Back to Journals » Cancer Management and Research » Volume 12
The Extent of Therapeutic Central Compartment Neck Dissection in Unilateral cT1N1a or cT2N1a Papillary Thyroid Carcinoma
Authors Liu N, Yang Y, Chen B , Li L, Zeng Q, Sheng L , Zhang B, Liang W , Lv B
Received 22 July 2020
Accepted for publication 28 November 2020
Published 14 December 2020 Volume 2020:12 Pages 12801—12809
DOI https://doi.org/10.2147/CMAR.S273316
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Nan Liu,1 Yupeng Yang,2 Bo Chen,1 Luchuan Li,1 Qingdong Zeng,1 Lei Sheng,1 Bin Zhang,1 Weili Liang,1 Bin Lv1
1Department of General Surgery, Qilu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China; 2Department of Thyroid Surgery, Jinan Zhangqiu District Hospital of TCM, Jinan 250200, Shandong, People’s Republic of China
Correspondence: Bin Lv
Department of General Surgery, Qilu Hospital of Shandong University, Jinan 250012, Shandong, People’s Republic of China
Email [email protected]
Purpose: Papillary thyroid carcinomas (PTCs) frequently metastasize to the central neck compartment. Therapeutic central compartment neck dissection (CCND) is a well-established treatment for PTC nodal metastases; however, the extent to which therapeutic CCND should be performed remains controversial. In this study, we investigated the predictive risk factors for contralateral paratracheal lymph node metastasis (LNM) in unilateral cT1N1a or cT2N1a PTC.
Patients and Methods: In this case–control study, which was conducted at a single center, demographic and pathological data from unilateral cT1N1a or cT2N1a PTC patients were collected from January 2017 to March 2019. All patients were treated with total thyroidectomy and bilateral CCND.
Results: Two hundred thirty-seven patients met the inclusion criteria. Forty-nine patients (20.7%) were diagnosed with positive lymph nodes in the contralateral paratracheal region. Male sex (p=0.003), T2 disease (21– 40 mm) (p< 0.001), inferior pole tumor (p=0.011), near isthmus tumor (p< 0.001), aggressive pathology (p< 0.001), intraglandular dissemination (p=0.009), pretracheal LNM (p< 0.001), > 5 metastatic lymph nodes (p< 0.001) and extranodal invasion (p=0.003) were significantly associated with contralateral paratracheal LNM in univariate analysis. Multivariate analysis showed that male sex (p=0.005, OR=17.545), T2 disease (p=0.003, OR=34.317), inferior pole tumor (p=0.022, OR=8.289), near isthmus tumor (p=0.001, OR=40.229), aggressive pathology (p=0.027, OR=48.063), pretracheal LNM (p=0.002, OR=14.235) and > 5 metastatic lymph nodes (p=0.025, OR=23.426) were independent risk factors for contralateral paratracheal LNM.
Conclusion: Male sex, T2 disease, a tumor located near the isthmus or inferior pole, aggressive pathology, pretracheal LNM and > 5 metastatic lymph nodes were predictive factors for contralateral paratracheal LNM in unilateral cT1N1a or cT2N1a PTC. These data may be useful to identify targets for surveillance or develop therapeutic interventions for patients with CCND.
Keywords: papillary thyroid carcinoma, central compartment neck dissection, predictive factor, lymph node metastasis
Introduction
Papillary thyroid carcinoma (PTC) is the most common malignant neoplasm of the thyroid gland. The incidence of PTC has increased significantly over the past several decades.1–3 An increasing number of small PTCs (T1 or T2) are being diagnosed due to the development of high-resolution ultrasound and widespread health screening. Regional lymph node metastases (LNMs) are present at the time of diagnosis in a majority of patients with PTC,4–6 and the most common site of nodal metastasis is the central neck compartment (level VI). The central neck compartment consists of the ipsilateral and contralateral paratracheal compartments, the prelaryngeal compartment and the pretracheal compartment. In previous studies, central lymph node metastasis (CLNM) was identified in 40–70% of patients with PTC and was detected even in patients with small PTCs.7–11 Contralateral paratracheal LNM was observed in 9.8–27.3% of patients with cN0 unilateral PTC.12–15 Lymph node metastasis is commonly associated with an increased rate of local recurrence and distant metastasis.16–19
The extent of thyroid resection that should be performed has been the subject of contentious debate regarding the surgical management of PTC.20 Therapeutic central compartment neck dissection (CCND) is commonly performed for the treatment of PTC nodal metastases in patients with cN1a disease because CCND is associated with improved outcomes, both in terms of recurrence and survival. The American Thyroid Association (ATA) guidelines suggest that therapeutic CCND should accompany total thyroidectomy in patients with clinically involved central nodes to ensure clearance of disease from the central neck region.2 However, the extent to which therapeutic CCND that should be used for unilateral cN1a disease remains unclear. Bilateral CCND has a high rate of postoperative complications, such as temporary or permanent hypoparathyroidism. Therefore, bilateral CCND should only be performed in unilateral cN1a PTC patients with a high risk of contralateral paratracheal LNM.
