Back to Journals » Cancer Management and Research » Volume 14
The Fibrinogen/Albumin Ratio Index as an Independent Prognostic Biomarker for Patients with Combined Hepatocellular Cholangiocarcinoma After Surgery
Authors Xu J, Li S, Feng Y, Zhang J, Peng Y, Wang X, Wang H
Received 7 February 2022
Accepted for publication 7 May 2022
Published 23 May 2022 Volume 2022:14 Pages 1795—1806
DOI https://doi.org/10.2147/CMAR.S361462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Jiake Xu,1 Shaochun Li,2 Ye Feng,1 Jie Zhang,1 Youduo Peng,2 Xiaohong Wang,2 Hongwei Wang2
1Department of Gastroenterology, Kunshan Second People’s Hospital, Kunshan, People’s Republic of China; 2Department of General Surgery, Huadong Hospital Affiliated to Fudan University, Shanghai, People’s Republic of China
Correspondence: Hongwei Wang, Tel +86 15021133649, Email [email protected]
Purpose: The fibrinogen/albumin ratio (FAR) is increasingly considered as a potential biomarker for predicting prognosis in various malignant tumors, whereas the value of the FAR in predicting the recurrence-free survival (RFS) in patients with combined hepatocellular cholangiocarcinoma (cHCC-CCA) after surgery has not been studied.
Patients and Methods: A total of 104 patients with surgical-pathologically proved cHCC-CCA were retrospectively analyzed. The best cut-off value of the FAR was calculated via receiver operating characteristic (ROC) curve analysis, and the cohort was then divided into two groups as high-FAR (H-FAR) group and low-FAR (L-FAR) group. The correlation between the preoperative FAR and clinicopathological characteristics was analyzed. Uni- and multi-variable analyses for RFS were evaluated using a Cox proportional hazards model to verify the predictive value of FAR on the RFS of cHCC-CCA. Additionally, a novel clinical nomogram based on FAR was developed to preoperatively predict the RFS of HCC-CCA. The C-index and calibration were conducted to evaluate the performance of the developed nomogram.
Results: According to the cut-off value of the FAR, the patients were grouped into the H-FARI (> 0.075) and L-FARI (≤ 0.075) groups. FAR was significantly correlated with several clinical-pathological features, including age, cirrhosis, AFP, CA19-9, BCLC staging, NLR, and PLR. In the multi-variate analysis, FAR, cirrhosis and tumor size were independent prognostic predictors for poor RFS in cHCC-CCA patients after surgery. Moreover, the clinical nomogram based on FAR was constructed, showing well-predictive accuracy.
Conclusion: The preoperative FAR is a convenient and feasible serum biomarker for predicting the RFS of cHCC-CCA after surgery. Such developed FAR-based nomogram integrating tumor size and cirrhosis could be served as a feasible and convenient tool to assist the decision-making of clinical strategy.
Keywords: combined hepatocellular cholangiocarcinoma, fibrinogen-to-albumin ratio, surgery, prognosis
Introduction
Combined hepatocellular cholangiocarcinoma (cHCC-CCA), accounting for approximately 2–5% of all primary liver malignancy, owned characteristics of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA).1,2 Due to its unique histopathology, hard diagnosis, and worse prognosis, cHCC-CCA has recently received more attention.3 Moreover, compared to the 2010 classification, the 2019 WHO classification of cHCC-CCA was obviously updated.4 However, owing to the low incidence and short study period of cHCC-CCA, there is still no consensus about several problems regarding its diagnostic and prognostic features.5,6
There are a large number of studies have focused on exploring preoperative clinical features to preoperatively predict the prognostic factors of HCC or ICC, including serum indexes (alpha-fetoprotein (AFP) or carbohydrate antigen 19–9 (CA19-9)) or histopathological features (microvascular invasion (MVI) or lymph node metastasis (LNM)).7–11 Unfortunately, the constructed predictive models targeting HCC or ICC seem not to be appropriate for cHCC-CCA, which may be caused by that the proportions of both tumoral characteristics cannot be assessed before biopsy or surgery. Thus, several studies have focused on the impact of conventional clinical features for predicting the prognosis of cHCC-CCA after surgical resection.12 For example, Minjae Kim and coworkers have reported that both 8th AJCC tumor stage and 2010 WHO histologic classification were independent prognostic factors for tumor recurrence and overall survival of cHCC-CCA.13 However, in another study, 2019 WHO classification was not associated with the prognosis of patients with cHCC-CCA after surgery.14 Considering that the prognostic factors of cHCC-CCA are less studied and no consensus, more potential biomarkers are urgent to be explored for precisely predicting the post-surgical prognosis of cHCC-CCA.
