Back to Journals » Journal of Inflammation Research » Volume 14
The Flavonoid Kurarinone Regulates Macrophage Functions via Aryl Hydrocarbon Receptor and Alleviates Intestinal Inflammation in Irritable Bowel Syndrome
Authors Xu X, Dong Q, Zhong Q, Xiu W, Chen Q, Wang J, Zhou Z
Received 15 July 2021
Accepted for publication 24 August 2021
Published 3 September 2021 Volume 2021:14 Pages 4347—4359
DOI https://doi.org/10.2147/JIR.S329091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiang Xu,1,* Qiwei Dong,1,* Qingling Zhong,2 Wenbo Xiu,1,2 Qinyuan Chen,1,2 Jinxia Wang,1 Zhou Zhou1,2
1Clinical Immunology Translational Medicine Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 2Department of Gastroenterology and Hepatology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhou Zhou
Clinical Immunology Translational Medicine Key Laboratory of Sichuan Province, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
Tel +86-028-87394201
Fax +86-028-87394201
Email [email protected]
Background: Irritable bowel syndrome (IBS) is characterized with abdominal pain, bloating, and changes in bowel habits, and dealing with IBS is still a clinical challenge. The pathogenesis of IBS has been reported to be linked to low-grade mucosal inflammation, and macrophages contribute to the pathological process of this disease. Kurarinone (KAR), a flavanoid derived from Sophora flavescens, has been found medically effective in many inflammatory conditions and cancers. KAR was previously reported to inhibit LPS-induced expression of inflammatory cytokines in macrophages, whether and how KAR regulates the functions of macrophage in IBS remains to be elusive.
Methods: We established a TNBS-induced IBS mouse model, in which KAR was administrated, and mucosal cytokine expression was measured by qRT-PCR. Additionally, mouse macrophages were generated in vitro and their responses to LPS were evaluated by flow cytometry and qRT-PCR. AhR+/+ or AhR−/- macrophages were transferred into DTx-treated CD11b-DTR transgenic mice to investigate the role of AhR in IBS. We collected colonic biopsies and peripheral blood samples from 64 patients with IBS, and analyzed AhR expression by qRT-PCR.
Results: We found KAR effectively alleviated visceral hypersensitivity and maintained intestinal barrier functions in mice with IBS. KAR inhibited LPS-induced macrophage activation and expression of pro-inflammatory genes, while increased anti-inflammatory gene expression including IL-10 in an AhR-dependent manner. Using macrophage-depleted mice, we found that chimera mice with AhR−/- macrophages were more susceptible to TNBS-induced IBS and the therapeutic effect of KAR on IBS was significantly impaired in mice with AhR−/- macrophages. Additionally, we found AhR expression in macrophages of IBS patients was associated with the disease severity.
Conclusion: Our findings provide new evidences that KAR regulates IBS development via macrophage-intrinsic AhR. KAR might show promise as an immunomodulatory therapeutic agent in treating IBS.
Keywords: aryl hydrocarbon receptor, irritable bowel syndrome, macrophage, kurarinone, flavonoid
Introduction
Irritable bowel syndrome (IBS) is a common condition affecting the digestive system, characterized with abdominal pain, bloating, and changes in bowel habits. The prevalence rate of patients with IBS in China who meet the Rome III diagnostic criteria has reached 12.9%, which has become one of the common diseases of the digestive tract.1 Therefore, dealing with IBS is still an important clinical challenge. Although the exact etiology and pathogenesis of IBS are still unclear, accumulating evidences also show that dysregulated gut microbiota and imbalanced mucosal immunity are critical factors in the pathogenesis of IBS.2
IBS has been reported to be linked to low-grade mucosal inflammation.3,4 About 25% of IBS patients had symptoms of gastroenteritis, dysentery and other infectious diseases. Mucosal inflammation in IBS patients is often observed, probably attributing to compromised epithelial barrier functions and the translocation of luminal bacteria, parasites or viruses, which might abnormally initiate immune responses in the lamina propria.2,5 The activation of mucosal immune system in IBS patients is significantly up-regulated, including increased immune cell infiltration and inflammatory cytokine expression.5 Intestinal mucosa biopsies from patients with IBS show an increased number of mast cells, T lymphocytes and other immune cells.6–8 In addition, there are a number of macrophages in the intestine, which play an important role in maintaining the microbial homeostasis on the surface of intestinal mucosa and regulating the continuous renewal of intestinal epithelial cells.9 Macrophages are not only an important part of innate immunity, but also participate in the development of adaptive immune cells, which they cooperate with to contribute to the pathological process of intestinal inflammation, such as inflammatory bowel disease (IBD) and IBS.10 However, the underlying pathogenesis of these changes in immune responses is still largely unclear.