Some studies have reported that a tumor size >1 cm, extrathyroidal extension and lateral lymph node metastasis may be important factors for predicting contralateral paratracheal LNM.15,21–25 However, few studies have examined contralateral paratracheal LNM in unilateral cT1N1a or cT2N1a PTC patients. Therefore, we aim to determine the incidence of occult contralateral paratracheal LNM in unilateral cTIN1a or cT2N1a PTC patients and identify the risk factors associated with contralateral paratracheal LNM.
Patients and Methods
Patient Selection
We performed total thyroidectomy (TT) and bilateral CCND in unilateral cT1N1a or cT2N1a PTC patients from January 2017 to March 2019. Patients with more than five metastatic lymph nodes or with the largest involved lymph node measuring >3 cm in diameter were treated with radioactive iodine. High-resolution ultrasound and computed tomography (CT) scans were performed preoperatively. All patients underwent fine needle aspiration (FNA) before surgery. A solid hypoechoic nodule measuring >5 mm with a highly suspicious sonographic pattern (irregular margins, microcalcifications, taller-than-wide shape, or evidence of extrathyroidal extension) were subjected to FNA to refute or confirm malignancy. In this study, nodules measuring <5 mm were not subjected to FNA or surgery. If ipsilateral paratracheal lymph nodes that were sonographically suspicious for PTC (microcalcifications, cystic aspect, round shape, peripheral vascularity, or hyperechogenicity) were detected by ultrasound, FNA of the suspicious lymph node was performed for cytology and washout for thyroglobulin (Tg) assessments. The CT scans were mainly used to detect superior mediastinal lymph nodes (level VII). Histological examinations were routinely performed postoperatively.
This study complies with the Declaration of Helsinki. All patients were provided with a description of the study in written and oral forms and signed an informed consent document to participate. This case–control study was reviewed by the Medical Ethics Committee of Qilu Hospital of Shandong University (project identification code: 2018149) and registered in the Research Registry (UIN: researchregistry5292). Reporting followed the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) criteria.26 The study design is depicted in Figure 1. With the exception of the surgeons and surgical assistants, all of the study members were blinded to the surgical method used for each patient to minimize bias.
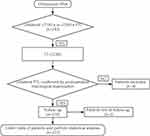 |
Figure 1 Flow chart of the study protocol. Abbreviations: FNA, fine needle aspiration; PTC, papillary thyroid carcinoma; TT, total thyroidectomy; CCND, central compartment neck dissection. |
The inclusion criteria for this study were as follows: (1) the absence of a previous history of thyroidectomy; (2) unilateral PTC verified by histological examination; (3) stage T1 or T2 primary carcinoma (tumor size ≤4 cm in the largest dimension and without gross extrathyroidal extension);27 (4) tumor not located within the isthmus or pyramidal lobe; (5) contralateral lobe without nodes or benign contralateral nodes confirmed by a postoperative pathological assessment; (6) unilateral cN1a disease (ipsilateral paratracheal lymph node metastases verified by preoperative imaging and FNA or by inspection at the time of surgery);27 and (7) the absence of distant metastasis. Patients with multifocal unilateral PTC were included. Patients who met the inclusion criteria were divided into two groups based on the status of the contralateral paratracheal LNM.
