Back to Journals » Risk Management and Healthcare Policy » Volume 15
The Evaluation of Gait and Balance for Patients with Early Diabetic Peripheral Neuropathy: A Cross-Sectional Study
Authors Jiang X, Deng F, Rui S, Ma Y, Wang M, Deng B, Wang H, Du C, Chen B, Yang X, Boey J , Armstrong DG, Deng W, Duan X
Received 9 February 2022
Accepted for publication 18 March 2022
Published 30 March 2022 Volume 2022:15 Pages 543—552
DOI https://doi.org/10.2147/RMHP.S361698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Xiaoyan Jiang,1,* Fang Deng,1,2,* Shunli Rui,1 Yu Ma,1 Min Wang,1 Bo Deng,1 Hongyan Wang,1 Chenzhen Du,1 Bing Chen,2 Xiuhua Yang,3 Johnson Boey,4 David G Armstrong,5 Wuquan Deng,1 Xiaodong Duan6
1Department of Endocrinology, Chongqing University Central Hospital, School of Medicine Chongqing University, Chongqing, 400014, People’s Republic of China; 2Department of Endocrinology, Chongqing Southwest Hospital, Chongqing, 400038, People’s Republic of China; 3Department of Lower Extremity Surgery, NO.1 Orthopedics Hospital of Chengdu, Chengdu City, 610000, Sichuan, People’s Republic of China; 4Department of Podiatry, National University Hospital, 169608, Singapore; 5Department of Surgery, Keck School of Medicine of University of Southern California, Los Angeles, CA, 90033, USA; 6Department of Rehabilitation, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wuquan Deng, Department of Endocrinology, Chongqing University Central Hospital, School of Medicine Chongqing University, No. 1 Jiankang Road, Yuzhong District, Chongqing, 400014, People’s Republic of China, Tel +86 23 63692185, Email [email protected] Xiaodong Duan, Department of Rehabilitation, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, 646000, People’s Republic of China, Email [email protected]
Objective: Falls often occur in patients with diabetic neuropathy due to biomechanical alternation. The implication of diabetic peripheral neuropathy (DPN) on gait and balance remains poorly understood.
Methods: A total of 11 dynamic gait, balance and electrophysiological parameters were evaluated in 176 participants. The biomechanical parameters were compared between groups.
Results: Stride length and stride velocity were significantly lower in all subgroups of DPN compared with healthy subjects (p< 0.05). Stance phase and double support phase were significantly higher, but swing phase were significantly lower across all subgroups of DPN than healthy subjects (p< 0.05). Under eyes-open standing, the ML and AP range parameters of CoM sway, ankle sway and hip sway, CoM sway index, ankle swing index in both subclinical and confirmed DPN patients were all significantly higher compared to healthy subjects (p< 0.05). Under eyes-closed standing, AP range parameters of CoM sway in subclinical DPN and confirmed DPN patients were significantly higher than healthy subjects (p< 0.05). The hip sway areas in diabetics were significantly higher compared to healthy subjects (p< 0.05).
Conclusion: The abnormal biomechanical parameters existed in the early stages of patients with DPN. The static balance under eyes-open and eye-closed condition is maintained by ankle joint compensation strategy and hip joint protection strategy. An early evaluation and better risk management is essential for diabetic patients with a history of more than 5 years even without DPN clinical symptoms and signs.
Clinical Trial Registration Number: No. ChiCTR1800019179, www.chictr.org.cn.