Kurarinone (KAR), a flavonoid derived from Sophora flavescens, has been reported to be medically effective in many inflammatory conditions and cancers.11–13 KAR exerts potent antioxidant and immunosuppressive functions via activating an array of antioxidant enzymes by abrogating the KEAP1 inhibition of Nrf2, which is well-known as a key transcription factor in the antioxidant defense system.14 Moreover, KAR was found to inhibit the process of experimental autoimmune encephalomyelitis (EAE) via blocking Th1 and Th17 cell differentiation12 Although KAR was previously reported to inhibit LPS-induced expression of inflammatory cytokines in RAW264.7 cells (a macrophage cell line),14 whether and how KAR regulated the functions of macrophage in IBS remains to be elusive. In this study, we found KAR effectively alleviated visceral hypersensitivity and inflammatory responses in a post-inflammatory (PI)-IBS mouse model, and its regulation of the anti-inflammatory functions of macrophage via aryl hydrocarbon receptor (AhR) was indispensable for this therapeutic effect. Additionally, we found AhR expression in macrophages of IBS patients was closely associated with the disease severity. Our results shed new lights on the mechanisms of how KAR regulates macrophage functions, which might be a potential therapeutic drug for IBS.
Materials and Methods
Patients
A total of 64 patients with IBS diagnosed according to the Rome III criteria and 37 healthy controls were all recruited from the Department of Gastroenterology and Hepatology, Sichuan Provincial People’s Hospital (Chengdu, China). Constipation-predominant IBS (IBS-C) was present in 21 patients (5/21 patients had a history of gastroenteritis), diarrhea-predominant IBS (IBS-D) in 26 patients (3/26 had suffered from gastroenteritis), and IBS with mixed bowel habits (IBS-M) in 17 patients. Colonic biopsies were collected during colonoscopy procedures, which were used for mRNA expression. For cell isolation, fresh peripheral blood samples were collected using EDTA-anticoagulated blood collection tubes (BD green cap, BD Biosciences, San Diego, CA, USA). Blood sera were collected using serum separator tube (BD red cap, BD Biosciences) and cytokine measurement was performed as described previously.15 The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board for Clinical Research of the Sichuan Provincial People’s Hospital. All subjects signed an informed consent before participation.
Animal
AhR± mice and CD11b-DTR transgenic mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Wild type (WT) mice were purchased from the Shanghai Model Organisms (Shanghai, China). All mice enrolled in the current study were male and on a C57BL/B6J background and used at their age of 8–10 weeks. All mice were maintained in our facility under specific pathogen-free (SPF) conditions. According to our recent report,16 macrophages were removed in CD11b-DTR transgenic mice using diphtheria toxin (DTx) and the removal efficiencies were verified by flow cytometry, showing over 75% of CD11b+ macrophages were removed. All animal experiments in this study complied with the ARRIVE guidelines, and were approved by the Animal Care and Use Committee at Sichuan Provincial People’s Hospital and performed in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
Peritoneal Macrophage Isolation and Adoptive Transfer
Mouse peritoneal cell suspension were prepared. After being sacrificed, mice were injected with 5 mL of PBS in the peritoneal cavity and the anterior and lateral walls of the abdomen were gently massaged for 5 minutes. Peritoneum washes were collected, which were then filtered through a nylon filter (70 µm) and macrophages (F4/80+ cells) were isolated from peritoneal cell suspension by using the Macrophage Isolation Kit (Peritoneum), an MS Column, and a MiniMACS™ Separator. Then, AhR+/+ or AhR−/- macrophages (2 × 106 per recipient mice) were transferred intravenously into CD11b-DTR transgenic mice, which had underwent macrophage depletion.