Surgical Procedure
All surgeries were performed by the same surgical team, which included two experienced thyroid surgeons. To ensure comprehensive removal of lymph nodes from the central compartment, a standardized CCND was performed in accordance with the method described by Pai.28 The recurrent laryngeal nerve was identified and preserved using intraoperative nerve monitoring (IONM). The parathyroid gland was routinely identified and preserved in situ or through autotransplantation. The central neck compartment was bounded superiorly by the hyoid bone, inferiorly by the innominate artery, laterally by the carotid artery and dorsally by the prevertebral fascia. The central compartment was further divided into the prelaryngeal, pretracheal and paratracheal regions (Figure 2). For accurate compartmentalization, the boundary of each region was marked by suturing prior to CCND. The specimens were isolated and sent for histological examinations.
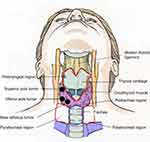 |
Figure 2 Schematic representation of the subsites of the central compartment and the primary tumor site. |
Clinical Assessment
The following data were recorded: patient age; patient sex; primary tumor site; primary tumor size (measured as the longest diameter of the largest lesion); whether the pathology was aggressive; multifocality; microscopic extrathyroidal extension; intraglandular dissemination; the number of metastatic lymph nodes in the ipsilateral paratracheal, pretracheal and paratracheal regions; the total amount of metastatic lymph nodes in these three regions; underlying conditions of chronic lymphocytic thyroiditis (CLT); and the presence of a proto-oncogene B-Raf mutation (BRAF V600E).
The tumor site was further divided into the following regions: left lobe or right lobe, inferior pole or superior pole, and far isthmus or near isthmus region (Figure 2). Inferior pole tumors were located in the lower half of the thyroid gland lobe. The borders of the near isthmus tumors were in contact with the isthmus lateral boundary. In contrast to the near isthmus tumors, the far isthmus tumors were those whose boundaries did not contact the isthmus lateral boundary. Microscopic extrathyroidal extensions were detected only by histological examination. Multifocality was defined as the presence of two or more lesions in a single lobe. Intraglandular dissemination was defined as cancerous embolisms in the lymphatic vessels surrounding a major thyroid carcinoma, with features of heterotypic cells and psammoma bodies.29 The aggressive pathologies included the hobnail variant, columnar cell variant, solid variant and tall cell variant.
Statistical Analysis
Based on our own clinical experience, the rates of contralateral paratracheal LNM reported in previous publications,12–15 and an estimated 10% loss to follow-up rate, we calculated that 243 total patients would be needed to achieve 90% power at the 5% significance level.
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, N.Y., USA). Continuous variables were reported as mean ± standard deviation. A t-test or Mann–Whitney U-test was used to compare continuous variables, while a Chi-square test or Fisher’s exact test was used for categorical variables. Logistic regression was used for multivariate analysis, and independent risk factors were defined as those with an odds ratio (OR) >1. Before logistic regression analysis, correlation analyses and collinearity tests were used for variable selection. P-values <0.05 indicated statistical significance.
Results
Of the 243 patients initially enrolled in this study, two patients were lost at follow-up. Four patients were excluded due to contralateral papillary thyroid microcarcinoma (PTMC) confirmed by postoperative histological examination. Two hundred thirty-seven patients (179 females and 58 males, age 45.9±9.3 years, range 25–68) met the inclusion criteria. The patient, tumor and lymph node characteristics are shown in Table 1. The size of the primary tumor ranged from 6 to 40 mm (average 14.3±7.8 mm). Forty-nine patients (49/237, 20.7%) had metastatic lymph nodes in the contralateral paratracheal region. In addition, 95 patients (40.1%) had positive LNM in the pretracheal region, while 54 (22.7%) had positive LNM in the prelaryngeal region (Table 1). A total of 1557 T1 or T2 PTC patients (86.5%) undergoing simultaneous surgeries had cN0 stage.
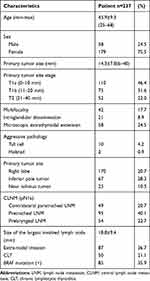 |
Table 1 Demographic, Tumor and Lymph Node Characteristics of Patients |
The follow-up period was 3–12 months (median 8 months). The rate of structural lymph node recurrence in the neck after the first surgery was 1.3% (3/237 patients). The time to recurrence among the 3 patients was 5.3±1.2 months (4 months, 5 months, and 7 months). All of these patients experienced recurrence in the ipsilateral lateral neck region. Among the 237 patients, seven had temporary recurrent laryngeal nerve injury confirmed by IONM and laryngoscopy within 7 days after surgery, and nerve function recovery was verified with laryngoscopy after 3 months. Eighteen patients had permanent hypoparathyroidism, and their parathyroid hormone (PTH) levels remained below the lower limit of the normal range at 3 months after surgery.