At present, inflammation of the tumoral microenvironment has shown a close association with cancer surveillance and elimination.15,16 Therefore, a series of inflammation-related biomarkers, such as platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), have been investigated as valuable prognostic factors in various malignant tumors.17–20 These inflammation-related biomarkers were often calculated in the light of ratios of peripheral circulating white blood cells or acute phase proteins, indicating systemic responses in the liver and the lymphoid/medullary tissue.21 Notably, among them, the fibrinogen/albumin ratio (FAR), as a new biomarker, has recently been discovered as a promising index for evaluating the prognosis of malignancy including HCC and ICC.22 However, the impact of FAR on the prognosis of cHCC-CCA patients undergoing surgery is rarely explored.
Therefore, the aim of our study was to investigate the value of various clinical features, especially FAR in the prediction of the recurrence-free survival (RFS) of cHCC-CCA, and a clinical nomogram based on these prognostic indexes was constructed and verified.
Materials and Methods
Study Population
A total of 104 patients with surgical-pathologically confirmed cHCC-CCA who were consecutively between January 2014 and January 2019 were enrolled. All patients received radical resection. In our study, the inclusion criteria of the patients were as follows: 1) patients with surgical-pathologically confirmed cHCC-CCA after R0 resection; 2) Child–Pugh A or B liver function; 3) patients with detailed clinical, laboratory and imaging data; and 4) no local or systemic treatment prior to surgery and no history of extra-hepatic tumor. Thirty-nine patients were excluded as follows: 1) patients received treatment before surgery, such as transarterial chemoembolization (TACE), ablation or neoadjuvant chemotherapy (n = 21); 2) lack of clinical or laboratory data (n = 9); 3) patients received R1 resection (n = 3) or the existence of synchronous extra-hepatic lesions (n = 6). Finally, 104 cHCC-CCA patients were enrolled in our study. In addition, in this study, cHCC-CCA was pathologically diagnosed in line with the 2010 WHO classification criterion. The study was approved by Huadong Hospital Research Ethics Committee (2019K050), and informed consent of all participants was obtained. And this study was strictly performed according to the Declaration of Helsinki.
Clinical Features and Inflammation-Related Biomarkers
The basic characteristics of patients, including gender, age, body mass index (BMI), tumor location, hepatitis B virus infection, cirrhosis, tumor number, tumor size, Child–Pugh score, tumor encapsulation, and Barcelona Clinic Liver Cancer (BCLC) staging were recorded. Besides, the pathology-related indicators including MVI and LNM was recorded. Blood- and inflammation-related biomarkers including ALT, AST, CA19-9, AFP, proteins induced by vitamin K absence or antagonist-II (PIVKA), NLR, PLR, and FAR was also recorded. Among them, the NLR was calculated by dividing the neutrophil count by the lymphocyte count, and the PLR was calculated by dividing the platelet count by the lymphocyte count, and the FAR was calculated by dividing the plasma fibrinogen level by the albumin level. All recorded data were obtained within 1 week before surgery.
Treatment and Follow-Up
In our cohort, all patients were followed up every 3 months in the first 2 years and every 3–6 months thereafter. Patients were subjected to follow-up examination by blood examination, biochemical tests, dynamic-enhanced abdominal computed tomography or magnetic resonance imaging or PET-CT. The follow-up methods included telephone follow-up and outpatient follow-up. Recurrence including local recurrence and distant metastatic diseases after radical surgery was diagnosed by postoperative imaging, and RFS was measured from the day of surgery to the time of recurrence, last clinical follow-up, or death.23 In this study, the final follow-up ended on October 30, 2021.
