors associated with HIV viral load suppression on antiretroviral therapy in Vietnam. J virus Erad. 2016;2(2):94–101. doi:10.1016/S2055-6640(20)30466-0
21. Rodriguez-fernandez R, Id YL, Fransisca L, Id RR. HIV treatment outcomes following antiretroviral therapy initiation and monitoring: a workplace program in Papua, Indonesia HIV treatment outcomes following antiretroviral therapy initiation and monitoring: a workplace program in Papua, Indonesia. Plos One. 2019;14:e0212432.
22. Cherutich P, Kim AA, Kellogg TA, et al. Detectable HIV viral load in Kenya: data from a population-based survey. PLoS ONE. 2016;11(5):e0154318. doi:10.1371/journal.pone.0154318
23. Davey J. Factors associated with unsuppressed viral load in HIV-1 infected patients on 1st line antiretroviral therapy therapy in South Africa. South African AIDS Conf. 2017;29:603–610.
24. Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to antiretroviral therapy among people living with HIV. North Am J Med Sci. 2013;5(3):220–223. doi:10.4103/1947-2714.109196
25. Chhim K, Mburu G, Tuot S, et al. Factors associated with viral non- suppression among adolescents living with HIV in Cambodia: a cross-sectional study. AIDS Res Ther;2018. 1–10. doi:10.1186/s12981-018-0188-9
26. International Epidemiology Databases to Evaluate AIDS (IEDEA). Report from the 12th International Conference on HIV Treatment, Pathogenesis and Prevention Research (INTEREST); 2018:1–39. Kigali, Rwanda. Available from http://interestworkshop.org/wp-content/uploads/2018/07/INTEREST-2018-Final-Report_10072018-1.pdf. August 5, 2022.
27. Rwanda Ministry of Health. Republic of Rwanda; Ministry of Health. Rwanda National HIV and viral hepatitis annual report; 2017-2018. Available from https://rbc.gov.rw/fileadmin/user_upload/report2019/report2019/Annual%20Report%20for%20HIV%20and%20Viral%20Hepatitis%202017-2018.pdf. Accessed August 5, 2022.
28. Mwumvaneza M, Hinda R, Nyankesha E, Mugwaneza P, Sebaziga G, Koleros A. HIV and AIDS in Rwanda, 2010 epidemiologic update. Change. 2010;2015:45.
29. El N, Head MPH. Xvii international aids conference. Aids. 2008;5:3–8.
30. Prevalence HI, Quintile HW. HIV prevalence: data from the 2010 Rwanda Demographic and Health Survey Demographic and Health Survey (RDHS). 2010. doi:10.1111/j.1756-185X.2010.01533.x
31. Collins JB, Verheyden CN, Mahabir RC. Core measures. Plast Reconstr Surg. 2013;131(6):1266–1271. doi:10.1097/PRS.0b013e31828bd17e
32. Rwanda Biomedical Center (RBC). Rwanda national HIV annual report 2015–2016. Rwanda Ministry of Health (RMOH); 2016:1–103. Available from: http://rbc.gov.rw/fileadmin/user_upload/report2019/report2019/Annual%20Report%20for%20HIV%202015-2016.pdf.
33. Negesa L. iMedPub Journals adherence to antiretroviral therapy and factors affecting among people living with HIV/AIDS and taking antiretroviral therapy, Dire Dawa Town, Eastern Ethiopia Abstract; 2017:1–6.
34. Davey DJ, Abrahams Z, Feinberg M, Prins M. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29:603–610.
35. Hicham T, Ilyas E, Tarik H, et al. Risk factors associated with unsuppressed viral load in HIV-1 infected patients at the first antiretroviral therapy in Morocco. Int J Mycobacteriol. 2019;8:113–117. doi:10.4103/ijmy.ijmy_41_19
36. Cherutich P, Kim AA, Kellogg TA, et al. Detectable HIV viral load in Kenya: data from a population-based survey. 2016;5:1–14.
37. Millum J, Wendler D, Emanuel EJ. Declaration of Helsinki and Protection for Vulnerable Research Participants—Reply. JAMA. 2014;311(12):1252–1253 doi: 10.1001/jama.2014.1281
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
