Back to Journals » International Journal of Women's Health » Volume 14
The Epidemiology, Associated Factors and Bacterial Profile of Asymptomatic Bacteriuria in Pregnant Women: A Retrospective Chart Review Study in Saudi Arabia
Authors AlShamlan NA , AlOmar RS , Aldossary R, Alahmari M, Alghamdi A, AlGhamdi M, Alkanaan N, AlReedy AH, AlOtaibi AS, Alghamdi NS
Received 1 November 2022
Accepted for publication 13 December 2022
Published 16 December 2022 Volume 2022:14 Pages 1749—1759
DOI https://doi.org/10.2147/IJWH.S394936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Nouf A AlShamlan,1 Reem S AlOmar,1 Roba Aldossary,2 Maha Alahmari,2 Asma Alghamdi,2 Mawaddah AlGhamdi,2 Najla Alkanaan,2 Abdullah H AlReedy,1 Amani S AlOtaibi,3 Nada S Alghamdi4
1Department of Family and Community Medicine, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 2College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 3Department of Obstetrics and Gynaecology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 4Department of Microbiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Correspondence: Nouf A AlShamlan, College of Medicine, Imam Abdulrahman Bin Faisal University, P.O. Box 1982, Dammam, 34224, Saudi Arabia, Tel +966504901406, Email [email protected]
Purpose: Asymptomatic bacteriuria (ASB) is the presence of significant amounts of bacteria within the urinary tract in the absence of urinary tract infection (UTI) symptoms, resulting in negative neonatal and pregnancy consequences. This study determined the prevalence, bacteriology patterns, and associated factors with ASB among pregnant women in both primary and hospital levels of care in the Eastern Province of Saudi Arabia.
Methods: This retrospective chart review study included pregnant women between 18 and 50 years who performed the screening urine culture test during their first antenatal visit between 2017 and 2021, without UTI symptoms. The collected data involved the demographic, medical, and obstetric characteristics, and urine culture results. T-tests and chi-squared tests were used for bivariate associations followed by binary logistic regression models.
Results: ASB was positive among 03.42% of the 6471 pregnant women included in the study. Logistic regression revealed that the risk of positive ASB increased in pregnant women in the first and second trimesters (OR = 2.04, 95% CI = 1.41– 2.93 and OR= 1.50, 95% CI = 1.03– 2.19, respectively), as well as pregnant women with a history of previous UTI (OR = 2.98, 95% CI = 2.14– 4.15). The predominant organism isolates were E. coli, followed by GBS, Klebsiella pneumoniae, and Enterococcus faecalis.
Conclusion: With limited data on ASB among pregnant women in Saudi Arabia, findings from the current study could help decision-makers in the country assess the epidemiological characteristics of the condition. Further study is recommended to investigate the susceptibility patterns of commonly prescribed antibiotics with different uropathogens to guide the clinicians who deal with these cases. Additionally, a large national study across the other regions in the kingdom is suggested to calculate the prevalence of ASB in Saudi Arabia.
