Back to Journals » OncoTargets and Therapy » Volume 13
The Expression of IgG and IgG4 in Orbital MALT Lymphoma: The Similarities and Differences of IgG4-Related Diseases
Authors Li KM, Xu MH, Wu X, He WM
Received 18 December 2019
Accepted for publication 9 April 2020
Published 17 June 2020 Volume 2020:13 Pages 5755—5761
DOI https://doi.org/10.2147/OTT.S242852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Kai-Ming Li,1,2 Man-Hua Xu,2 Xia Wu,3 Wei-Min He1
1Department of Ophthalmology, West China Hospital of Sichuan University, Chengdu 610000, People’s Republic of China; 2Department of Ophthalmology, Affiliated Hospital of Southwest Medical University, Luzhou 646000, People’s Republic of China; 3Department of Pathology, West China Second Hospital of Sichuan University, Chengdu 610000, People’s Republic of China
Correspondence: Wei-Min He
Department of Ophthalmology, West China Hospital of Sichuan University, No. 37 Guoxue Lane, Wuhou District, Chengdu 610000, People’s Republic of China
Tel +86 18980601753
Email [email protected]
Objective: This study aims to study the expression and role of IgG and IgG4 in orbital MALT lymphoma and to compare the characteristics of IgG4-related diseases.
Patients and Methods: Patients with orbital MALT lymphoma, treated in the West China Hospital of Sichuan University from 2012 to 2017, were enrolled in the current study. The immunological examination of the wax blocks of orbital masses was performed again and the expression level of IgG and IgG4 in pathological tissue was analyzed.
Results: The results presented that the positive rates of IgG and IgG4 in the cases of orbital MALT lymphoma were 90.91% and 61.98% respectively, of which IgG4/IgG > 40% accounted for 49.33%. The positive rates of IgG and IgG4 in relapse cases were 94.60% and 70.27% respectively, and IgG4/IgG > 40% accounted for 42.31%. There was no significant change in the expression of IgG and IgG4 in cases of lymphoproliferation converting to MALT lymphoma whereas, in cases of MALT lymphoma postoperatively converting to lymphoproliferation, there was an increase in IgG and IgG4 expression, with the change of IgG4 being significant.
Conclusion: IgG and IgG4 have a high correlation in the pathogenesis of MALT lymphoma and may even play an important role in the transformation of MALT lymphoma into orbital lymphoid hyperplasia. Given the association of IgG4 with inflammation and tumors and as an important diagnostic indicator for IgG4-RD and IgG4-related ophthalmic diseases, IgG4 may play an important role in these diseases.
Keywords: MALT lymphoma, IgG, IgG4, IgG4-related ophthalmic diseases, inflammation
Introduction
Malignant lymphomas include three major types of lymphoid tumors: B cell lymphoma, T and NK cell lymphoma, and Hodgkin’s lymphoma.1 The extranodal marginal zone B cell lymphoma (MALT lymphoma), which is a unique B-cell lymphoma, is a clinically heterogeneous entity in a set of low-grade B-cell lymphomas in a wide range of different extranodal areas, including the stomach, lung, ocular annex, and skin.2–4 MALT lymphoma was first reported in 19835 and this report showed that some of the gastric lymphoma cases did not present as a lymph node structure but was clearly characterized by a Peyer patch, which is the physiological set of the terminal lymphoid cells of the ileum. It was also shown that MALT lymphoma is a common lymphoma which accounts for 7 to 8% of new lymphoma cases; it is rare in follicular and diffuse large B-cell lymphomas, but is similar to the incidence of mantle cell lymphoma.6,7
In recent years, a new type of disease, IgG4 related disease (IgG4-RD), has also been widely recognized and accepted. It is a new clinical disease that behaves like many malignant, infectious, and inflammatory diseases8 and is characterized by an increase in serum IgG4 concentration and swelling or tissue infiltration of IgG4 positive plasma cells.9 As a new clinical entity, IgG4-RD has been placed into a unique, clinically independent disease category but there are still many issues to be elucidated, including its pathogenesis, establishment of diagnostic criteria, and the role of IgG4.10–13 There is evidence that malignant orbital tumors can be caused by IgG4-related orbital inflammation.14 Indirect infection or chronic local antigen stimulation can lead to the occurrence of ectopic B-cell lymphoma.15 While MALT lymphoma is also the most common lymphoma in the orbit,16 the association of IgG4 with MALT lymphoma has also been gradually noted and studied.17
Therefore, we conducted this trial to investigate the expression and role of IgG and IgG4 in orbital MALT lymphoma and to compare the characteristics of IgG4-related diseases.
