Back to Journals » International Journal of General Medicine » Volume 12
The expression of microRNA 146a in patients with ischemic stroke: an observational study
Authors Kotb HG, Ibrahim AH, Mohamed EF, Ali OM, Hassanein N, Badawy D, Abdelatty Aly E
Received 26 April 2019
Accepted for publication 18 July 2019
Published 9 August 2019 Volume 2019:12 Pages 273—278
DOI https://doi.org/10.2147/IJGM.S213535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hend G Kotb,1 Amal H Ibrahim,1 Eman F Mohamed,1 Omaima M Ali,2 Nagwa Hassanein,3 Dina Badawy,3 Ehab Abdelatty Aly4
1Department of Internal Medicine, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 2Department of Internal Medicine, Faculty of Medicine, Aswan University, Aswan, Egypt; 3Department of Clinical Pathology, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt; 4Health Radiation Research Department, National Center of Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority, Cairo, Egypt
Aim: We conducted the present prospective study to assess the level of microRNA (miRNA) 146a in patients with ischemic stroke and its correlation with patients’ characteristics.
Methods: We conducted an observational study that included adult patients (≥18 years old) who presented within 24 hrs after the onset of the symptoms of acute ischemic stroke. In addition, age- and sex-matched healthy volunteers were included as control group. The primary outcome in the present study was the difference in miRNA 146a expression between patients with ischemic stroke and control group participants. The expression of miRNA 146a was measured using quantitativereal-time PCR. Quantitativereal-time PCR amplification and analysis were performed using Rotor-Gene Q thermal cycler.
Results: The present study included 44 patients with ischemic stroke and 22 matched controls. Regarding the primary outcome of the present study, the median expression of miRNA 146a in patients with ischemic stroke was −1.98 fold (IQR −27.1–3.9) compared to 1.75 fold (IQR −2.25–5.27) in control group (P<0.001). However, the subgroup analysis showed that the expression of miRNA 146a was significantly downregulated in comatosed patients only (P<0.001). The expression of miRNA 146a correlated negatively with Glasgow Coma Scale score in comatose patients (r=−0.352, P=0.022).
Conclusion: In conclusion, the expression of miRNA 146a is significantly downregulated in ischemic stroke patients. Further studies are needed to assess its diagnostic utility and therapeutic potentials.
Keywords: ischemic stroke, coma, cerebral infarction, miRNA 146a
Introduction
Stroke is one of the major public health burdens worldwide and a leading contributor to the global mortality and morbidities. According to recent epidemiological figures, stroke affects 16.9 million people worldwide every year and accounts for 6.5 million deaths globally in 2016 alone; which ranked it as the second most common cause of global mortality.1,2 The condition is defined as the acute development of vascular insult (whether ischemic or hemorrhagic) that leads to the sudden onset of acute, symptomatic, neurological deficit lasting for >24 hrs or accompanying by death.3 Ischemic strokes account for the vast majority of (≈85%) of cerebrovascular accident and characterized by sustained occlusion of the cerebral blood vessel, with the subsequent reduction in regional blood flow and central area of irreversibly damaged tissue within few hours.4 Patients with ischemic strokes can present with a wide range of clinical features, according to the size and location of the vascular insult, including aphasia, hemiparesis, and cranial nerve dysfunction.5 In addition to the increased risk of mortality, ischemic stroke is a leading cause of short- and long-term disabilities in affected individuals; patients with ischemic strokes are at increased risk of cerebral edema, hemorrhage within the infarct area, venous thrombosis, seizures, depression, and functional disabilities.6,7 Moreover, improper management of ischemic strokes can increase health care expenditure and represents an economic burden.8 Therefore, prompt diagnosis and treatment of ischemic strokes are critical for improving patients’ outcomes, the current mainstay management options for ischemic strokes include thrombolytic and/or endovascular treatment.9
 |
Figure 2 The correlation between miRNA 146a expression and GCS.Abbreviations: GCS, Glasgow Coma Scale; miRNA, microRNA. |