In the univariate analysis, a larger number of male patients (p=0.003) were diagnosed with contralateral paratracheal LNM. The patients with contralateral paratracheal LNM had larger tumors (p<0.001) and more commonly had T2 diseases (21–40 mm) (p<0.001). More patients in the contralateral paratracheal LNM (+) group had metastatic lymph nodes in the pretracheal region (p<0.001) but not in the prelaryngeal region. In the contralateral paratracheal LNM (+) group, a larger number of patients had more than five metastatic lymph nodes. Contralateral paratracheal LNM was more likely to occur in patients with aggressive pathology (p<0.001), inferior pole (p=0.011) or near isthmus (p<0.001) tumors, extranodal invasion (p=0.003) and intraglandular dissemination (p=0.009) (Table 2). Age, multifocality, microscopic extrathyroidal extension, the size of the largest involved lymph node, CLT and BRAF gene mutations were not associated with contralateral paratracheal LNM. In the final multivariate analysis, the male sex (p=0.005, OR=17.545), T2 disease (p=0.003, OR=34.317), an inferior pole (p=0.022, OR=8.289) or near isthmus tumor (p=0.001, OR=40.229), aggressive pathology (p=0.027, OR=48.063), pretracheal LNM (p=0.002, OR=14.235) and having more than five metastatic lymph nodes (p=0.025, OR=23.426) were independent predictive factors of contralateral paratracheal LNM (Table 3).
 |
Table 2 Univariate Analysis of Clinical and Pathological Factors Related to Contralateral Paratracheal LNM |
 |
Table 3 Multivariate Logistic Regression for Contralateral Paratracheal LNM |
Discussion
Bilateral CCND has many advantages, including the fact that it leads to a low local recurrence rate because other potentially metastatic lymph nodes are removed in the process.21,22 However, unilateral CCND can obviate the possibility of bilateral recurrent laryngeal nerve injury and permanent hypoparathyroidism because it does not involve exploration of the contralateral central neck compartment. Therefore, it is very important to evaluate preoperative and intraoperative predictive factors of contralateral paratracheal LNM and avoid unnecessary neck dissections. In this study, contralateral paratracheal LNM was uncommon in the cN1a PTC patients (20.7%; 49/237). Other studies have reported similar rates of contralateral paratracheal LNM (9.8–27.3%) in cN0 PTC patients.12–15
Previous studies have shown that PTC with a maximal diameter of >10 mm is associated with a high rate of concomitant contralateral paratracheal LNM.9,30–33 However, this study showed that the presence of a T2 disease (21–40 mm) was an independent predictive factor of contralateral paratracheal LNM (p=0.003, OR=34.317). This inconsistency may be attributable to the fact that patients with tumors measuring >40 mm and gross extrathyroidal extension (stage T3 and T4) were included in previous studies. In this study, we analyzed the relationship between the primary tumor site and contralateral paratracheal LNM and observed that both inferior pole (p=0.022, OR=8.289) and near isthmus tumors (p=0.001, OR=40.229) were independent predictive factors of contralateral paratracheal LNM in T1N1a or T2N1a PTC. Previous studies have reported that patients with PTC located in the isthmus are more likely to have CLNM, especially pretracheal and prelaryngeal LNM, and bilateral CCND should be considered.34–36
According to this study, male sex (p=0.005, OR=17.545) and the presence of an aggressive pathology (p=0.027, OR=48.063) were independent predictive factors of contralateral paratracheal LNM. Men are known to be at high risk for thyroid cancer.35,37–40 One previous study reported that male sex was associated with a high rate of contralateral paratracheal LNM.30 Aggressive pathologies have also been identified as risk factors for PTC invasion and metastasis.2 PTC with aggressive pathology was more likely to have extrathyroidal extension and lymph node metastasis than typical PTC.41–43
Although previous studies have reported that multifocality is predictive of CLNM,38–40 we found that multifocality was not associated with contralateral paratracheal LNM. Genetic studies have shown that some multifocal PTC are caused by intraglandular dissemination of a single cancer focus.44,45 In this study, cancers with scattering foci that were detectable only by microscopy were classified as PTC with intraglandular dissemination. Previous studies have suggested that patients with intraglandular dissemination have a higher likelihood of developing LNM.46 In this study, univariate analyses confirmed that patients with intraglandular dissemination are more likely to develop contralateral paratracheal LNM (p=0.009); however, intraglandular dissemination was not an independent predictor in the multivariate analysis. Patients with intraglandular dissemination are more likely to have LNM, but the specific metastatic direction may be more strongly influenced by other factors.