Patients and Methods
General Information
We collected information and samples from patients who were pathologically diagnosed with orbital MALT lymphoma from 2009 to 2016 for inpatient surgery at the West China Hospital of Sichuan University. All patients received surgical treatment. The ethics committee of our hospital approved this study and all patients signed informed consent.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) patients who were diagnosed with orbital MALT lymphoma; (2) and were older than 18. Exclusion criteria: (1) patients whose clinical data were incomplete; (2) poor preservation of specimens.
Methods
This retrospective study was conducted in a group of previously unselected patients with MALT lymphoma of the ocular adnexal in Sichuan, China. The pathological specimens of all the above patients that were fixed in formalin and paraffin-embedded were retrieved from the pathology file of the West China Hospital of Sichuan University and we re-sliced the samples for the pathological examination. Immunofluorescence double staining was used to detect IgG and IgG4. Steps: 1. Paraffin section dewaxing and rehydration; 2. Antigen repair: PH6.0 citrate buffer as the repair solution, the slice was placed in a 97°C water bath for 40 minutes and then slowly recovered to room temperature; 3. Block: 3%H2O2-methanol was blocked at room temperature for 30 minutes and then washed with water; 4. We marked the specimens with a marker; 5. Close: we covered the tissue section with 5% BSA for 30 minutes in a 37 °C incubator; 6. We added the first antibody: no washing was completed, we only removed the blocking solution and directly added the prepared first antibody working solution Anti-IgG4 (concentration 1:5000) and Anti-Human IgG (concentration: 1:500) and incubated this overnight in a refrigerator at 4 °C; 7. We took them out the next morning, slowly rewarmed them for about 1/2 an hour to room temperature, and washed them many times; 8. We added a secondary antibody: added IgG4 secondary antibody working solution and IgG secondary antibody working solution, in turn, incubated them in the 37°C incubator for 1 hour and washed them several times; 9. The fluorescent markers Rhod, FITC, and DAPI were added in turn to label IgG4, IgG, and the nucleus respectively and the slices were finally closed (Figure 1). Each pathological section was photographed 3 times under a high-power fluorescence microscope, IgG+ and IgG4+ cells were counted, and the average of the counts of the three pictures was recorded. The serum IgG4 test results and the MRI findings of the patients during hospitalization were collected and used as a reference for diagnosis and comparison.
 |
Figure 1 Fluorescent images of different channels: (A) Rhod; (B) FITC; (C) DAPI; (D) Three channels. |
Statistic Analysis
We used the software program SPSS 20.0 (IBM, Chicago, USA) to conduct the statistical analysis. Continuous variables were expressed as mean± SD. Discontinuous variables were expressed as a percentage (%). A value of P<0.05 was considered statistically significant.
Results
The General Characteristics
A total of 121 patients were included in this study. Among these 121 patients, 80 participants were male and 41 participants were female. Their ages ranged from 42 to 71, with an average onset age (53.7 ± 12.5).
The Main Outcomes
Of the 121 cases diagnosed with MALT lymphoma, 110 were IgG+, accounting for 90.91%; 75 were IgG4+, accounting for 61.98%, of which 37 of IgG4/IgG>40% accounted for all MALT cases. 30.58%, accounted for 49.33% in IgG+ cases, and 3 cases (2.48%) in compliance with IgG4-ROD criteria. Of the 37 cases of recurrent MALT lymphoma, 35 were IgG+, accounting for 94.60%; 26 were IgG4+, accounting for 70.27%, of which 11 of IgG4/IgG>40% accounted for 29.72% of all MALT cases, accounting for 42.31% of IgG+ cases. There were also 3 cases with orbital lymphoid hyperplasia, recurrence, and worsening of the MALT lymphoma and 1 case showed elevated IgG expression with no change in IgG4 and no significant changes in IgG and IgG4 expression in the other 2 cases.