On the other hand, the diagnosis of acute ischemic stroke is challenging due to the wide range of possible differential diagnosis, in which thrombolysis is contraindicated in some of them, and the time‐sensitive nature of the condition.10 Owing to the major technological advances in neuroimaging, the diagnostic accuracy and prognosis of stroke have improved dramatically in the past few decades. However, imaging studies are limited by low sensitive to infarction in the first 2 hrs and unavailability in limited-resource areas.11 Thus, innovative diagnostic tools and biomarkers have been proposed including acute phase reactants, IL-6, and microRNAs (miRNA).12
miRNAs are a group of non-protein encoding, small, RNAs that act as inhibitors of translation or degradation of messenger RNAs (mRNAs).13 Over the past decade, a growing body of evidence showed that many circulating miRNAs are major regulators of different cellular processes and pathological conditions such as neurodegenerative diseases and tumorigenesis.14,15 Recently, it was reported that miRNAs are key regulators of many pathological processes in ischemic stroke such as excitotoxicity, oxidative injury to neurons, and post-ischemic inflammation.16 Particularly, miRNA 146a was shown to be significantly involved in downregulation of pro-inflammatory cytokines involved in neurological disorders.17
Nevertheless, the role of miRNA 146a in the diagnosis and prognosis of ischemic stroke is still unclear. Therefore, we conducted the present prospective study to assess the level of miRNA 146a in patients with ischemic stroke and its correlation with patients’ characteristics.
Materials and methods
We followed the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement during the preparation of the present study.18 The present study runs in concordance with the Declaration of Helsinki principles and the guidelines of the International Committee of Medical Journal. The study’s protocol gained the approval of the local ethics and research committee of Al-Zahraa university hospital.
Study design and patients selection
We conducted an observation, prospective, study at Internal Medicine Department and intensive care unit of Al-Zhraa University Hospital through the period from August 2018 to February 2019. Adult patients (≥18 years old) who presented to the Emergency Department within 24 hrs after the onset of the symptoms of acute ischemic stroke were included. Only patients with thrombotic stroke due to the affection of anterior circulation were included. The eligible patients were then grouped according to the presence of coma. Patients with intracerebral hemorrhage, hemorrhagic infarction, or extensive infarction who received anticoagulants or thrombolytic therapy, and patients with other neurological disorders, were excluded. Also, we excluded only patients with extensive infarction who received anticoagulants and/or thrombolysis.19 In addition, age- and sex-matched healthy volunteers were included as control group. A non-probability convenient sampling method was utilized to enroll eligible participants. Written informed consents were obtained from eligible participants or their first-degree relatives prior to study enrollment.
Data collection and mirna 146a assessment
We collected the following data from eligible participants: demographic characteristics, complete medical history, full medical examination, Glasgow Coma Scale (GCS),20 lipid profile, complete blood count (CBC), serum creatinine level, and miRNA 146a level. Blood specimens (5 mL) were collected immediately after admission. The CBC profile was done using Sysmex KX21N (Kobe, Japan); while the biochemical analysis using Cobas C311 (Mannheim, Germany) was done. The expression of miRNA 146a was measured using quantitative real-time PCR.
Quantitative PCR for serum gene expression of miR-146a
Total RNA was extracted from serum samples using miRNeasy Mini kit (Qiagen, Hilden, Germany) according to instructions of manufacturer. The isolated miRNA was reverse transcribed into cDNA using miScript II Reverse Transcription Kit (Qiagen). Quantitative real-time PCR amplification and analysis were performed using Rotor-Gene Q thermal cycler using 20 µL reaction mixture consisting of 10 µL SYBR Green PCR Master Mix (Qiagen), 1 µL forward primer (nM), 1 µL reverse primer (nM), 3 µL cDNA and 5 µL RNase free water performing the following thermal cycling conditions: 95°C for 10 mins, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. All values of miRNA 146a were normalized to the snoRD68 gene (housekeeping gene). The primer sequences for miRNA 146a was as follow: sense, 5′-CAG-CTG-CAT-TGG-ATTTAC-CA-3′ and anti-sense, 5′-GCC-TGA-GAC-TCT-GCC-TTC-TG-3′. Relative expression of the miRNA 146a was calculated using by 2−ΔΔCT method.