Keywords: gait, balance, diabetic peripheral neuropathy, risk assessment, wearable biomechanical system
Introduction
Diabetic neuropathy and vascular disease are common serious complications of diabetes. At present, diabetic microvascular complications are widely studied. Some newly identified markers were considered to be a good predictor for one or more diabetic microvascular complications.1–3 However, early detection of diabetic neuropathy remains poorly understood. Diabetic peripheral neuropathy (DPN) is likely to affect up 50% of individual diagnosed with diabetes mellitus, their incidence of which increases with duration of diabetes mellitus.4–6 The most typical form of DPN is a chronic, symmetrical, distal sensorimotor polyneuropathy. The diagnosis of DPN for clinical practice generally depends on a combination of neuropathy symptoms, signs, and nerve conduction study (NCS). However, many factors affects dynamic balance and increase the risk of falls.7,8 The implication of biomechanical abnormalities caused by DPN is leading factors to the development diabetic foot problems and traumatic falls.9–11 A systematic review revealed that diabetic neuropathy has a negative impact on postural balance and gait kinematics for an increased fall risk, but further study is needed because of limited number of studies available.12 A recent study has explored the association between electrophysiological parameters and biomechanical parameters. Interestingly, the results revealed that gait was associated with clinical DPN parameters, but balance was associated with electrophysiological parameters.13 Nevertheless, the question on whether early stage of DPN, as diagnosed by NCS, can influence or alter biomechanical parameters remains to be explored. A further understanding in biomechanical performance at early stage of DPN, especially for the people with long diabetes duration, may provide greater insight into development of gait and balance alterations and falling prevention. In present study, we hypothesized that gait and balance abnormalities could occur at the early stage of DPN without clinical symptoms and signs. Therefore, a novel real-time wearable wireless sensor systems and electrophysiological device were used for verification of our hypothesis.
Materials and Methods
Participants
A total of 176 participants included 144 patients with type 2 diabetes mellitus (T2DM) and 32 healthy volunteers were recruited in a cross sectional study at the University-affiliated Hospital based on a Footwear and Offloading Optimum Therapy (FOOT) study. The assessment of DPN were performed using physical examinations and NCS testing, consistent with the methods described in our previous study.7 Patients with T2DM were allocated into three groups according to clinical symptoms of DPN and results from the NCS test, with reference to the standard of American Diabetes Association recommendation:5 Group A (Non-DPN: no DPN signs or symptoms with negative NCS; n=48), Group B (subclinical DPN: no DPN signs or symptoms with NCS positive; n=45), and Group C (confirmed DPN: both DPN signs, symptoms and NCS positive; n=51). Demographics data were collected from all subjects including gender, age, body mass index (BMI), diabetes duration, Hemoglobin A1c (HbA1c), diabetic retinopathy, diabetic kidney disease, and history of smoking and drinking.
Inclusion and Exclusion Criteria
The participants with T2DM were enrolled and provided a written statement certifying existence of at least one of the following conditions: 1) 18–75 years old; 2) BMI: 18–35kg⁄m2; 3) were able to move or walk independently; Conversely, a participant was excluded if he or she: 1) had lower extremity amputation (toe) in any part, overt foot deformity (eg, Charcot’s arthropathy); 2) had active lower extremity ulcer; 3) Had a history of brain injury (trauma, inflammation, tuberculosis, tumor, epilepsy, etc.); 4) Had visual impairment; 5) Had a history of vertigo, otitis interna or any other vestibular disease; 6) Had spinal diseases (trauma, inflammation, tuberculosis, tumor, disc herniation and compression of nerves, etc.); 7) Had muscle defects or rheumatic diseases; 8) Had orthostatic hypotension, alcoholism, liver and kidney insufficiency; 9) Had pregnancy, special type of diabetes, acute complications of diabetes, HIV positive.
Assessment the Performance of Gait and Balance
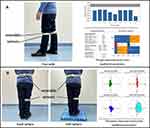
The performance of gait and balance were assessed using validated body-worn sensor technology (LegSysTM and BalanSensTM, BioSensics LLC, Watertown, MA, USA).14 This system is equipped with a triaxial accelerometer and gyroscope that estimates temporal gait, balance, and spatial parameters described in our previous study.8 It generates digitized signals at a frequency of 100 Hz, which is transmitted in real-time via Bluetooth to a laptop. The participants were assessed on multiple static, dynamic parameters of gait and balance. The gait parameters were assessed by a single task free walking that participant walked in one minute with habitual speed without any distraction (Figure 1A). The dynamic gait parameters include stride length, stride length height ratio, stride time, stride velocity, stride velocity height ratio, swing phase, stance phase, and double support phase. The balance parameters were evaluated under double-limb stance eyes open and eyes closed condition, which includes center of mass (CoM), ankle sways, and hip sways in medio-lateral (ML) and anterior-posterior (AP) direction, sway area, sway index, and sway velocity (Figure 1B).