Keywords: asymptomatic bacteriuria, pregnant, prevalence, E. coli, epidemiology, Saudi Arabia
Introduction
Asymptomatic bacteriuria (ASB) is the existence of ≥105 colony-forming units per milliliter (CFU)/mL of a single uropathogen in the urine sample in the absence of urinary tract infection (UTI) symptoms.1,2 Although all age groups and both genders are susceptible to ASB, it is more prevalent in women due to the anatomical characteristics of the urogenital system and the proximity of the anal orifice to the urethra that enables colonization of the periurethral area with pathogens from the gastrointestinal tract.1 During pregnancy, the prevalence of ASB and symptomatic UTI is increased due to the associated physiological changes in the urinary tract that may lead to a relaxation of smooth muscle and a dilation of the renal pelvis and ureter, as well as a decline in immunity due to pregnancy.1–3 Moreover, pregnant women are prone to ASB and UTI complications such as pyelonephritis which in turn may progress to other outcomes such as preterm birth, low birth weight, respiratory distress, and septicemia.2,4 Evidence has shown that treatment of ASB could reduce these complications. Therefore, different guidelines recommend screening pregnant women for ASB, eg, the US Preventive Services Task Force recommends that clinicians should screen pregnant women at the 12th to 16th gestational age week or at the first antenatal care visit by using a urine culture obtained by a midstream and clean-catch urine sample and start treatment for pregnant women who screen positive.2
ASB affects around 2 to 10% of pregnant women worldwide.2,5 However, the prevalence varies across different countries. Data from several countries in Africa demonstrated that the prevalence was 5.5% to 24.7%.1,5,6 In Bangladesh, the prevalence of ASB in early pregnancy was 4.5%.7 A meta-analysis of 31 Iranian studies concluded that the estimated prevalence of ASB in pregnant women in Iran was 8.7% (95% CI 7.2 to 10.4).3 Although the data concerning the factors associated with ASB are conflicting across the countries, some studies reported that increasing maternal age, parity, low educational status, presence of diabetes, and history of UTI were associated with an increased risk of ASB in pregnant women.1,3,6
Escherichia coli (E. coli) is the most common bacterial species detected in pregnant women with ASB in most studies.3,4,6,7 However, other species such as Proteus species, Klebsiella species, Staphylococcus aureus, and group B streptococcus (GBS) are also identified.1,7,8 Estimating the prevalence and factors associated with ASB among pregnant women, in addition to the identification of the most common bacterial species involved, are crucial initial steps from the epidemiological perspective to understand the burden of this condition. Moreover, it is noticed that such data are sparse in Saudi Arabia, and the prevalence and bacteriology patterns could vary from region to region. Thus, this study aims to estimate the prevalence and bacteriology patterns, as well as the related factors, of ASB among pregnant women who had undergone the screening test in either the primary care or hospital in the Eastern Province of Saudi Arabia.
Materials and Methods
Ethical Consideration
The Institutional Review Board Committee of Imam Abdulrahman Bin Faisal University approved the study (IRB: IRB-2022-01-018). Data is kept confidential and will not be used for other purposes. The data were anonymized, and patient’s informed consent was not needed. The research is in accordance with the Declaration of Helsinki.
Study Design and Setting
This retrospective chart review study was conducted at the Obstetric and Family Medicine clinics in the King Fahd Hospital of the University (KFHU) and its Family and Community medicine center, located in Khobar, Saudi Arabia. KFHU is a teaching hospital of Imam Abdulrahman Bin Faisal University; it is a large hospital and serves patients from the Eastern province of Saudi Arabia and Family and Community Medicine center is a large center incorporating several specialized daily clinics. A full electronic record system was launched in KFHU in the middle of 1997. However, at that time, it was only covering the laboratory and pharmacy orders with little documentation. Lately, it expanded to more than that where in the early months of 2017, nursing and physicians’ notes were included alongside the corresponding ICD coding. Thus, this study included pregnant females who presented during five years from January 2017 to December 2021.
Study Population
The study included all pregnant women, aged between 18 and 50 years old, who performed the screening urine culture test during their first antenatal visit in the Obstetric or Family Medicine clinics, and without having UTI symptoms. Files with missing data were excluded from the study.
Data Collection
Data were obtained from the electronically stored patients’ files. The data collection tool was designed after reviewing studies with similar objectives.1,5,8 It included variables on the patients’ demographics, medical, and obstetric characteristics recorded in the patients’ files at the first antenatal visit (Table 1), as well as their urine culture results. To enhance its content validity, three professional experts revised the data collection sheet. The data were collected between February and June 2022.