Statistical Analysis
SPSS 26.0 (Armonk, NY, USA) and R software (version 4.1.2, http://www.r-project.org/) were performed to statistical analyses. Based on the receiver operating characteristic (ROC) curve, the maximum value of the Youden index (sensitivity + specificity −1) was performed to obtain the optimal cut-off values of FAR, PLR, and NLR.24 And the cut-off values of AFP and CA19-9 were referred to the previous literature.23 All categorical variables were expressed by numbers and percentages. The correlation between FAR and other clinical features was analyzed by Chi-square test. Then, the uni- and multi-variate Cox proportional hazard models were performed to assess the risk factors of recurrence in cHCC-CCA following surgery, and corresponding hazard ratios (HR) and 95% confidence interval (CI) were also calculated. The Kaplan–Meier (K-M) analysis was conducted to depict the survival curves via the Log rank test. Based on the multi-variate Cox regression analysis, the clinical nomogram for RFS was developed. The calibration curves and the consistency index (C-index) were performed to further assess the predictive effect of the constructed nomogram. All statistical analyses were considered as statistically significant at two-sided P ≤ 0.05.
Results
Patients Characteristics
A total of 104 patients (45 females (43.3%) and 59 males (56.7%)) with cHCC-CCA was enrolled in present study. In this cohort, the follow-up time was 19.5 ± 9.8 months (median 19.8, range 4.0–38.5 months). The clinical characteristics are listed in Table 1. According to the 2010 WHO classification criterion, in our cohort, 83 cases (79.8%) were defined as classical type, 12 cases (11.5%) were defined as typical subtype of stem cell features, 6 cases (5.8%) were defined as intermediate cell subtype of stem cell features and 3 cases (2.9%) were defined as cholangiolocellular subtype of stem cell features. In aspects of histopathological results, 52 (50.0%) patients and 52 (50.0%) patients exhibited MVI and non-MVI, respectively. Twelve (11.5%) patients and 92 (88.5%) patients exhibited LNM and non-LNM, respectively.
 |  |  |
Table 1 The Clinical-Pathological Characteristics of the cHCC-CCA Population |
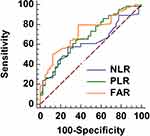
Through a ROC analysis approach (Figure 1), the cut-off values were calculated as 0.075 for FARI, 3.26 for NLR, and 64.8 for PLR, respectively. And the corresponding area under the curves (AUC) for RFS were 0.726 for FAR (P ≤ 0.001), 0.616 for NLR (P = 0.035), and 0.681 for PLR (P = 0.007), respectively (Figure 1). Then, based on the cut-off value of FAR, the patients were divided into two groups as high-FAR (H-FAR, n = 66) and low-FAR (L-FAR, n = 38).
The Correlations Between FAR and Clinical-Pathological Features
According to the results, FAR were statistically correlated with several clinical-pathological features, including age (χ2 = 4.782, P = 0.029), cirrhosis (χ2 = 4.532, P = 0.033), AFP (χ2 = 5.218, P = 0.022), CA19-9 (χ2 = 7.932, P = 0.005), MVI (χ2 = 4.417, P = 0.042), BCLC staging (χ2 = 8.706, P = 0.033), NLR (χ2 = 5.456, P = 0.020), and PLR (χ2 = 6.380, P = 0.012). However, there were no significant correlations between FAR and other clinical features, including gender, location, hepatitis B virus infection, BMI, tumor number, tumor size, ALT, AST, PIVKA, Child–Pugh score, tumor encapsulation, LNM (all P > 0.05). The detailed associations between the FARI and various variables are presented in Table 1.