Assessment of Electrophysiological Parameters
An electromyography machine (Medtronic KeyPoint 4, Skovlunde, Denmark) was used for NCS assessment according to our previous study.7 The test of NCS was performed by single qualified medical technologist. Electrophysiological parameters included 26 items: sensory nerve conduction velocity (SCV); sensory nerve action potential amplitude; motor nerve conduction velocity (MCV), motor conduction distal latency, compound muscle action potential (CMAP) amplitude (proximal and distal); and H-reflex latency of the tibial nerve and shortest F-wave latency of the median nerve. The normal reference range of NCS was established based on different age groups based on our previous study. Two or more abnormal index of nerve conduction velocity or nerve action potential amplitude was considered as NCS positive.
Statistical Analysis
All statistical analyses of the experimental date were performed using IBM SPSS version 22.0 (IBM, Armonk, N.Y., USA). Continuous variables are presented as the means ± standard deviations. The enumeration data were described using ratios or rates. The normality of data was analyzed using the Kolmogorov-Smirnov test. For normally distributed data, the comparison between groups was analyzed by one-way ANOVA, and the comparison between the two groups was performed with the least-significant difference (LSD) test. For non-normally distributed data, multiple groups of rank sum test were used for comparison between groups, and further analysis was performed by Kruskal Wallis method. A p-value < 0.05 was considered to be statistically significant for all of the analyses.
Results
General clinical characteristics and biomechanical parameters showed in Table 1.
 |
Table 1 The General Characteristics of Participants |
All three diabetes groups had significantly higher HbA1c values than the healthy controls (p<0.001) and the two DPN groups had significantly higher HbA1c values than the non-DPN diabetic group (p<0.001). There were no significant differences in sex, age, body mass index (BMI), smoking and drinking in all groups (p>0.05). No significant difference in diabetes duration, diabetic retinopathy, and diabetic kidney disease was observed in all diabetics groups (p>0.05).
Gait Parameters Assessment
Stride length, including adjusted for body height, was significantly lower across all subgroups of DPN (absence, subclinical and confirmed) compared with healthy subjects (p<0.05). Further analysis of the subgroups of DPN found that stride length was significantly lower in confirmed DPN than non-DPN diabetics after adjusting for body height. Stride time was significantly longer in both subclinical-DPN and confirmed DPN compared to healthy subjects (p<0.05). Further comparison between different subgroups of DPN revealed that patients with confirmed DPN has significantly protracted stride time as compared to no-DPN patients and subclinical DPN patients (p<0.05). Stride velocity, even adjusting for body height, was significantly lower in all subgroups of DPN versus healthy subjects (p<0.05). Among them, confirmed DPN patients registered significantly lower stride velocity than those without DPN, and stride velocity was the lowest among the subgroups of DPN after adjusting for body height. The steps per minutes was significantly lower in subclinical and confirmed DPN compared with healthy subjects (p<0.05), and steps per minutes was the lowest among the subgroups of DPN. Stance phase and double support phase were significantly higher, but swing phase were significantly lower across all-groups of DPN than healthy subjects (p<0.05) (Table 2), but no significant difference between all diabetic groups (p>0.05).