 |
Table 1 Demographic, Medical, and Obstetrics Characteristics of Pregnant During Their First Visit Diagnosed Between 2017 and 2021 in the Eastern Province of Saudi Arabia |
According to the laboratory procedures, the urine samples were transported to the microbiology laboratory and processed within 2 hours of collection. Using a 0.001 calibrated loop, a loopful of well-mixed urine samples were inoculated on cysteine-lactose-electrolyte-deficient (CLED), MacConkey, and Columbia Naladixic Acid (CNA) agar medias. Plates were incubated aerobically at 37°C for 24 hours. To determine the colony-forming units per milliliter (CFU/mL) of the original urine specimen, the number of colonies grown on CLED media was counted and multiplied by 1000. A specimen was considered significant for ASB (the main outcome) if colony counts were equal to or exceeded 105 of pure bacterial isolates.1,5 GBS is reported even in a growth of less than 105 CFU/mL as it indicates vaginal colonization that necessitates in-labor prophylaxis.2,9 Preliminary identification of gram-positive and gram-negative bacteria was made based on growth on CNA and MacConkey media, respectively. Complete identification of bacterial isolates was performed using VlTEK 2 Compact bacterial identification system or VITEK Mass spectrometry microbial identification system (bioMérieux Inc., France).
Statistical Analysis
The outcome of this study was the presence of ASB upon the first antenatal visit (positive/negative). The Body Mass Index (BMI) (kg/m2) of pregnant women was obtained from their electronic record. BMIs were classified as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2) overweight (25–29.9kg/m2), and obese (≥30kg/m2), respectively. Descriptive statistics were presented as frequencies and percentages for categorical variables and means with standard deviations for continuous variables. Bivariate associations were performed through t-tests for continuous comparisons and chi-squared tests for categorical ones. A p-value <0.05 was considered statistically significant. Univariable and multivariable binary logistic regression models were used to obtain Odds Ratios (ORs) of risk of ASB and their accompanying 95% Confidence Intervals (CIs). Certain variables such as parity and the number of abortions were not included to avoid multicollinearity. Post-hoc model fit diagnostics were performed and the model with the best fit was chosen. All analyses were performed in STATA software version 15.0 (Stata Corporation).10
Results
Participants’ Characteristics
The data included 6471 pregnant women. The overall mean age was 31.66 ± 05.76 years. Around 80% of these patients were Saudi nationals. Of the total patients, 39.30% were overweight and only 02.21% were underweight. About 40% of pregnant women had their first visit recorded in the third trimester; further examination of these visits found that 92.04% of them were in obstetric clinics. Examining gravidity and parity, 79.11% were multigravida, whereas 44.24% were multiparous women. Around 12% of this sample had two or more abortions. Over 80% of the entire recorded visits were in the obstetric clinics. Concerning chronic disease history, 08.51% had a history of diabetes and 08.38% had a history of hypertension. Of the total sample, 09.82% had a history of gestational diabetes mellitus (GDM) in a previous pregnancy (Table 1).
Bacteriology Patterns of Positive Samples
The prevalence of ASB was 03.42%. A total of 244 bacterial pathogens were isolated from the 221 patients who tested positive for ASB. E. coli was the most frequently detected organism in the urine samples (44.26%), followed by GBS (23.77%), Klebsiella pneumoniae (15.57%), and Enterococcus faecalis (09.02%). The other less commonly identified organisms are shown in Table 2. Interestingly, of the total sample, GBS was identified in 352 pregnant women (05.44%), and only 58 patients had a growth of ≥105 CFU/mL among them.
 |
Table 2 Bacteriology Patterns Isolated from Pregnant Women’s Urine Samples During Their First Antenatal Care Visit Diagnosed Between 2017 and 2021 in the Eastern Province of Saudi Arabia |
ASB Positivity and Pregnant Characteristics
Table 3 presents the characteristics of pregnant women and its associations with the presence of ASB. Among the sample, 03.42% were found to be positive, compared to 96.58% negative. A statistically significant difference was observed for BMI, where a higher percentage of positive cases for ASB was found in women with underweight and normal weight (04.90% and 04.87% respectively). A similar but highly statistically significant difference was observed for trimesters, where women in the third trimester had the lowest percentage of positive ASB (02.33%). History of GDM during a previous pregnancy was significantly associated with the presence of ASB, where a high difference can be observed for ASB between those with GDM and those without (06.61% and 03.07% respectively). Also, a history of UTI prior to pregnancy was significantly associated with ASB during pregnancy (P-value < 0.001). No other statistically significant differences were observed.