The Uni- and Multi-Variate Survival Analyses
The correlations between clinical-pathological characteristics and RFS are shown in Table 2. In a univariate analysis, the presence of cirrhosis (HR = 0.528, P = 0.023), tumor size >5 cm (HR = 0.356, P = 0.001), AFP > 100 ng/mL (HR = 0.325, P ≤ 0.001), CA19-9 > 37 U/mL (HR = 0.426, P = 0.001), the presence of MVI (HR = 0.397, P = 0.001), PPLR >64.8 (HR = 0.528, P = 0.013), and FAR > 0.075 (HR = 0.425, P = 0.006) were significantly correlated with poor RFS. Furthermore, the multivariate analysis revealed that a FAR (HR = 0.446, 95% CI: 0.213–0.936, P = 0.033), tumor size (HR = 0.252, 95% CI: 0.089–0.715, P = 0.010) and cirrhosis (HR = 2.927, 95% CI: 1.117–7.671, P = 0.029) were independent risk factors for recurrence of patients with cHCC-CCA following surgery (Figure 2).
 |  |  |
Table 2 Uni- and Multi-Variate Cox Analyses for Risk Factors for RFS in cHCC-CCA |
Construction and Validation of the Nomogram
In line with the multivariate analysis, a novel nomogram incorporating the significant risk factors was constructed (Figure 3). In the present study, the C-index was used to efficiency of the constructed nomogram in the evaluation of RFS. As shown in Table 3, the results investigated that the C-index for RFS evaluation with the overall nomogram was 0.684 (95% CI: 0.621–0.748), which was significantly higher than that for FAR (0.610, 95% CI: 0.549–0.671), tumor size (0.608, 95% CI: 0.543–0.673), cirrhosis (0.575, 95% CI: 0.507–0.643), FAR + tumor size (0.663, 95% CI: 0.596–0.730), FAR + cirrhosis (0.646, 95% CI: 0.576–0.717), and cirrhosis + tumor size (0.611, 95% CI: 0.541–0.681). By calculating the points of each factor, the 1- and 2-year recurrence probabilities of patients could be individually predicted before treatment. Moreover, calibration curves were performed to verify the performance of the developed nomogram, showing that the nomogram-predicted RFS was well-consistent with the observed RFS at 1 and 2 years after radical resection (Figure 4).
 |
Table 3 The C-Index of RFS in the Nomogram and Other Risk Factors |
Discussion
In the present study, the FAR was not only significantly correlated with several clinical features including FAR, cirrhosis, and tumor size, butalso served as an independent risk factor for postoperative recurrence in cHCC-CCA patients. The results showed that the FAR >0.075, the presence of cirrhosis, and tumor size (>5 cm) were independent prognostic factors for predicting poor RFS of cHCC-CCA after surgery. In addition, we constructed and verified a preliminary clinical nomogram model incorporating FAR, cirrhosis, and tumor size to help the clinician make clinical decisions.