 |
Table 2 Comparison of Gait Performance |
Balance Parameters in Eyes Open Standing
For static balance parameters with eyes open, the ML and AP range parameters (including CoM sway, ankle sway and hip sway), CoM sway index, ankle swing index in both subclinical and confirmed DPN patients were significantly higher compared to healthy subjects (p<0.05). Though these parameters were increased across all subgroups of DPN but only the AP range parameters of CoM sway and ankle sway, CoM sway index, ankle swing index in confirmed DPN patients were significantly higher than those without DPN (p<0.05). The CoM sway area and ankle sway area in subclinical and confirmed DPN were significantly higher compared to healthy subjects (p<0.05). The CoM sway velocity in confirmed DPN or without DPN was significantly higher than healthy subjects. The highest CoM sway velocity was in confirmed DPN among the subgroups of DPN. The ankle sway velocity in confirmed DPN was significantly higher than healthy subjects, and subclinical DPN. No significant differences were observed in hip sway area, hip sway index, and hip sway velocity among all groups (Table 3).
 |
Table 3 Assessment Balance Performance with Eyes Open |
Balance Parameters in Eyes Closed Standing
For static balance parameters with eyes closed, AP range parameters of CoM sway in subclinical and confirmed DPN patients were significantly lower than healthy subjects (p<0.05), and significantly higher in confirmed DPN than those with diabetes but without DPN (p<0.05). The CoM sway areas and CoM sway velocity in confirmed DPN were significantly higher compared to healthy subjects (p<0.05). The hip sway areas in subject with diabetes were significantly higher compared to healthy subjects (p<0.05). No significant differences were observed in all groups for ankle ML range, ankle AP range, ankle sway area, ankle swing index and ankle sway velocity (Table 4).
 |
Table 4 Assessment Balance Performance with Eyes Closed |
Discussion
It is well known that patients with DPN have an increased risk of falls that result in hospitalization. The American Diabetes Association guidelines for DPN suggest that tests assessing gait and balance could be considered in clinical practice to evaluate risk of falls in patients at risk.15 HbA1c, the average of blood glucose level, was strongly correlated to severity of DPN in present study. A review article involved 336 studies suggested that HbA1c played an important role in DPN, and was considered as a biomarker.16 Our previous study also revealed that not only confirmed DPN but also subclinical DPN had a higher HbA1c.7 Moreover, asymptomatic DPN suggested a high incidence of electrophysiological abnormalities including slowing of motor nerve conduction velocity and lowering of sensory amplitudes.17 As age increased in T2DM, the development and complication of DPN was influenced. So it need further study to explore the difference of gait and balance at the various state of DPN identified by NCS. The wearable wireless real-time acceleration sensor was successfully used to examine the biomechanical parameters, gait and balance in patients with type 2 diabetes, DPN, subclinical DPN, and healthy populations in present study. To the best of our knowledge, it is the first study to report that the association between gait, balance and subclinical DPN tested by electrophysiological device and wearable device.
It is known that people with DPN have an increased risk of falls, but, it is still unknown whether patients without clinical symptoms and signs of DPN would change gait biomechanics and increase the risk of falling in DPN. Therefore, understanding mechanisms of changes in the gait parameters has facilitated understandings about fall risk prediction in the DPN population. Various factors cause gait abnormalities in DPN patients. Our study’s aim is to evaluate the possible association between the presence and severity of DPN and both the balance impairment and the risk of falls in patients with diabetes. The gait performance of diabetic patients was significantly impaired, and the presence of DPN further aggravated its damage. The results suggested that stride length and gait velocity even after adjusting for body height were significantly reduced in confirmed DPN, consistent with previous studies.18,19 Although there was no significant difference between diabetes without DPN and subclinical DPN, a decreased trend was found in stride length and stride velocity. In addition to gait, gait initiation variables also indicated to be important outcomes for clinical management of DPN.20 Moreover, stance phase and double-limb support phase were significantly increased in persons with diabetes. Double-limb support phase tended to be increased in T2DM with subclinical DPN compared with T2DM without DPN, although this difference did not reach statistical significance. The change perhaps was the possible reason for the decline in gait performance of DPN patients. Ankle equinus, as defined by the lack of ankle dorsiflexion during stance phase, is found to 2.8 times more prevalent in patients with DPN.21 This observation is likely to be contributed by the glycosylation of tendo-ligamentous structures which result in the thickening of the Achilles tendon, leading to ankle joint stiffness.22 These compensatory mechanisms will exacerbate limitation of joint mobility and fragility, while increases plantar peak pressures during forefoot loading.23 As previous results, they have shown to have reduced walking velocity, cadence and stride length.19,24 DPN developed clinical signs or symptoms usually later than NCS alternation. The decline of gait parameters could occur at the early stage of even only NCS abnormality without any DPN signs or symptoms.