Seven cases were converted to lymphocyte proliferation. Among these 7 cases, the proportions of cases found in IgG+ were 71.43% (5 cases), the proportions of cases found in IgG4+ were 42.86% (3 cases). Another case showed no significant change in the expression of IgG and IgG4. However, only 1 patient with MALT lymphoma who converted to lymphoid hyperplasia had IgG4/IgG >40%, while 4 patients with lymphoproliferative had IgG4/IgG >40% (Table 1).
 |
Table 1 The Expression Level of IgG and IgG4 Among Seven Cases Which Were Converted to Lymphocyte Proliferation |
IgG+ cells and IgG4+ cells showed diffuse distribution (Figure 2), focal distribution (Figure 3), scattered distribution (Figure 4), and no expression (Figure 5). The distribution was not correlated with IgG4/IgG.
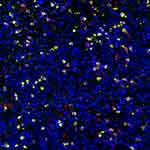 |
Figure 2 Diffusion distribution of IgG4+ cells. |
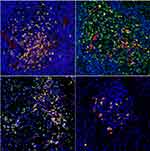 |
Figure 3 Local distribution of different forms of IgG4+ cells. |
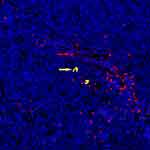 |
Figure 4 Scattered distribution of IgG4 cells (red →: red blood cells; yellow→: IgG4+ cells). |
 |
Figure 5 No IgG 4 or IgG expression. |
Discussion
Patients with organ-specific criteria can be diagnosed with IgG4-RD. IgG4-RD was identified as a refractory disease in 2015 and it is currently believed that further research is needed to clarify whether IgG4-RD is indeed a unique disease entity or a complex disease with different etiology and clinical conditions.18–22 Orbit is one of the most easily involved sites of IgG4-RD and it is also the preferred site of non-gastric MALT lymphoma.23–26 It has been reported that IgG4 positive plasma cells may appear in MALT lymphoma and there was also a correlation between IgG4-RD and MALT lymphoma. Therefore, there is a view that MALT lymphoma belongs to IgG4-RD.27 In our study, there was a significant correlation between IgG and IgG4 and orbital MALT lymphoma. The percentage of IgG+ specimens and IgG4+ was 90.91% and 61.98% respectively, which indicated that IgG and IgG4 played an important role in the pathogenesis of MALT lymphoma. There were only 30.58% of our patients with IgG4/IgG > 40% in the MALT lymphoma cases and less than half in the IgG4+ patients. Fewer cases met the diagnostic criteria of IgG4-RD or IgG4 related ophthalmic disease.
This means that the correlation between MALT lymphoma and IgG4-RD may not be as certain as some literature suggests, that is to say, the incidence of MALT lymphoma is significantly related to IgG and IgG4 but it does not prove that MALT lymphoma is IgG4-RD. Although the cases with IgG4/IgG > 40% accounted for a certain proportion, most of our cases do not meet the diagnostic criteria of IgG4-RD or IgG4-related ophthalmopathy, the main reason being the serum IgG4 < 135 mg/dL.
Although the cases with IgG4/IgG > 40% accounted for a certain proportion, most of our cases do not meet the diagnostic criteria of IgG4-RD or IgG4-related ophthalmic disease.28–31 Because the immune system is exposed to tumor-associated antigens for a relatively long time and the tumor itself can cause and promote inflammation, the inflammatory state in the tumor supports the increase of IgG4.32 IgG4 may even promote tumor-related immune surveillance escape and its effect on cancer immunotherapy through some mechanisms, while it has also been reported that lymphoid cells may produce monoclonal IgG-kappa.33
At the same time, the relationship between inflammation and tumors has been confirmed,34 including lymphoma.35 Among them, MATL lymphoma is considered to be a tumor model mediated by chronic inflammation.36 This suggests that the increase of IgG and IgG4 is significantly associated with the recurrence of MALT lymphoma, but it is not directly related to the criteria of IgG4/IgG > 40% or even IgG4-RD. There was no marked change in the expression of IgG4 in a small number of cases, which converts from the orbital lymph hyperplasia into MALT lymphoma. This perhaps suggests that the deterioration of the lesion does not significantly affect the expression of IgG and IgG4. In the cases showing a conversion from MALT lymphoma into orbital lymphoproliferative lesion, the expression of IgG and IgG4 increased significantly and the proportion of IgG4/IgG > 40% was also increased in patients with orbital lymphohyperplastic lesions. Lymphoproliferative lesions were also caused by inflammatory stimuli, confirming the association of IgG+ and IgG4+ with inflammation again and even IgG or/and IgG4 play an important role in the transformation of MALT lymphoma into orbital lymphoproliferative lesions. Because of the small number of cases, such conclusions should be more cautious and require a larger sample study to confirm it.