IBS Mouse Model, Abdominal Withdrawal Reflex (AWR), and Visceromotor Response (VMR) Test
Mice were received with a single enema of 2,4,6-trinitrobenzenesulfonic acid (TNBS) to induce colonic inflammation as described previously.15 Five weeks after TNBS insults, the AWR test was used to evaluate the visceral hypersensitivity in response to colorectal distention (CRD) in mice.17 Briefly, anesthetized mice were inserted intrarectally with a silicone balloon-urethral catheter and the balloon was distended at indicated volumes for 30 seconds every 4 minutes one hour after these mice revived. The AWR scoring standard was documented elsewhere.18 In addition, we also measured the abdominal electromyography activity to determine the visceral hypersensitivity. Briefly, CRD was performed at indicated volumes as described above, and then VMR was recorded by quantifying reflex contractions of the abdominal musculature. Two electrodes (diameter: 0.08 mm) were implanted in the abdominal external oblique muscle and a third in the abdominal skin. Abdominal electromyography activity was recorded (amplification: 10,000×). The baseline of electromyographic activity was subtracted from that recorded during distension. All animal experiments were approved by the Animal Care and Use Committee at Sichuan Provincial People’s Hospital.
Colon Transportation Test (CTT) and Stool Consistency Evaluation
The status of mouse intestinal motility was evaluated using CTT as reported elsewhere.18 Briefly, mice were administered intragastrically with active carbon in the stomach, and the time these mice took to defecate the first black stool was monitored. In addition, we collected the total stool within 8 hours and evaluated their consistency by Bristol stool grade. Normal shaped stool was defined as 1 point, soft or deformed stool as 2 points, and water-like stool as 3 points.
Generation of Mouse Bone Marrow-Derived Macrophages (BMDM) and Human Monocyte-Derived Macrophages (MDM)
Mouse bone marrow cells and human peripheral blood monocytes were obtained as described previously.16 For mouse BMDM generation, bone marrow cells were incubated with macrophage colony-stimulating factor (M-CSF, 10 ng/mL) in the Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% fetal bovine serum (FBS).19 After 7 days of incubation, flow cytometry was performed to evaluate the formation of mature BMDM, and > 90% of these cells co-expressed CD11b and F4/80. For human MDM, purified peripheral monocytes were cultured in the presence of M-CSF (10 ng/mL) plus granulocyte–macrophage colony-stimulating factor (GM-CSF, 1 ng/mL) in RPMI-1640 supplemented with 10% FBS for 5 days. The formation evaluation was performed as described above.20
Measurement of Intestinal Barrier Function
Intestinal barrier function was determined as reported previously.16 After being starved overnight, mice were orally administrated with FITC-dextran (2 mg/10 g body weight, Sigma-Aldrich, St. Louis, MO, USA) by gastric gavage 4 hours prior to sacrifice. Blood samples were obtained by cardiac puncture, and serum separator tubes (BD red cap) were used to collected mouse sera. The fluorescence intensity of FITC-dextran in sera was assessed (excitation: 485nm, emission: 525 nm) using an Epoch 2 reader (BioTek, Winooski, VT). Serum LPS concentration was measured with a quantitative chromogenic limulus amoebocyte lysate (LAL) QCL-1000 test kit (LONZA), following the manufacturer’s protocols.
Quantitative Real-Time PCR (qRT-PCR)
As reported previously,16 Trizol (TransGen Biotech, Beijing, China) was used to extracted total RNA from cells or tissues. Reversible transcription PCR was performed to transcribe the total RNA into cDNA by a reverse transcription kit (TransGen Biotech). A SYBR Green PCR kit (TaKaRa, Japan) was used to carried out qRT-PCR in a fluorescence thermocycler (Roche Diagnostics, Germany) and primers used in this study were listed in Supplementary Table 1. An Epoch 2 reader (BioTek, Winooski, VT) was used to acquire the data from qRT-PCR assays.
Flow Cytometry
Flow cytometric analysis of cells cultured in vitro and cells obtained from tissues was carried out as described previously.16 Briefly, intracellular cytokine staining was measured using the Mouse IL-10 Secretion Assay-Detection Kits purchased from Miltenyi Biotec (Bergisch Gladbach, Germany), cell surface molecules were examined using anti-mouse antibodies including APC-conjugated CD80, PE-conjugated CD86 (Miltenyi Biotec), and PE-conjugated, CD45, PerCP/Cy5.5-conjugated CD11b, PE-conjugated CD11b, PE/Cy7-conjugated Ly6G, Pacific Blue-conjugated Ly6C, FITC-conjugated F4/80, Brilliant Violet 510-conjugated MHC II, FITC-conjugated IFN-γ (BioLegend, San Diego, CA, USA), and T cell proliferation was evaluated using a CellTrace™ CFSE Cell Proliferation Kit (Invitrogen, Thermo Fisher Scientific).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 7 (San Diego, CA, USA). Data were presented as mean values ± SD. Student’s t test (paired, two-tailed, between 2 groups) or one-way analysis of variance (ANOVA, ≥ 3 groups) was used to perform comparisons. *P < 0.05 was accepted as significantly different.