Patients with DPN are more likely to fall than age-matched controls, although their underlying risk factors are not yet fully understood.11,25 The present study will be of interest to demonstrate that static balance performance damage could be occurred in the early stage of DPN under eye-open condition. However, this phenomenon was almost not found under eye-closed condition. In AP direction, static balance performances in patients with DPN were significantly worse, suggesting that it prone to fall. A previously study have suggested that balance is markedly impaired in patients with DPN and the impairment was predominantly in the ML plane and was greatest during stair descent.8 Perhaps it may contribute to explaining why the higher ML separations found in patients with DPN will require greater muscular demands to control AP posture in order to maintain balance to avoid fall risk. In addition, reduced knee flexion and hip adduction of the swing limb were considered as risk factors for tripping during obstacle-crossing in T2DM patients without or at an early stage of DPN. Increased mechanical demands on the ankle plantarflexors suggest that weakness of these muscles may further reduce the already compromised performance of obstacle-crossing in these patients.26 Muscular atrophy and weakness may be accelerated in elderly patients with known history of hyperglycemia.27
Our results also revealed the joint compensation strategy changed under different situation in the attempt to maintain balance. In eyes open standing, the static balance is maintained by the ankle joint compensation strategy. In contrast, under eye-closed condition, the static balance is maintained by the hip joint protection strategy. As it has been known that peripheral sensory reception, including somatosensory receptors, vision system, and vestibular system was the key controlling systems to maintain body balance. Somatosensory receptors usually represent the ability of joints, muscles, tendons, ligaments, connective tissue and skin. We speculated that the patients without visual input may have a stronger caution and joint protection strategy to avoid to be fallen. So hip joint protection strategy could supply stronger support to avoid body fallen when eyes closed.
Biomechanical factors have been implicated in the development of foot ulceration. Furthermore, a preliminary study altered biomechanical characteristics are likely to influence the healing of plantar DFUs. Therefore, gait retaining technology and off-loading could reduce mechanical loading and improve worsened biomechanical characteristics.8,28,29 A randomized controlled trial performed by Najafi et al with the same sensor technology has proved that patients with DPN could significantly improve their postural balance with specific, tailored, sensor-based exercise training.30 It may indicate that early lesion of biomechanical alternation induced by DPN could be intervened by physical therapy, such as interactive visual joint movement feedback. Similarly, the other specific training also showed it can improve gait speed, balance, muscle strength and joint mobility in diabetic patients after 12 weeks twice weekly with function orientated strengthening.31 Most importantly, neuroimaging evidence for disrupted central sensorimotor circuitry suggested that there may be unrecognized behavioral impairments in individuals with diabetes. Central nervous system (CNS) degeneration might interact with the loss of sensory feedback from the limbs due to DPN to cause motor impairments in individuals with diabetes.32 Moreover, low Vitamin D level is an independent risk factor for muscle weakness in elderly men.33 Therefore, early assessment of the biomechanical parameters among the middle age and elderly patients with DPN symptoms and signs may be the key to reducing the risk of falls, which contributes to search for new prevention measures.19 Efforts to reduce the risk of falling can improve the quality of life of people with diabetes and reduce the use of medical resources. These findings suggest that even patients without symptoms and signs of DPN also should be assessed for gait and balance before safe exercise, and further effective interventions should be acted to prevent falls.