Figure 1 shows the presence of ASB by year of diagnosis. Although the association was statistically insignificant (P-value=0.535), an increase in the positivity of ASB was seen gradually across 2017 and 2018 and reached its peak in 2019. A notable decrease in cases was observed in 2020.
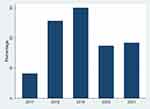 |
Figure 1 Distribution of positive asymptomatic bacteriuria cases diagnosed between 2017 and 2021 in the Eastern Province of Saudi Arabia. |
Predictors of ASB Positivity According to Multivariable Regression Analysis
Table 4 shows the results of both the unadjusted and adjusted binary logistic regression models for ASB positivity. Neither age nor hemoglobin levels were significant predictors for ASB in this patient population. Before adjustment for all other variables, the normal weight category had a 53% increased risk of ASB compared to the overweight category (95% CI = 1.10–2.13). However, this association became non-significant after controlling for other variables. Both before and after adjustment, a significant 2-fold increased risk of ASB was observed for women in their first trimester (95% CI = 1.48–2.85 and 1.41–2.93, respectively) and a 50% increased risk of ASB for women in their second trimester (95% CI = 1.03–2.13 and 1.03–2.19, respectively) compared to pregnant women in their third trimester. Women who were primigravida had a lower albeit non-significant risk of ASB. Similarly, patients who received their care at the Family Medicine clinic tended to have a lower non-significant risk of ASB. In the unadjusted model, the results show a 2.42 OR of ASB risk for women with GDM history (95% CI = 1.71–3.44), although this became non-significant after controlling for all other variables. Analyses also show that women with a history of UTI before pregnancy had a higher risk of ASB (Unadjusted OR = 3.92, 95% CI = 2.98–5.16, and adjusted OR = 2.98, 95% CI = 2.14–4.15).
 |
Table 4 Univariable and Multivariable Binary Logistic Regression Models of Asymptomatic Bacteriuria Positivity Diagnosed Between 2017 and 2021 in the Eastern Province of Saudi Arabia |
Discussion
Screening pregnant women for ASB during their first antenatal care visit and treatment of positive cases is an important preventive measure recommended by different guidelines to avoid the associated neonatal and maternal complications.2 The positivity of ASB among pregnant women in the current study was 03.42%. This is comparable to the prevalence of ASB in other developed countries (2–10%).2 Furthermore, the ASB prevalence in this study was lower than reports from other countries like Ethiopia (13.78–19.90%),1,6,11 Nigeria (18.21%),12 India (17.40%),13 Egypt (10%),14 Iran (08.70%),3 Cameroon (07.80%),4 United Arab Emirates (04.80%),15 and Bangladesh (04.50%).7 The differences between the prevalence of ASB in this study and others could be explained by variations in methodologies and sampling, setting of the study (primary care, hospital level of care, or community-based), patient’s background, socioeconomic levels, social habits, genital hygienic practices, and methods of urine collection among patients from different studies.1,14 Moreover, the low prevalence of ASB in the current study could reflect the improvement in the socio-economic status, level of education, and awareness of pregnant women in the country. In addition, easy accessibility to well-established primary care centers may also have attributed to this relatively small prevalence. These factors were also addressed in other studies and were found to have an inverse association with ASB during pregnancy.3,4
Gram-negative bacteria, especially E. coli and Klebsiella pneumoniae, were common etiologies of ASB in the current study. Other studies of ASB bacteriology patterns among pregnant women worldwide reported a similar finding with the predominance of gram-negative organisms, especially E. coli.1,3–5,7,8,13 GBS was the second most reported uropathogen that caused ASB in this study and interestingly, of the total sample, it was identified in 05.44% of pregnant. A previous Saudi study reported that the prevalence of ASB due to GBS organisms was 02.1%.16 Another Saudi study of women during labor and which utilized data obtained from vaginal swabs, rectal swabs, and midstream urine samples revealed that the prevalence of GBS was 19%.17 GBS bacteriuria receives special attention with updated recommendations since its presence indicates heavy colonization and has been reported to be associated with maternal and neonatal morbidity and mortality, such as chorioamnionitis, premature rupture of membranes, preterm delivery, and neonatal sepsis. Thus, its presence in the urine cultures, regardless of colony count, is an indication of intrapartum antibiotic prophylaxis.18–20