Results
KAR Alleviates Visceral Hypersensitivity and Mucosal Inflammatory Responses in Mice with IBS
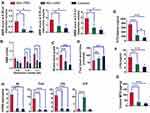
To investigate whether KAR was effective for IBS, we established a TNBS-induced IBS mouse model. As shown in Figure 1A, IBS mice suffered from significantly increased visceral hypersensitivity compared with TNBS-unexposed control mice. Next, we found that KAR treatment potently reduced the AWR scores at all distention volumes in a dose-dependent manner (Figure 1A, Supplementary Figure 1). This effect was also verified by visceromotor response (VMR) assessment (Figure 1B). In addition, the colon transportation test (CTT) was used to evaluate the intestinal motility alteration. We used the Bristol stool grade and monitored the first black stool time to show that the intestinal motility was obviously increased in IBS mice compared with TNBS-unexposed control mice, which was largely reversed by KAR treatment (Figure 1C and D). These data suggest that KAR could effectively relief the clinical presentations in IBS mice. Furthermore, compromised intestinal barrier function and overexpression of inflammatory mediators have been indicated in the development of IBS. To this end, we first measured the permeability of the intestinal barrier by analyzing the FITC-dextran and LPS concentrations in the serum of mice, and found that serum FITC-dextran and LPS levels were greatly elevated in IBS mice, both of which were reduced by KAR treatment (Figure 1E and F). Subsequently, we examined levels of several inflammatory cytokines in the colon, and showed that KAR remarkably decreased MPO expression (an indicator of neutrophil activity, Figure 1G) and suppressed the expression of IL-6, TNF-α, and IL-1β in IBS mice. Notably, we found that colonic IL-10, a potent anti-inflammatory cytokine, was significantly up-regulated in KAR-treated IBS mice (Figure 1H). These data suggest that KAR might exert a therapeutic effect on IBS via inhibiting mucosal inflammation and enhancing anti-inflammatory responses.
KAR Mediates Macrophage Phenotype in the Colon of Mice with IBS
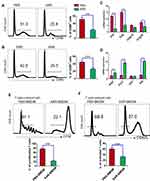
Since macrophages were well-known to play a role in mucosal inflammation in intestinal inflammatory diseases. We analyzed the phenotype and function of colonic macrophages in IBS mice. We used an established flow cytometric gating strategy21,22 to determine macrophages (Figure 2A) and found that KAR treatment reduced the frequency of total macrophages (CD45+ CD11b+ CD11c− Ly6G− F4/80+) in IBS colons (Figure 2B). Next, we analyzed the phenotype of macrophages by using additional markers (Ly6C and MHC II) and found that the frequency of pro-inflammatory macrophages (CD45+ CD11b+ CD11c− Ly6G− F4/80+ Ly6C+ MHCII−) was significantly decreased (Figure 2C) but the anti-inflammatory subset (CD45+ CD11b+ CD11c− Ly6G− F4/80+ Ly6C−) was up-regulated (Figure 2D) in KAR-treated IBS mice than PBS-treated IBS mice. Moreover, we sorted colonic macrophages from IBS mice with or without KAR by flow cytometry and measured a panel of cytokines and transcriptional factors. qRT-PCR revealed that KAR decreased colonic macrophage expression of IL-1β, TNF-α and iNOS, while increased their expression of Arg1 and IL-10 (Figure 2E), suggesting that KAR might be able to inhibit pro-inflammatory responses in macrophages by imprinting an immunoregulatory property on them.