Conclusions
It is the first time to report that early DPN has an important influence on balance control both of open- or closed eyes condition. These changes, which are likely caused by altered activation of the extensor muscles, increase the likelihood of instability and may be important contributory factors for the increased risk of falling. The wearable biomechanical monitoring system may be a potential device for predicting these factors and thereby potentially beneficial for reducing fall risk. We recommend an early screening and better risk factor management for diabetic patients when their diabetes duration is more than 5 years even without clinical symptoms and signs of DPN.
Data Sharing Statement
All data generated and analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Statement of Ethics
All participating subjects signed informed consent for this study and subsequent publications, and all identifying features were removed. The ethical approval to report this work was obtained from the Ethical Committee Board of Chongqing university central hospital and was in accordance with the declaration of Helsinki.
Acknowledgments
The authors thank all of the patients and control subjects for participation in the study. The authors thank Dr. He Zhou and Dr. Bijan Najifi for data analysis. Parts of this study will be presented in abstract form at the ADA’s 79th scientific sessions in San Francisco, CA on June 7–11, 2019.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Joint Medical Research Programs of Chongqing Science and Technology Bureau and Health Commission Foundation (No.2020GDRC023 & No.2022QNXM018), and the Fundamental Research Funds for the Central Universities of Chongqing University (No.2021CDJYGRH012) awarded to Dr. Wuquan Deng and Dr. Min Wang. This study is also partially supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Award Number 1R01124789-01A1 and National Science Foundation (NSF) Center to Stream Healthcare in Place (#C2SHiP) CNS Award Number 2052578 awarded to Prof. DG Armstrong.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Kocak MZ, Aktas G, Erkus E, et al. Neuregulin-4 is associated with plasma glucose and increased risk of type 2 diabetes mellitus. Swiss Med Wkly. 2019;149:w20139. doi:10.4414/smw.2019.20139
2. Kin TB, Tekce H, Aktas G, et al. Evaluation of the urinary kidney injury molecule-1 levels in patients with diabetic nephropathy. Clin Invest Med. 2014;37(6):E377–83. doi:10.25011/cim.v37i6.22242
3. Kocak MZ, Aktas G, Erhus E, et al. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018;28(11):844–847. doi:10.29271/jcpsp.2018.11.844
4. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi:10.2337/dc10-1303
5. Watson JC, Dyck PJ. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90(7):940–951. doi:10.1016/j.mayocp.2015.05.004
6. Deng W, Dong X, Zhang Y, et al. Transcutaneous oxygen pressure (TcPO2): a novel diagnostic tool for peripheral neuropathy in type 2 diabetes patients. Diabetes Res Clin Pract. 2014;105(3):336–343. doi:10.1016/j.diabres.2014.05.012
7. Anyanwu EG, Onuchukwu CL. Does plantar lipoatrophy affect dynamic balance in HIV infected persons? Gait Postures. 2021;86:101–105. doi:10.1016/j.gaitpost.2021.02.015
8. Jiang X, Li N, Yuan Y, et al. Limb salvage and prevention of ulcer recurrence in a chronic refractory diabetic foot osteomyelitis. Diabetes Metab Syndr Obes. 2020;13:2289–2296. doi:10.2147/DMSO.S254586
9. Brown SJ, Handsaker JC, Bowling FL, Boulton AJ, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care. 2015;38(6):1116–1122. doi:10.2337/dc14-1982
10. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi:10.1056/NEJMra1615439
11. Du C, Wang H, Chen H, et al. The feasibility and effectiveness of wearable sensor technology in the management of elderly diabetics with foot ulcer remission: a proof-of-concept pilot study with six cases. Gerontology. 2021;3:1–10.