Limitations. Firstly, this trial was only a retrospective study, not a randomized controlled trial. Secondly, this study was only a single-center trial and the sample size was limited. Thirdly, the relationship between MALT lymphoma and IgG4-RD remains uncertain and should be studied further. Fourthly, IgG4 plays an important role in these diseases but the specific mechanism was unknown and should be studied further.
Conclusion
IgG and IgG4 have a high correlation in the pathogenesis of MALT lymphoma and may even play an important role in the transformation of MALT lymphoma into orbital lymphoid hyperplasia. Given the association of IgG4 with inflammation and tumors and as an important diagnostic indicator for IgG4-RD and IgG4-related ophthalmic diseases, IgG4 may play an important role in these diseases.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bledsoe JR, Wallace ZS, Deshpande V, et al. Atypical IgG4+ plasmacytic proliferations and lymphomas: characterization of 11 cases. Am J Clin Pathol. 2017;148(3):215–235. doi:10.1093/AJCP/AQX067
2. Schreuder MI, van den Brand M, Hebeda KM, Groenen PJTA, van Krieken JH, Scheijen B. Novel developments in the pathogenesis and diagnosis of extranodal marginal zone lymphoma. J Hematopathol. 2017;10(3–4):91–107. doi:10.1007/s12308-017-0302-2
3. Nasu A, Igawa T, Sato H, Yanai H, Yoshino T, Sato Y. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue with plasma cell differentiation: periodic acid-schiff reaction-positive Dutcher body is a diagnostic clue to distinguish it from plasmacytoma. Diagn Cytopathol. 2017;45(6):547–551. doi:10.1002/dc.23691
4. NHL Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89(11):3909–3918. doi:10.1182/blood.V89.11.3909
5. Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52(8):1410–1416. doi:10.1002/1097-0142(19831015)52:8<1410::AID-CNCR2820520813>3.0.CO;2-3
6. Meng J, Chang C, Pan H, et al. Epidemiologic characteristics of malignant lymphoma in Hubei, China: a single-center 5-year retrospective study. Medicine (Baltimore). 2018;97(35):e12120. doi:10.1097/MD.0000000000012120
7. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87.
8. Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64(10):3061–3067. doi:10.1002/art.34593
9. Huijbers MG, Plomp JJ, van der Maarel SM, Verschuuren JJ. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Ann N Y Acad Sci. 2018;1413(1):92–103. doi:10.1111/nyas.13561
10. Umehara H, Okazaki K, Kawano M, Tanaka Y. The front line of research into immunoglobin G4-related disease - Do autoantibodies cause immunoglobin G4-related disease? Mod Rheumatol. 2019;29(2):214–218. doi:10.1080/14397595.2018.1558519
11. Yamamoto M, Hashimoto M, Takahashi H, Shinomura Y. IgG4 disease. J Neuroophthalmol. 2014;34(4):393–399. doi:10.1097/WNO.0000000000000172
12. Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. doi:10.1038/modpathol.2012.72
13. Defrancesco I, Arcaini L. Overview on the management of non-gastric MALT lymphomas. Best Pract Res Clin Haematol. 2018;31(1):57–64. doi:10.1016/j.beha.2017.11.001
14. Takahira M, Ozawa Y, Kawano M, et al. Clinical aspects of IgG4-related orbital inflammation in a case series of ocular adnexal lymphoproliferative disorders. Int J Rheumatol. 2012;2012:635473. doi:10.1155/2012/635473
15. Decaudin D, Ferroni A, Vincent-Salomon A, et al. Ocular adnexal lymphoma and Helicobacter pylori gastric infection. Am J Hematol. 2010;85(9):645–649. doi:10.1002/ajh.21765
16. Sohn EJ, Ahn HB, Roh MS, Jung WJ, Ryu WY, Kwon YH. Immunoglobulin G4 (IgG4)-positive ocular adnexal mucosa-associated lymphoid tissue lymphoma and idiopathic orbital inflammation. Ophthalmic Plast Reconstr Surg. 2018;34(4):313–319. doi:10.1097/IOP.0000000000000965
17. Thompson A, Whyte A. Imaging of IgG4-related disease of the head and neck. Clin Radiol. 2018;73(1):106–120. doi:10.1016/j.crad.2017.04.004
18. Johnston J, Allen JE. IgG4-related disease in the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2018;26(6):403–408. doi:10.1097/MOO.0000000000000487
19. Okazaki K, Kawa S, Kamisawa T, et al. Research committee of intractable diseases of the pancreas. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41(7):626–631. doi:10.1007/s00535-006-1868-0
20. Masaki Y, Sugai S, Umehara H. IgG4-related diseases including Mikulicz’s disease and sclerosing pancreatitis: diagnostic insights. J Rheumatol. 2010;37(7):1380–1385. doi:10.3899/jrheum.091153
21. Kawano M, Saeki T, Nakashima H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15(5):615–626. doi:10.1007/s10157-011-0521-2
22. Hamano H, Tanaka E, Ishizaka N, Kawa S. IgG4-related disease - A systemic disease that deserves attention regardless of one’s subspecialty. Intern Med. 2018;57(9):1201–1207. doi:10.2169/internalmedicine.9533-17
23. Chidambaram VA, Anita CSY, Robert C, Garry C. IgG4 related orbit disease - An unusual cause of an orbital mass. Med J Malaysia. 2018;73(6):415–417.