Study’s outcomes
The primary outcome in the present study was the difference in miRNA 146a expression between patients with ischemic stroke and control group. The secondary outcomes included: 1) the difference in miRNA 146a expression between patients with and without coma and 2) the correlation between miRNA 146a and clinical characteristics of the patients.
Statistical analysis
Data entry, processing, and statistical analysis were carried out using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) and SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 22 for Microsoft Windows. Quantitative data were described in terms of mean±SD, while qualitative data were expressed as frequencies (number of cases) and relative frequencies (percentages). Comparisons of quantitative variables between stroke patients and control were done using unpaired Student t-test for parametric data or Mann–Whitney Rank Sum test for non-parametric data. While the comparisons between the three study’s groups were done using ANOVA for parametric data and Kruskal–Wallis H test for non-parametric data. Chi-square test was performed for categorical variables. A P<0.05 was considered statistically significant.
Results
The present study included 44 patients with ischemic stroke and 22 matched controls. The mean ages of the included patients and control group were 63.7±6.4 and 64.4±6.2, respectively (P=0.05). Patients with ischemic stroke had significantly higher cholesterol level (P<0.001), serum urea level (P<0.001), and serum creatinine level (P<0.001). On the other hand, patients group had significantly lower serum hemoglobin and hematocrit values (P<0.05). Table 1 shows the baseline demographic and laboratory characteristics of the included participants.
 |
Table 1 The baseline demographic and laboratory characteristics of the included participants |
Regarding the primary outcome of the present study, the median expression of miRNA 146a in patients with ischemic stroke was −1.98 (−27.1–3.9), compared to 1.75 (−2.25–5.27) in control group (P<0.001). However, the subgroup analysis showed that the expression of miRNA 146a was significantly downregulated in comatose patients compared to control group (P<0.001), while there was no statistically significant difference in the levels of miRNA 146a between non-comatose patients and healthy individuals (P=0.53; Figure 1). The expression of miRNA 146a correlated negatively with GCS in comatose patients (r=−0.352, P=0.022; Figure 2). Similarly, the expression of miRNA 146a correlated positively with hemoglobin and hematocrit values, and negatively with platelet count (P<0.05; Table 2).
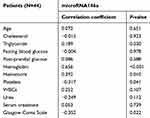 |
Table 2 The correlation between miRNA 146a and the clinical/laboratory parameters of the included patients |
 |
Figure 1 The distribution of miRNA 146a levels across studied groups.Note: The * symbols represent outliers.Abbreviation: miRNA, microRNA. |
Discussion
Over the last few years, a growing body of evidence showed that miRNAs play key regulatory roles in many physiological processes that can limit the onset and progression of ischemic strokes. However, there is no consensus regarding their diagnostic and therapeutic values.16 In the present study, we found that the expression of miRNA 146a was significantly downregulated in comatose patients with severe ischemic stroke, compared to healthy volunteers and ischemic patients without coma. The expression of miRNA 146a was negatively correlated with GCS as well.