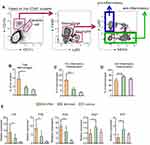 |
Figure 2 KAR mediates macrophage phenotype in the colon of mice with IBS. IBS mouse model was established and KAR was administrated as described in Figure 1. Colonic macrophages were determined by flow cytometry. (A) Gating strategy. (B) The frequencies of total macrophages (CD45+ CD11b+ CD11c− Ly6G− F4/80+), (C) pro-inflammatory subpopulation (CD45+ CD11b+ CD11c− Ly6G− F4/80+ Ly6C+ MHCII−), and (D) anti-inflammatory subpopulation (CD45+ CD11b+ CD11c− Ly6G− F4/80+ Ly6C−) were presented in bar charts. (E) Colonic macrophages were sorted by flow cytometry and their transcript expression levels of IL-1β, TNF-α, iNOS, Arg1 and IL-10 were measured by qRT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA). n = 8 mice in each group. Representative results from one of three independent experiments were shown. |
KAR Mediates Macrophage Phenotype and Function via AhR
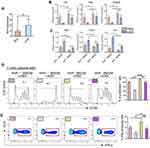
We next sought to further investigate whether KAR could indeed modulate macrophage function and explore the underlying mechanisms. To this end, we generated BMDM and stimulated in vitro them with LPS in the presence or absence of KAR to evaluate its effect on macrophages. KAR markedly down-regulated LPS-induced macrophage activation, characterized by lower levels of CD86 and CD80 than controls (Figure 3A and B). We also analyzed the expression of pro- and anti-inflammatory genes, and found that LPS-stimulated macrophages with KAR were less pro-inflammatory in nature as evidenced by lower expression of inflammatory genes (Il1b, Tnfa, Il12) (Figure 3C) but higher expression of anti-inflammatory genes (Arg1, Fizz1, Il10) (Figure 3D). As presented in Supplementary Figure 2, the effect of KAR on LPS-stimulated BMDM was dose-dependent. Given macrophages have capacities to affect T cell activation and proliferation, we next co-cultured KAR-treated or non-treated BMDM with naïve CD4+ T cells and used a CFSE Cell Proliferation Kit to determine T cell proliferation. KAR-treated macrophages greatly inhibited T cell proliferation (Figure 3E). Flow cytometric staining for CD62L, a naïve T cell marker, showed that T cell activation was suppressed by KAR-treated macrophages either (Figure 3F). These data are indicative of an immunoregulatory phenotype of KAR-treated macrophages.
Next, we further investigated the downstream pathways in the KAR regulation of macrophage functions. Previous studies have suggested a potential role of AhR in shifting macrophages toward an anti-inflammatory phenotype,23 we asked whether AhR was involved in the effect of KAR on macrophages. Towards this direction, we first verified that KAR treatment indeed up-regulated AhR expression in BMDM (Figure 4A). We found that LPS induced a pro-inflammatory nature in AhR−/- BMDM to a larger extent than AhR+/+ BMDM (Figure 4B). Additionally, AhR deficiency greatly impaired the inhibitory effect of KAR on BMDM inflammatory cytokine expression under LPS stimulation, compared with AhR+/+ BMDM (Figure 4B). Importantly, AhR deficiency abrogated KAR-induced anti-inflammatory mediator expression in BMDM (Figure 4C). Expectedly, AhR deficiency impaired the ability of KAR-treated BMDM to inhibit T cell proliferation (Figure 4D) and IFN-γ expression (Figure 4E). Taken together, KAR might imprint a regulatory phenotype on macrophages via AhR.
AhR Deficiency in Macrophages Impaired the Effect of KAR on IBS Mice
Next, we wanted to investigate the role of macrophage-derived AhR in the effect of KAR on IBS. First of all, we found that AhR−/- mice were more susceptible to TNBS-induced IBS than AhR+/+ littermates (Supplementary Figure 3) and AhR expression in colonic macrophages was up-regulated in IBS mice with KAR compared to those without KAR (Supplementary Figure 4), prompting us to believe that macrophage-derived AhR might be crucial for intestinal homeostasis and involved in the effect of KAR on IBS.