12. Khan KS, Andersen H. The impact of diabetic neuropathy on activities of daily living, postural balance and risk of falls - a systematic review. J Diabetes Sci Technol. 2022;16(2):289–294. doi:10.1177/1932296821997921
13. Shin KJ, Kang JW, Sung KH, et al. Quantitative gait and postural analyses in patients with diabetic polyneuropathy. J Diabetes Complications. 2021;35(4):107857. doi:10.1016/j.jdiacomp.2021.107857
14. Najafi B, Horn D, Marclay S, Crews RT, Wu S, Wrobel JS. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J Diabetes Sci Technol. 2010;4(4):780–791. doi:10.1177/193229681000400403
15. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
16. Casadei G, Filippini M, Brognara L. Glycated hemoglobin (HbA1c) as a biomarker for diabetic foot peripheral neuropathy. Diseases. 2021;9(1):16. doi:10.3390/diseases9010016
17. de Souza RJ, de Souza A, Nagvekar MD. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. J Diabetes Complications. 2015;29(6):811–817. doi:10.1016/j.jdiacomp.2015.05.009
18. Hazari A, Maiya AG, Shivashankara K, et al. Kinetics and kinematics of diabetic foot in type 2 diabetes mellitus with and without peripheral neuropathy: a systematic review and meta-analysis. Springerplus. 2016;5(1):1819. doi:10.1186/s40064-016-3405-9
19. Zhang X, Wang H, Du C, et al. Custom-molded offloading footwear effectively prevents recurrence and amputation, and lowers mortality rates in high-risk diabetic foot patients: a multicenter, prospective observational study. Diabetes Metab Syndr Obes. 2022;15:103–109. doi:10.2147/DMSO.S341364
20. Kang GE, Zhou H, Varghese V, Najafi B. Characteristics of the gait initiation phase in older adults with diabetic peripheral neuropathy compared to control older adults. Clin Biomech. 2020;72:155–160. doi:10.1016/j.clinbiomech.2019.12.019
21. Frykberg RG, Bowen J, Hall J, Tallis A, Tierney E, Freeman D. Prevalence of equinus in diabetic versus nondiabetic patients. J Podiatr Med Assoc. 2012;102(2):84–88.
22. Giacomozzi C, D’Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech. 2005;20(5):532–539. doi:10.1016/j.clinbiomech.2005.01.011
23. Searle A, Spink MJ, Ho A, Chuter VH. Association between ankle equinus and plantar pressures in people with diabetes. A systematic review and meta-analysis. Clin Biomech. 2017;43:8–14. doi:10.1016/j.clinbiomech.2017.01.021
24. Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85(2):245–252. doi:10.1016/j.apmr.2003.06.015
25. Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol a Biol Sci Med Sci. 2005;60(9):1157–1162. doi:10.1093/gerona/60.9.1157
26. Hsu WC, Liu MW, Lu TW. Biomechanical risk factors for tripping during obstacle–Crossing with the trailing limb in patients with type II diabetes mellitus. Gait Posture. 2016;45:103–109. doi:10.1016/j.gaitpost.2016.01.010
27. Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38(1):82–90. doi:10.2337/dc14-1166
28. Allet L, Armand S, de Bie RA, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–466. doi:10.1007/s00125-009-1592-4
29. Du C, Wang H, Chen H, et al. The feasibility and effectiveness of wearable sensor technology in the management of elderly diabetics with foot ulcer remission: a proof-of-concept pilot study with six cases. Gerontology. 2021;67(4):493–502. doi:10.1159/000513729
30. Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology. 2016;62(5):553–563. doi:10.1159/000442253
31. Fernando ME, Crowther RG, Cunningham M, Lazzarini PA, Sangla KS, Golledge J. Lower limb biomechanical characteristics of patients with neuropathic diabetic foot ulcers: the diabetes foot ulcer study protocol. BMC Endocr Disord. 2015;15:59. doi:10.1186/s12902-015-0057-7
32. Ferris JK, Inglis JT, Madden KM, Boyd LA. Brain and Body: a review of central nervous system contributions to movement impairments in diabetes. Diabetes. 2020;69(1):3–11. doi:10.2337/db19-0321
33. Kocak MZ, Aktas G, Atak B, et al. The association between vitamin D levels and handgrip strength in elderly men. Acta Endocrinologica. 2020;16(2):263. doi:10.4183/aeb.2020.263
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.