Recently, the concept of NeurimmiR has emerged in line with the dramatic increase in our understanding of the impact of the biological functions of several miRNAs on the pathogenic processes of neurological disorders. Based on the findings of Iyer et al,17 the expression of miRNA 146a exhibited anti-inflammatory effect during the astrocyte-mediated inflammation by suppressing pro-inflammatory cytokines, as IL-1β and IL-6. Moreover, the miRNA 146a was found to downregulate cyclooxygenase-2 (COX-2) mRNA in the glial cell.21 Thus, we hypothesized that the expression of miRNA 146a is significantly reduced in the setting of ischemic stroke which can be used as a promising biomarker. The present study showed that the expression of miRNA 146a was correlated with the severity of the ischemic stroke and it was significantly downregulated in patients with coma, compared to healthy controls. In agreement with our findings, Li et al22 reported that the level of miRNA 146a was significantly lower in ischemic stroke patients in the acute phase, compared to patients in the subacute phase and healthy controls. Such findings were further supported by genetic studies which demonstrated a significant association between miRNA 146a polymorphisms and the risk of ischemic stroke23,24; however, this association is still debatable.25
Despite the exact function of miRNA 146a downregulation during the acute phase of ischemic stroke is unclear, the previous work by Zhou et al26 demonstrated that the miRNA 146a downregulation has a protective effect on neural cells and it targeted pro-apoptotic genes to reduce apoptosis. Notably, a more recent report found that miRNA 146a/b significantly downregulated tumor necrosis factor receptor-associated factor 6 and IL-1 receptor-associated kinase 1 during the recovery phase of ischemic stroke, which in return favor the proliferation and migration of endothelial progenitor cells.27 miRNA 146a was found to enhance stroke-induced oligodendrogenesis as well.28 These findings, along with the finding of Iyer et al17 about the inhibitors of miRNA 146a on pro-inflammatory cytokines and COX-2 in various brain cells, highlight the potential role of miRNA 146a as promising therapeutic target during the acute and recovery phases of ischemic stroke.29 Therefore, future research is still needed to evaluate the potential therapeutic implications of miRNA 146a in ischemic stroke.
We acknowledge that the present study has a number of limitations. The study included a small number of patients and the power analysis was not planned prior to study enrollment. Moreover, the study was a single-center experience. Such factors may affect the generalizability of our findings. Moreover, patient-centered and long-term outcomes were not utilized in the present study.
Conclusion
The expression of miRNA 146a is significantly downregulated in patients with severe ischemic stroke. The expression of miRNA 146a showed a declining trend in patients with more severe status. Nevertheless, further studies are needed to assess its diagnostic utility and therapeutic potentials.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Béjot Y, Daubail B, Giroud M. Epidemiology of stroke and transient ischemic attacks: current knowledge and perspectives. Rev Neurol (Paris). 2016;172(1):59–68. doi:10.1016/j.neurol.2015.07.013
2. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018. doi:10.1055/s-0038-1649503
3. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2013. doi:10.1161/STR.0b013e318296aeca
4. Meschia JF, Brott T. Ischaemic stroke. Eur J Neurol. 2018;25(1):35–40. doi:10.1111/ene.13409
5. Hasan TF, Rabinstein AA, Middlebrooks EH, et al. Diagnosis and management of acute ischemic stroke. Mayo Clin Proc. 2018;93(4):523–538. doi:10.1016/j.mayocp.2018.02.013
6. van der Worp HB, Raaijmakers TWMD, Kappelle LJ. Early complications of ischemic stroke. Curr Treat Options Neurol. 2008;10(6):440–449. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18990312. Accessed April 8, 2019.