Next, we administrated CD11b-DTR transgenic mice with TNBS to establish the IBS model and macrophages were depleted in these mice using diphtheria toxin (DTx) 7 days after TNBS enema. As shown in Supplementary Figure 5, there were no evident differences in the susceptibility to TNBS between CD11b-DTR and their wild type (WT) littermates. In addition, we observed that CD11b-DTR mice with macrophage depletion suffered from milder visceral hypersensitivity (Supplementary Figure 6), and transfer of macrophages restored the visceral hypersensitivity to a level comparable to CD11b-DTR mice without DTx (Supplementary Figure 6). Subsequently, peritoneal macrophages isolated from AhR+/+ or AhR−/- mice were transferred into DTx-treated CD11b-DTR transgenic mice to reconstitute them (Figure 5A). The visceral sense and intestinal motility were measured in all groups of mice. We found that IBS mice with AhR−/- macrophages exhibited increased visceral sense and intestinal motility compared to those with AhR+/+ macrophages, and the therapeutic effect of KAR on IBS was significantly impaired in TNBS-exposed chimeras with AhR−/- macrophages compared to that in their counterparts with AhR+/+ macrophages (Figure 5B–E). Regarding intestinal barrier permeability, KAR-treated IBS chimeras with AhR−/- macrophages exhibited much higher FITC Dextran concentration in the serum compared to KAR-treated IBS chimeras with AhR+/+ macrophages (Figure 5F). Additionally, we investigated whether AhR deficiency in macrophages could abrogate the suppressive effect of KAR on mucosal inflammation in IBS mice. Colonic inflammatory gene expression including Il1b, Il12, Tnfa and Inos was potently reduced and anti-inflammatory gene expression including Arg1, Fizz1, Ym1, and Il-10 was increased by KAR in IBS chimeras with AhR+/+ macrophages compared to non-treated IBS mice, but AhR deficiency in macrophages significantly abrogated these effects (Figure 5G and H). Collectively, these findings suggest that KAR could ameliorate IBS in mice and macrophage-derived AhR plays a crucial role in this effect.
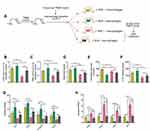 |
Figure 5 AhR deficiency in macrophages impaired the effect of KAR on IBS mice. (A) CD11b-DTR transgenic mice were received with TNBS to induce colonic inflammation as in Figure 1, and DTx (2 mg/g) was injected intraperitoneally into them to deplete macrophages 7 days post TNBS exposure. Peritoneal macrophages from AhR+/+ or AhR−/- mice were purified and were transferred intravenously into DTx-treated mice. 2×106 macrophages for each DTx-treated mouse. After 3 days of reconstitution, KAR (100 mg/kg/day) was administrated intraperitoneally and these chimeras were divided into four groups (n = 8): chimeras with AhR+/+ macrophages (AhR+/+ chimeras) without KAR, AhR+/+ chimeras with KAR treatment, chimeras with AhR−/- macrophages (AhR−/- chimeras) without KAR, and AhR−/- chimeras with KAR treatment. The (B) AWR test and (C) VMR in response to CRD at 0.5 mL distending volume. (D) Bristol stool grade. (E) The first black stool time. (F) Intestinal permeability was minored by serum FITC-dextran concentrations. The transcript expression levels of colonic (D) inflammatory mediators (IL-1β, TNF-α, Il-12p35, and iNOS) and anti-inflammatory mediators (Arg1, Fizz1, Ym1, and IL-10) were measured by qRT-PCR. *P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance (ANOVA). Representative results from one of three independent experiments were shown. |
KAR Regulates the Functions of Human Monocyte-Derived Macrophages (MDM) via AhR, Which is Down-Regulated in Patients with IBS
We next sought to validate our findings above in human macrophages. We first generated human MDM and confirmed that KAR indeed up-regulated AhR expression in MDM (Figure 6A). Next, we treated MDM with or without KAR under stimulation of LPS. Similar with observations in mice, KAR significantly decreased LPS-induced TNFa and Il1b gene expression but increased Il10 gene expression in MDM, and knockdown of AhR significantly abrogated these effects (Figure 6B). Subsequently, we analyzed AhR expression profile in patients with IBS. We found that AhR expression in either mucosal tissues (Figure 6C) or MDM (Figure 6D) was significantly decreased in all three IBS groups (IBS-C, IBS-D, and IBS-M) compared to that in healthy control group. Furthermore, we revealed that AhR expression in MDM from IBS patients was positively correlated with serum IL-10 protein level (Figure 6E) and yet inversely correlated with serum TNF-α protein level (Figure 6F). As shown in Figure 6G, we used serum LPS concentration to mirror the intestinal barrier permeability in IBS patients, and found that AhR expression in MDM was negatively correlated with serum LPS level. It has been well-known that IBS patients often suffer from anxiety and depression during the course of the disease.24 We found that the levels of AhR in MDM were negatively correlated with anxiety scores (HAD-A) in patients with IBS (Figure 6H).