cHCC-CCA is an uncommon type of primary liver cancer, which is differed from HCC and ICC in the origin and intrinsic properties of the tumor.3,25 Previous literatures have reported that the recurrence rate of cHCC-CCA after surgical resection was relatively high, and its OS rate has been even lower than that of ICC.26,27 In our study, of 104 patients, 64 (61.5%) experienced recurrences after curative surgery during the follow-up period, which is in line with the previous results. Unfortunately, owing to the relatively low incidence and less research, there is no consensus about the prognostic factors of cHCC-CCA after surgical resection. To our knowledge, this study was the first study to focus on the impact of FAR in the prediction of prognosis of cHCC-CCA patients following surgery. In the present study, 104 patients with cHCC-CCA patients who underwent surgical resection were enrolled and the corresponding cut-off value for the FAR was calculated to be 0.075 for RFS. Intriguingly, the FAR was associated with several clinical characteristics, containing age, cirrhosis, AFP, CA19-9, MVI, and BCLC staging, NLR, and PLR. This may imply that patients with a relatively high FAR may be more invasive. More importantly, the patients with a FAR > 0.075 exhibited worse RFS than those with a FAR ≤ 0.075, indicating that a high FAR is an independent risk factor for postoperative recurrence in cHCC-CCA patients. Fibrinogen, acted as an important acute-phase protein, is closely associated with coagulation cascade reaction, tumor immune, and systemic inflammation.28 Notably, fibrinogen was able to serve as a cytoskeleton to support the extracellular matrix of tumors for facilitating tumor angiogenesis and amplifying tumor cell adhesion and invasion, which might be the implying reasons that the level of fibrinogen is associated with poor prognosis with malignancies.29 Furthermore, albumin acts as an important protein in body, commonly reflects the nutritional status of patients. As usually, low level of albumin commonly indicated malnutrition and cachexia in malignant tumor patients, which are closely correlated with worse prognosis.30 Similar to our results, the FAR, as a new biomarker combining with blood nutritional status and blood coagulation function, has already been proven as a valuable prognostic factor in the prediction of prognosis of various malignancies, such as HCC, ICC, pancreatic cancer, etc.21,22,31
The prediction of recurrence cHCC-CCA following surgical resection is of great importance in managing the patients in the clinic.5 Thereby, we further investigated the prognostic biomarkers for the postoperative recurrence of cHCC-CCA. The results represented that both tumor size and cirrhosis are another two predictive factors for RFS of cHCC-CCA patients. In our cohort, a large tumor size (>5 cm) and the presence of cirrhosis were significant risk factors for recurrence of cHCC-CCA patients at 1-year and 2-year following surgery. Understandably, large tumors are more likely with worse prognoses. Similarly, Zeng’s team has reported that the large size (>5 cm) of cHCC-CCA was one of the significant factors for predicting the presence of MVI32 Moreover, the presence of cirrhosis was similarly often reported as a risk factor for a worse prognosis of the tumor. In a recent study focused on the impact of FAR on the prognosis of HCC, it is also reported that cirrhosis is one of the prognostic factors for overall survival (OS) of HCC after surgical resection.22 However, in another recent study reported by Zhou, 160 patients with cHCC-CCA underwent surgical resection were enrolled and analyzed. The results showed that age and CA19-9 are independent clinical predictors for RFS in cHCC-CCA, which is not consistent with our findings.33 The reasons may be caused by that sample sizes and enrolled criterion are variant in different centers. For example, cirrhosis is not analyzed in the Zhou’s study. Therefore, multi-center, large sample size and unified standard studies are needed to be further implemented in the future. In addition, we also constructed and validated a clinical nomogram including FAR, tumor size and cirrhosis, which enabled the visual prediction of 1-year and 2-year RFS in cHCC-CCA patients treated with surgical resection. Since there is no globally accepted consensus for the first-line treatment of cHCC-CCA and comparative studies on different curative strategies are rare, the constructed nomogram, to some extent, was helpful for guiding the clinician to conveniently evaluate the patient’s condition and plan more appropriate treatments.
There are several limitations in this study. First, this is a single-center and single-region retrospective study with inevitable selection bias and the validation cohort was not included owing to the limit of the sample size. Therefore, multi-center and multi-region studies with larger samples are needed to further verify our results. Second, owing to the long time-span of sample and data collection, there are unavoidable selection bias about diagnosis and clinical treatment. Meanwhile, the differences in the inclusion criterion and observed indicators of the enrolled patients may influence the conclusions to some extent. And the optimal cut-off values of FAR, PLR, and NLR were screened according to the data of our cohort, leading to the cohort dependence and selection bias of our conclusion. Thereby, multi-center studies with unified standard needed to be performed to validate our conclusion. Third, since cHCC-CCA patients with radical surgical resection were enrolled in this study, the incidence of lymph node metastasis and MVI may be low, which may cause selection bias in the results. Finally, we did not classify cHCC-CCA into different subtypes according to the latest WHO classification system. The main aim of this study focused on the impact of FAR on the prediction of RFS of cHCC-CCA, and more studies concerning various cHCC-CCA types are needed to be performed in the future.