7. Civelek GM, Atalay A, Turhan N. Medical complications experienced by first-time ischemic stroke patients during inpatient, tertiary level stroke rehabilitation. J Phys Ther Sci. 2016;28(2):382–391. doi:10.1589/jpts.28.382
8. Roth EJ, Lovell L, Harvey RL, Heinemann AW, Semik P, Diaz S. Incidence of and risk factors for medical complications during stroke rehabilitation. Stroke. 2001. doi:10.1161/01.STR.32.2.523
9. Musuka TD, Wilton SB, Traboulsi M, Hill MD. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ. 2015. doi:10.1503/cmaj.140355
10. Erbguth F. Stroke mimics and stroke chameleons: differential diagnosis of stroke. Fortschritte der Neurol Psychiatr. 2017. doi:10.1055/s-0043-111889
11. Chiu AH, Phillips TJ, Phatouros CC, et al. CT perfusion in acute stroke calls: a pictorial review and differential diagnoses. J Med Imaging Radiat Oncol. 2016. doi:10.1111/1754-9485.12422
12. Cucchiara B, Nyquist P. Blood markers in TIA: array of hope? Neurology. 2011. doi:10.1212/WNL.0b013e318236f110
13. Liu J. Control of protein synthesis and mRNA degradation by microRNAs. Curr Opin Cell Biol. 2008. doi:10.1016/j.ceb.2008.01.006
14. Bourassa MW, Ratan RR. The interplay between microRNAs and histone deacetylases in neurological diseases. Neurochem Int. 2014. doi:10.1016/j.neuint.2014.03.012
15. Mocellin S, Pasquali S, Pilati P. Oncomirs: from tumor biology to molecularly targeted anticancer strategies. Mini Rev Med Chem. 2009. doi:10.2174/138955709787001802
16. Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. J Stroke. 2017;19(2):166–187. doi:10.5853/jos.2016.01368
17. Iyer A, Zurolo E, Prabowo A, et al. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One. 2012;7(9):e44789. doi:10.1371/JOURNAL.PONE.0044789
18. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies*. Int J Surg. 2014;12(12):1495–1499. doi:10.1016/j.ijsu.2014.07.013
19. Shahpouri MM, Mousavi SA, Khorvash F, Mousavi SM, Hoseini T. Anticoagulant therapy for ischemic stroke: a review of literature. J Res Med Sci. 2012;17(4):396–401.
20. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974. doi:10.1016/S0140-6736(74)91639-0
21. Li X, Gibson G, Kim JS, et al. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene. 2011. doi:10.1016/j.gene.2011.03.003
22. Li S-H, Su S-Y, Liu J-L. Differential regulation of microRNAs in patients with ischemic stroke. Curr Neurovasc Res. 2015;12(3):214–221. doi:10.2174/1567202612666150605121709
23. Zhu R, Liu X, He Z, Li Q. miR-146a and miR-196a2 polymorphisms in patients with ischemic stroke in the Northern Chinese Han population. Neurochem Res. 2014;39(9):1709–1716. doi:10.1007/s11064-014-1364-5
24. Jeon YJ, Kim OJ, Kim SY, et al. Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arterioscler Thromb Vasc Biol. 2013;33(2):420–430. doi:10.1161/ATVBAHA.112.300251
25. Li C-X, Weng H, Zheng J, Feng Z-H, Ou J-L, Liao W-J. Association between microRNAs polymorphisms and risk of ischemic stroke: a meta-analysis in Chinese individuals. Front Aging Neurosci. 2018;10:82. doi:10.3389/fnagi.2018.00082
26. Zhou X, Su S, Li S, et al. MicroRNA-146a down-regulation correlates with neuroprotection and targets pro-apoptotic genes in cerebral ischemic injury in vitro. Brain Res. 2016. doi:10.1016/j.brainres.2016.07.034
27. Su Z-F, Sun Z-W, Zhang Y, Wang S, Yu Q-G, Wu Z-B. Regulatory effects of miR-146a/b on the function of endothelial progenitor cells in acute ischemic stroke in mice. Kaohsiung J Med Sci. 2017;33(8):369–378. doi:10.1016/J.KJMS.2017.05.010
28. Liu XS, Chopp M, Pan WL, et al. MicroRNA-146a promotes oligodendrogenesis in stroke. Mol Neurobiol. 2017;54(1):227–237. doi:10.1007/s12035-015-9655-7
29. Martinez B, Peplow P. Immunomodulators and microRNAs as neurorestorative therapy for ischemic stroke. Neural Regen Res. 2017;12(6):865. doi:10.4103/1673-5374.208540
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