Discussion
A great number of studies have provided compelling evidences that macrophages are critical players as a member of innate immunity and a trainer of adaptive immune responses in the development of IBS, suggesting that targeting macrophages could assist to develop potential drugs for IBS. Our current study demonstrated a therapeutic role of KAR on IBS in mice via regulating macrophage functions through activating AhR signaling.
So far, many medicines currently used in clinic have various bioactive compounds derived from natural plants. Flavonoids widely exist in natural plants, which are a group of naturally-occurring botanical compounds derived from 2-phenylchromone.25 KAR, a member of the flavonoid family is derived from Sophora flavescens, has been found to exert potent therapeutic effects in many disorders, including inflammation and cancers. KAR was able to inhibit the growth and induce the mitochondria apoptosis of non-small cell lung cancer (NSCLC) cells both in vivo and in vitro without noticeable toxic effects.11 In EAE, a mouse model of human multiple sclerosis, KAR greatly relieved clinical symptoms and suppressed T cell-mediated autoimmune responses in the CNS of EAE mice, suggesting an immunomodulatory role of KAR.12 Moreover, KAR could abrogate CD4+ T cell differentiation via inhibiting JAK/STAT signaling and TCR activation and thus repressed the development of two inflammatory skin diseases.26 Previous data demonstrated that KAR was able to suppress macrophage production of inflammatory mediators upon LPS stimulation,14 we further investigated this effect in IBS and the underlying mechanisms. We found that KAR markedly inhibited LPS-induced BMDM activation, inflammatory gene expression, and their capacities of assisting T cell proliferation. More than that, KAR was found to up-regulate IL-10 expression in BMDM in vitro and in the colon of IBS mice, suggesting that KAR might imprint macrophage an immunoregulatory nature.
Additionally, we found that AhR was functionally involved in the effect of KAR on macrophage functions. AhR is widely expressed among immune, epithelial, and endothelial cells, and a member of the basic helix-loop-helix transcription factor family.27 AhR is able to bind with a panel of exogenous ligands including natural plant flavonoids, and regulate the maturation and function of many important immune cells in a ligand-dependent manner at multiple levels, including T cells, dendritic cells, and macrophages.27 Recent evidences suggest that macrophages displayed rapid activation of AhR upon exposure to apoptotic cells, which led to massive IL-10 production. Myeloid cells deficient in AhR led to exaggerative systemic autoimmune responses in lupus prone mice.28 In the current study, we found that KAR significantly up-regulated AhR expression in both mouse and human macrophages, and KAR failed to efficiently induce anti-inflammatory gene expression including IL-10 by macrophages in the absence of AhR. Mice with specific deficiency of AhR in macrophages were more susceptible to IBS and the effect of KAR treatment on IBS was significantly impaired in these mice, suggesting a critical role of AhR in KAR regulation of macrophage functions and in the protection of IBS. Of note, the effect of KAR was not completely abrogated by AhR deficiency in IBS, indicating that there might be other downstream pathways involved in the pathogenesis of KAR in regulation of IBS.
In summary, we here revealed that KAR, a natural product derived from the roots of Sophora flavescens, acted as an effective drug for visceral hypersensitivity in IBS, and an essential regulator of mucosal inflammation through macrophage-intrinsic AhR signaling. Our findings provide new evidence that KAR might be a promising candidate as a therapeutic cure for IBS.
Abbreviations
IFN, interferon; IL, interleukin; TCR, T cell receptor; TNF, tumour necrosis factor; PBS, phosphate buffered saline; PCR, polymerase chain reaction; LPS, lipopolysaccharide; MPO, myeloperoxidase.
Ethics Approval and Consent to Participate
All procedures involving animals were approved and performed in accordance with the Animal Care and Use Committee at Sichuan Provincial People’s Hospital. Human studies were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board for Clinical Research of the Sichuan Provincial People’s Hospital.
Funding
This research was funded by Scientific Research Fund of Sichuan Provincial People’s Hospital (No. 2016LY11).
Disclosure
The authors declare that they have no competing interests.