Conclusion
In summary, we investigated that FAR is a simple and valuable indicator that is associated with several clinical characteristics and prognosis of cHCC-CCA. Moreover, FAR >0.075, the presence of cirrhosis, and tumor size >5 cm are poor prognostic factors for RFS in cHCC-CCA patients underwent surgical resection, and the clinical nomogram integrating FAR, tumor size and cirrhosis was helpful for guiding the clinician to conveniently evaluate the individual prognosis and plan more appropriate treatments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: an update. J Hepatol. 2021;74(5):1212–1224. doi:10.1016/j.jhep.2021.01.035
2. Leoni S, Sansone V, Lorenzo S, et al. Treatment of combined hepatocellular and cholangiocarcinoma. Cancers. 2020;12(4):794. doi:10.3390/cancers12040794
3. Schizas D, Mastoraki A, Routsi E, et al. Combined hepatocellular-cholangiocarcinoma: an update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat Dis Int. 2020;19(6):515–523. doi:10.1016/j.hbpd.2020.07.004
4. Kim TH, Kim H, Joo I, Lee JM. Combined hepatocellular-cholangiocarcinoma: changes in the 2019 World Health Organization histological classification system and potential impact on imaging-based diagnosis. Korean J Radiol. 2020;21(10):1115–1125. doi:10.3348/kjr.2020.0091
5. Sempokuya T, Wien EA, Pattison RJ, Ma J, Wong LL. Factors associated with 5-year survival of combined hepatocellular and cholangiocarcinoma. World J Hepatol. 2020;12(11):1020–1030. doi:10.4254/wjh.v12.i11.1020
6. Yamashita YI, Aishima S, Nakao Y, et al. Clinicopathological characteristics of combined hepatocellular cholangiocarcinoma from the viewpoint of patient prognosis after hepatic resection: high rate of early recurrence and its predictors. Hepatol Res. 2020;50(7):863–870. doi:10.1111/hepr.13507
7. Fang Q, Chen H. The significance of m6A RNA methylation regulators in predicting the prognosis and clinical course of HBV-related hepatocellular carcinoma. Mol Med. 2020;26(1):60. doi:10.1186/s10020-020-00185-z
8. Sartoris R, Gregory J, Dioguardi BM, Ronot M, Vilgrain V. HCC advances in diagnosis and prognosis: digital and Imaging. Liver Int. 2021;41(Suppl 1):73–77. doi:10.1111/liv.14865
9. Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;9(6):1370. doi:10.3390/cells9061370
10. Gao YX, Yang TW, Yin JM, et al. Progress and prospects of biomarkers in primary liver cancer (Review). Int J Oncol. 2020;57(1):54–66. doi:10.3892/ijo.2020.5035
11. Zhang XF, Xue F, Dong DH, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann Surg. 2021;274(6):e1187–e1195. doi:10.1097/SLA.0000000000003788
12. Matsukuma KE, Yeh MM. Update on the pathology of liver neoplasms. Ann Diagn Pathol. 2019;38:126–137. doi:10.1016/j.anndiagpath.2018.10.005
13. Kim M, Hwang S, Ahn C-S, et al. Postresection prognosis of combined hepatocellular carcinoma-cholangiocarcinoma according to the 2010 World Health Organization classification: single-center experience of 168 patients. Ann Surg Treat Res. 2021;100(5):260–269. doi:10.4174/astr.2021.100.5.260
14. Kim M, Hwang S, Ahn CS, et al. Post-resection prognosis of combined hepatocellular carcinoma-cholangiocarcinoma cannot be predicted by the 2019 World Health Organization classification. Asian J Surg. 2021;44(11):1389–1395. doi:10.1016/j.asjsur.2021.03.002
15. Wang G, Wang Q, Liang N, et al. Oncogenic driver genes and tumor microenvironment determine the type of liver cancer. Cell Death Dis. 2020;11(5):313. doi:10.1038/s41419-020-2509-x