This study showed that pretracheal LNM (p=0.002, OR=14.235) and the presence of more than five metastatic lymph nodes (p=0.025, OR=23.426) were independent predictive factors of contralateral paratracheal LNM. Previous studies have reported that prelaryngeal/pretracheal LNM is a potential independent predictor of concomitant contralateral paratracheal LNM.31,33 In this study, however, prelaryngeal LNM was not an independent predictor of contralateral paratracheal LNM. As previously reported, the presence of >5 positive nodes and extranodal invasion were associated with a moderate risk of recurrence and low disease-specific survival in pN1 patients.6 Some studies have reported that intraoperative frozen section examinations of ipsilateral lymph nodes have high sensitivity, specificity and accuracy in predicting the contralateral nodal status and may be useful in determining the appropriate extent of central compartment node dissection.47–50 The presence of extranodal invasion greatly influences the risk of contralateral paratracheal LNM.51 In this study, extranodal invasion was significant in the univariate analysis but was not an independent predictor of contralateral paratracheal LNM.
The aim of this prospective study was to identify risk factors for contralateral paratracheal LNM in unilateral cT1N1a or cT2N1a PTC; however, long-term follow-up data on the complications, recurrence, and mortality were not available. We analyzed the BRAF gene mutation status in this study but were unable to include other genetic factors that may impact CLNM. Our study was also limited by a small sample size and absence of randomization. A randomized controlled trial with a large sample size is necessary to verify our conclusions.
Conclusion
In conclusion, our data indicate that the male sex and the presence of a T2 disease (21–40 mm), an inferior pole or near isthmus tumor, an aggressive pathology, pretracheal LNM and >5 metastatic lymph nodes may predict contralateral paratracheal LNM in unilateral cT1N1a or cT2N1a PTC. These data may be useful for identifying targets for surveillance or therapeutic interventions for CCND in the future.
Abbreviations
PTC, Papillary thyroid carcinoma; LNM, Lymph node metastasis; CLNM, Central lymph node metastasis; CCND, Central compartment neck dissection; ATA, American Thyroid Association; TT, Total thyroidectomy; FNA, Fine needle aspiration; IONM, Intraoperative nerve monitoring; CT, computed tomography; CLT, Chronic lymphocytic thyroiditis.
Acknowledgments
This research was funded by the Key Technology Research and Development Program of Shandong Province (grant number: 2019GSF108072) and the Natural Science Foundation of Shandong Province (grant number: ZR2019PH082).
Disclosure
The authors declare that they have no conflicts of interest related to this work.
References
1. La Vecchia C, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–2195. doi:10.1002/ijc.29251
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
3. Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. 2014;24(3):472–479. doi:10.1089/thy.2013.0257
4. Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134(6):946–955. doi:10.1016/s0039-6060(03)00424-0
5. Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148(6):1100. doi:10.1016/j.surg.2010.09.019
6. Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22(11):1144–1152. doi:10.1089/thy.2012.0043
7. Eun YG, Lee YC, Kwon KH. Predictive factors of contralateral paratracheal lymph node metastasis in papillary thyroid cancer: prospective multicenter study. Otolaryngol Head Neck Surg. 2014;150(2):210–215. doi:10.1177/0194599813514726
8. Lee KE, Chung IY, Kang E, et al. Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck. 2013;35(5):672–676. doi:10.1002/hed.23016
9. Koo BS, Choi EC, Yoon YH, Kim DH, Kim EH, Lim YC. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009;249(5):840–844. doi:10.1097/SLA.0b013e3181a40919
10. Lee SH, Lee SS, Jin SM, Kim JH, Rho YS. Predictive factors for central compartment lymph node metastasis in thyroid papillary microcarcinoma. Laryngoscope. 2008;118(4):659–662. doi:10.1097/MLG.0b013e318161f9d1
11. Delogu D, Pisano IP, Pala C, et al. Prophylactic central neck lymphadenectomy in high risk patients with T1 or T2 papillary thyroid carcinoma: is it useful? Ann Ital Chir. 2014;85(3):225–229.
12. Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol. 2011;18(8):2245–2250. doi:10.1245/s10434-011-1600-z
13. Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg. 2006;30(1):91–99. doi:10.1007/s00268-005-0113-y
14. Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131(3):249–256. doi:10.1067/msy.2002.120657
15. Wada N, Duh QY, Sugino K, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237(3):399–407. doi:10.1097/01.SLA.0000055273.58908.19
16. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71(9):731–734. doi:10.1177/000313480507100907
17. Leboulleux S, Rubino C, Baudin E, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90(10):5723–5729. doi:10.1210/jc.2005-0285
18. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135(2):139–148. doi:10.1016/s0039-6060(03)00384-2
19. Adam MA, Pura J, Goffredo P, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. 2015;33(21):2370–2375. doi:10.1200/JCO.2014.59.8391
20. Miccoli P, Bakkar S. Surgical management of papillary thyroid carcinoma: an overview. Updates Surg. 2017;69(2):145–150. doi:10.1007/s13304-017-0449-5
21. Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144(6):980–988. doi:10.1016/j.surg.2008.08.035
22. Cappelli C, Castellano M, Braga M, et al. Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol. 2007;95(7):555–560. doi:10.1002/jso.20746
23. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see commetns]. Cancer. 1998;83(12):2638–2648. doi:10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1
24. Gharib H, Papini E, Paschke R, et al. American association of clinical endocrinologists, associazione medici endocrinologi, and EuropeanThyroid association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010;16(Suppl 1):1–43. doi:10.4158/10024.GL
25. Cooper DS, Doherty GM, Haugen BR, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi:10.1089/thy.2009.0110
26. Agha R, Abdall-Razak A, Crossley E, et al. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156–165. doi:10.1016/j.ijsu.2019.11.002
27. Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017;27(6):751–756. doi:10.1089/thy.2017.0102
28. Pai SI, Tufano RP. Central compartment neck dissection for thyroid cancer. Technical considerations. ORL J Otorhinolaryngol Relat Spec. 2008;70(5):292–297. doi:10.1159/000149831
29. Jin H, Yan H, Tang H, Zheng M, Wu C, Liu J. Internal spreading of papillary thyroid carcinoma: a case report and systemic review. Case Rep Endocrinol. 2018;2018:7618456.
30. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009;96(3):253–257. doi:10.1002/bjs.6484
31. Wei T, Chen R, Zou X, Liu F, Li Z, Zhu J. Predictive factors of contralateral paratracheal lymph node metastasis in unilateral papillary thyroid carcinoma. Eur J Surg Oncol. 2015;41(6):746–750. doi:10.1016/j.ejso.2015.02.013
32. Moo TA, Umunna B, Kato M, et al. Ipsilateral versus bilateral central neck lymph node dissection in papillary thyroid carcinoma. Ann Surg. 2009;250(3):403–408. doi:10.1097/SLA.0b013e3181b3adab
33. Chen Q, Wei T, Wang XL, Li ZH, Du ZH, Zhu JQ. The total number of prelaryngeal and pretracheal lymph node metastases: is it a reliable predictor of contralateral central lymph node metastasis in papillary thyroid carcinoma? J Surg Res. 2017;214:162–167. doi:10.1016/j.jss.2015.02.056
34. Lee YC, Na SY, Chung H, Kim SI, Eun YG. Clinicopathologic characteristics and pattern of central lymph node metastasis in papillary thyroid cancer located in the isthmus. Laryngoscope. 2016;126(10):2419–2421. doi:10.1002/lary.25926
35. Vasileiadis I, Boutzios G, Karalaki M, Misiakos E, Karatzas T. Papillary thyroid carcinoma of the isthmus: total thyroidectomy or isthmusectomy? Am J Surg. 2018;216(1):135–139. doi:10.1016/j.amjsurg.2017.09.008