References
1. Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473–486. doi:10.1038/s41575-020-0286-8
2. Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS. J Inflamm Res. 2018;11:345–349. doi:10.2147/JIR.S174982
3. Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi:10.1053/j.gastro.2007.01.046
4. El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Low-grade inflammation in the rectum of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2013;7:1081–1085. doi:10.3892/mmr.2013.1320
5. Chang L, Adeyemo M, Karagiannidis I, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107:262–272. doi:10.1038/ajg.2011.423
6. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi:10.1053/j.gastro.2003.11.055
7. Lee KJ, Kim YB, Kim JH, et al. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi:10.1111/j.1440-1746.2008.05574.x
8. Park JH, Rhee P-L, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:71–78. doi:10.1111/j.1440-1746.2005.04143.x
9. Ruder B, Becker C. At the Forefront of the Mucosal Barrier: the Role of Macrophages in the Intestine. Cells. 2020;9:2162. doi:10.3390/cells9102162
10. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675–1688. doi:10.1016/S0140-6736(20)31548-8
11. Yang J, Chen H, Wang Q, et al. Inhibitory Effect of Kurarinone on Growth of Human Non-small Cell Lung Cancer: an Experimental Study Both in Vitro and in Vivo Studies. Front Pharmacol. 2018;9:252. doi:10.3389/fphar.2018.00252
12. Xie L, Gong W, Chen J, et al. The flavonoid kurarinone inhibits clinical progression of EAE through inhibiting Th1 and Th17 cell differentiation and proliferation. Int Immunopharmacol. 2018;62:227–236. doi:10.1016/j.intimp.2018.06.022
13. Chung TW, Lin CC, Lin SC, Chan HL, Yang CC. Antitumor effect of kurarinone and underlying mechanism in small cell lung carcinoma cells. Onco Targets Ther. 2019;12:6119–6131. doi:10.2147/OTT.S214964
14. Nishikawa S, Inoue Y, Hori Y, et al. Anti-Inflammatory Activity of Kurarinone Involves Induction of HO-1 via the KEAP1/Nrf2 Pathway. Antioxidants (Basel. 2020;9:842.
15. Yi Q, Wang J, Song Y, et al. Ascl2 facilitates IL-10 production in Th17 cells to restrain their pathogenicity in inflammatory bowel disease. Biochem Biophys Res Commun. 2019;510(3):435–441. doi:10.1016/j.bbrc.2019.01.123
16. Xiu W, Chen Q, Wang Z, Wang J, Zhou Z. Microbiota-derived short chain fatty acid promotion of Amphiregulin expression by dendritic cells is regulated by GPR43 and Blimp-1. Biochem Biophys Res Commun. 2020;533:282–288. doi:10.1016/j.bbrc.2020.09.027
17. Zhao Q, Yang W-R, Wang X-H, et al. Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells. World J Gastroenterol. 2019;25:5469–5482. doi:10.3748/wjg.v25.i36.5469
18. He Z, Sun X, Ma Z, et al. Heat shock protein 70 protects mouse against post-infection irritable bowel syndrome via up-regulating intestinal gammadelta T cell’s Th17 response. Cell Biosci. 2018;8:38. doi:10.1186/s13578-018-0237-z
19. Beattie L, Sawtell A, Mann J, et al. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol. 2016;65:758–768. doi:10.1016/j.jhep.2016.05.037
20. Nielsen MC, Andersen MN, Moller HJ. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology. 2020;159:63–74. doi:10.1111/imm.13125
21. Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi:10.1002/eji.201242847
22. He C, Yu T, Shi Y, et al. MicroRNA 301A Promotes Intestinal Inflammation and Colitis-Associated Cancer Development by Inhibiting BTG1. Gastroenterology. 2017;152(1434–1448):e1415. doi:10.1053/j.gastro.2017.01.049
23. Yang X, Liu H, Ye T, et al. AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization. Theranostics. 2020;10:12011–12025. doi:10.7150/thno.51144
24. Black CJ, Yiannakou Y, Houghton LA, et al. Anxiety-related factors associated with symptom severity in irritable bowel syndrome. Neurogastroenterol Motil. 2020;32:e13872. doi:10.1111/nmo.13872
25. Zeinali M, Rezaee SA, Hosseinzadeh H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed Pharmacother. 2017;92:998–1009. doi:10.1016/j.biopha.2017.06.003
26. Kim BH. Kurarinone regulates immune responses through regulation of the JAK/STAT and TCR-mediated signaling pathways. Biochem Pharmacol. 2013;85:1134–1144. doi:10.1016/j.bcp.2013.01.005
27. Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi:10.1038/nrc3846
28. Shinde R, Hezaveh K, Halaby MJ, et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat Immunol. 2018;19:571–582. doi:10.1038/s41590-018-0107-1
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.