24. Yamada K, Kawano M, Inoue R, et al. Clonal relationship between infiltrating immunoglobulin G4 (IgG4)-positive plasma cells in lacrimal glands and circulating IgG4-positive lymphocytes in Mikulicz’s disease. Clin Exp Immunol. 2008;152(3):432–439. doi:10.1111/j.1365-2249.2008.03651.x
25. Goto H, Takahira M, Azumi A. Japanese study group for IgG4-related ophthalmic disease. Diagnostic criteria for IgG4-related ophthalmic disease. Jpn J Ophthalmol. 2015;59(1):1–7. doi:10.1007/s10384-014-0352-2
26. Nakayama R, Matsumoto Y, Horiike S, et al. Close pathogenetic relationship between ocular immunoglobulin G4-related disease (IgG4-RD) and ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma. 2014;55(5):1198–1202. doi:10.3109/10428194.2013.823494
27. Hara S, Kawano M, Mizushima I, et al. A condition closely mimicking IgG4-related disease despite the absence of serum IgG4 elevation and IgG4-positive plasma cell infiltration. Mod Rheumatol. 2016;26(5):784–789. doi:10.3109/14397595.2014.916836
28. Li P, Chen H, Deng C, et al. Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related disease in Chinese population. Mod Rheumatol. 2016;26(4):583–587. doi:10.3109/14397595.2015.1117171
29. Otani K, Inoue D, Fujikura K, et al. Idiopathic multicentric Castleman’s disease: a clinicopathologic study in comparison with IgG4-related disease. Oncotarget. 2018;9(6):6691–6706. doi:10.18632/oncotarget.24068
30. Qi R, Chen LYC, Park S, et al. Utility of serum IgG4 levels in a multiethnic population. Am J Med Sci. 2018;355(1):61–66. doi:10.1016/j.amjms.2017.08.014
31. Crescioli S, Correa I, Karagiannis P, et al. IgG4 characteristics and functions in cancer immunity. Curr Allergy Asthma Rep. 2016;16(1):7. doi:10.1007/s11882-015-0580-7
32. Sakai A, Katayama Y, Mizuno A, et al. MALT lymphoma producing IgG-kappa type M-protein. Rinsho Ketsueki. 2000;41(8):658–663.
33. Bahiraee A, Ebrahimi R, Halabian R, Aghabozorgi AS, Amani J. The role of inflammation and its related microRNAs in breast cancer: a narrative review. J Cell Physiol. 2019;234(11):19480–19493. doi:10.1002/jcp.28742
34. Zhang L, XH L, Jin F, et al. Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) associated with or without lymphoma: comparison of clinical features and risk factors suggestive of underlying lymphomas. J Clin Neurosci. 2019;66:156–164.
35. Marcelis L, Tousseyn T, Sagaert X. MALT lymphoma as a model of chronic inflammation-induced gastric tumor development. Curr Top Microbiol Immunol. 2019;421:77–106. doi:10.1007/978-3-030-15138-6_4
36. Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550(7676):402–406. doi:10.1038/nature24050
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