36. Li G, Lei J, Peng Q, et al. Lymph node metastasis characteristics of papillary thyroid carcinoma located in the isthmus: a single-center analysis. Medicine (Baltimore). 2017;96(24):e7143. doi:10.1097/MD.0000000000007143
37. Zhang Q, Wang Z, Meng X, Duh QY, Chen G. Predictors for central lymph node metastases in CN0 papillary thyroid microcarcinoma (mPTC): a retrospective analysis of 1304 cases. Asian J Surg. 2019;42(4):571–576. doi:10.1016/j.asjsur.2018.08.013
38. So YK, Son YI, Hong SD, et al. Subclinical lymph node metastasis in papillary thyroid microcarcinoma: a study of 551 resections. Surgery. 2010;148(3):526–531. doi:10.1016/j.surg.2010.01.003
39. Chang YW, Kim HS, Kim HY, Lee JB, Bae JW, Son GS. Should central lymph node dissection be considered for all papillary thyroid microcarcinoma? Asian J Surg. 2016;39(4):197–201. doi:10.1016/j.asjsur.2015.02.006
40. Zhang L, Wei WJ, Ji QH, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. 2012;97(4):1250–1257. doi:10.1210/jc.2011-1546
41. Nath MC, Erickson LA. Aggressive variants of papillary thyroid carcinoma: hobnail, tall cell, columnar, and solid. Adv Anat Pathol. 2018;25(3):172–179. doi:10.1097/PAP.0000000000000184
42. Kuo EJ, Goffredo P, Sosa JA, Roman SA. Aggressive variants of papillary thyroid microcarcinoma are associated with extrathyroidal spread and lymph-node metastases: a population-level analysis. Thyroid. 2013;23(10):1305–1311. doi:10.1089/thy.2012.0563
43. Cho J, Shin JH, Hahn SY, Oh YL. Columnar cell variant of papillary thyroid carcinoma: ultrasonographic and clinical differentiation between the indolent and aggressive types. Korean J Radiol. 2018;19(5):1000–1005. doi:10.3348/kjr.2018.19.5.1000
44. Park SY, Park YJ, Lee YJ, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer. 2006;107(8):1831–1838. doi:10.1002/cncr.22218
45. Jovanovic L, Delahunt B, McIver B, Eberhardt NL, Grebe SK. Most multifocal papillary thyroid carcinomas acquire genetic and morphotype diversity through subclonal evolution following the intra-glandular spread of the initial neoplastic clone. J Pathol. 2008;215(2):145–154. doi:10.1002/path.2342
46. Sheng L, Shi J, Han B, et al. Predicting factors for central or lateral lymph node metastasis in conventional papillary thyroid microcarcinoma. Am J Surg. 2020;220(2):334–340. doi:10.1016/j.amjsurg.2019.11.032
47. Lee DH, Yoon TM, Kim HK, Lee JK, Kang HC, Lim SC. Intraoperative frozen biopsy of central lymph node in the management of papillary thyroid microcarcinoma. Indian J Otolaryngol Head Neck Surg. 2016;68(1):56–59. doi:10.1007/s12070-015-0900-1
48. Raffaelli M, De Crea C, Sessa L, Giustacchini P, Bellantone R, Lombardi CP. Can intraoperative frozen section influence the extension of central neck dissection in cN0 papillary thyroid carcinoma? Langenbecks Arch Surg. 2013;398(3):383–388. doi:10.1007/s00423-012-1036-3
49. Raffaelli M, De Crea C, Sessa L, Fadda G, Bellantone C, Lombardi CP. Ipsilateral central neck dissection plus frozen section examination versus prophylactic bilateral central neck dissection in cN0 papillary thyroid carcinoma. Ann Surg Oncol. 2015;22(7):2302–2308. doi:10.1245/s10434-015-4383-9
50. Zhou L, Li H, Liang W, Gao C, Chen B. Pretracheal-laryngeal lymph nodes in frozen section predicting contralateral paratracheal lymph nodes metastasis. Eur J Surg Oncol. 2020;46(10 Pt A):1829–1834. doi:10.1016/j.ejso.2020.06.048
51. Hei H, Song Y, Qin J. A nomogram predicting contralateral central neck lymph node metastasis for papillary thyroid carcinoma. J Surg Oncol. 2016;114(6):703–707. doi:10.1002/jso.24403
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