16. Luo D, Zhao D, Zhang M, et al. Alternative splicing-based differences between hepatocellular carcinoma and intrahepatic cholangiocarcinoma: genes, immune microenvironment, and survival prognosis. Front Oncol. 2021;11:731993. doi:10.3389/fonc.2021.731993
17. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. doi:10.21037/tlcr.2019.11.16
18. Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26(3):372–376. doi:10.3109/14397595.2015.1091136
19. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi:10.1016/j.lungcan.2017.07.024
20. Erre GL, Paliogiannis P, Castagna F, et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest. 2019;49(1):e13037. doi:10.1111/eci.13037
21. Liu H, Qiu G, Hu F, Wu H. Fibrinogen/albumin ratio index is an independent predictor of recurrence-free survival in patients with intrahepatic cholangiocarcinoma following surgical resection. World J Surg Oncol. 2021;19(1):218. doi:10.1186/s12957-021-02330-2
22. Xu Q, Yan Y, Gu S, et al. A novel inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. 2018;2018:1–11. doi:10.1155/2018/4925498
23. Ishii T, Ito T, Sumiyoshi S, et al. Clinicopathological features and recurrence patterns of combined hepatocellular-cholangiocarcinoma. World J Surg Oncol. 2020;18(1):319. doi:10.1186/s12957-020-02099-w
24. Deng S, Fan Z, Xia H, et al. Fibrinogen/Albumin ratio as a promising marker for predicting survival in pancreatic neuroendocrine neoplasms. Cancer Manag Res. 2021;13:107–115. doi:10.2147/CMAR.S275173
25. Xue R, Chen L, Zhang C, et al. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell. 2019;35(6):932–947. doi:10.1016/j.ccell.2019.04.007
26. Liu WR, Tian MX, Tao CY, et al. Adjuvant Transarterial chemoembolization does not influence recurrence-free or overall survival in patients with combined hepatocellular carcinoma and Cholangiocarcinoma after curative resection: a propensity score matching analysis. BMC Cancer. 2020;20(1):642. doi:10.1186/s12885-020-07138-z
27. Wang T, Yang X, Tang H, et al. Integrated nomograms to predict overall survival and recurrence-free survival in patients with combined hepatocellular cholangiocarcinoma (cHCC) after liver resection. Aging. 2020;12(15):15334–15358. doi:10.18632/aging.103577
28. Xue GQ, Li CP, Lv A, et al. Predictive value of preoperative controlling nutritional status score combined with Fibrinogen-Albumin ratio in postoperative local recurrence-free survival of patients with retroperitoneal liposarcoma. Cancer Manag Res. 2021;13:6157–6167. doi:10.2147/CMAR.S307920
29. Gao W, Li M, Zhang Y. Fibrinogen/Albumin ratio (FAR) in patients with triple negative breast cancer and its relationship with epidermal growth factor receptor expression. Onco Targets Ther. 2021;14:5403–5415. doi:10.2147/OTT.S339973
30. Chen J, Hao L, Zhang S, et al. Preoperative Fibrinogen-Albumin ratio, potential prognostic factors for bladder cancer patients undergoing radical cystectomy: a two-center study. Cancer Manag Res. 2021;13:3181–3192. doi:10.2147/CMAR.S300574
31. Guo Z, Liang J. Fibrinogen-Albumin ratio index (FARI) as a certain prognostic biomarker in pretreated patients with immunotherapy. Cancer Manag Res. 2021;13:4169–4180. doi:10.2147/CMAR.S307272
32. Wang Y, Zhou CW, Zhu GQ, et al. A multidimensional nomogram combining imaging features and clinical factors to predict the invasiveness and metastasis of combined hepatocellular cholangiocarcinoma. Ann Transl Med. 2021;9(20):1518. doi:10.21037/atm-21-2500
33. Zhou C, Wang Y, Ma L, Qian X, Yang C, Zeng M. Combined hepatocellular carcinoma-cholangiocarcinoma: MRI features correlated with tumor biomarkers and prognosis. Eur Radiol. 2022;32(1):78–88. doi:10.1007/s00330-021-08188-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.