Findings from the current study did not show an association of ASB with either patients’ hemoglobin level or gravidity. This is in agreement with reports from Eastern Ethiopia.5 Pregnant women in the study with a normal weight had an increased risk of ASB compared to those with an overweight category. The reason for this observation was not clear; however, this association became non-significant after controlling for other variables. Similarly, we reported a lower nonsignificant proportion of ASB cases in pregnant women who followed up in the Family Medicine clinics than their counterparts in the Obstetric clinics. However, this could be attributed to the fact that pregnant women attending obstetric clinics are usually at a higher risk for pregnancy or delivery complications or have comorbidities that could increase their risk of having ASB.
Previous studies concluded that pregnant women with diabetes mellitus or GDM are at risk of having UTI or ASB.21 In line with these findings, the current study found that women with a GDM history in a previous pregnancy had a higher risk of ASB; however, this became non-significant after controlling for all other variables in the regression analysis. Furthermore, the present study shows that women with a history of UTI before pregnancy had a higher risk of ASB during pregnancy. This result is aligned with previous investigations that have been conducted in India and Ethiopia,6,11,13 and it could be attributed to the fact that UTI is a recurrent infection and that there is a possibility of having remnant antibiotic-resistant organisms from a previous UTI attack.11,13
Reports from studies that address the associations between ASB and gestational age are conflicting. Some findings reported insignificant associations.1,4,5,8 On the other hand, many studies are in agreement with our findings that a higher positivity of ASB was seen more in the first trimester, followed by the second trimester.3,6 These findings emphasize the recommendations of screening pregnant women for ASB at the first antenatal care visit, which is usually during the first trimester or the early weeks of the second trimester.2
With very limited data on ASB among pregnant women in Saudi Arabia, epidemiological and clinical findings from this large study could help public health experts in the country assess the burden of ASB. Moreover, studies on patients from both primary and hospital levels of care are even more scarce, hence, giving strength to this study. However, we acknowledge some limitations; the data are collected from the patients’ electronic medical records, although this is considered a strong point since recall bias is minimized, patients’ records do not include information on patients’ income, education level, occupation, smoking status, sexual activity, and toileting practice which can be addressed in future studies using other epidemiologic designs.
Conclusion
This study gave an insight on the epidemiology, bacterial profile, and associated factors of ASB among pregnant women in Saudi Arabia. In the current study, ASB is prevalent among 03.42% of pregnant women, and positivity rates were reported more during the first and second trimesters and in pregnant women with a pre-pregnancy history of UTI. Thus, addressing this issue as early as possible during pregnancy is crucial. Moreover, the most common bacteria identified in the etiology of ASB was E. coli. Additionally, GBS was detected in 05.44% of pregnant women. Further studies to investigate the susceptibility patterns of commonly prescribed antibiotics with different uropathogens to guide clinicians in the management of such cases and decrease antibiotic resistance are recommended. Moreover, a large multicenter national study across all regions of the country is necessary to further understand the varying bacteriology patterns across Saudi Arabia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bizuwork K, Alemayehu H, Medhin G, Amogne W, Eguale T. Asymptomatic bacteriuria among pregnant women in addis ababa, Ethiopia: prevalence, causal agents, and their antimicrobial susceptibility. Int J Microbiol. 2021;2021:8418043. doi:10.1155/2021/8418043
2. Owens DK, Davidson KW, Krist AH, et al. Screening for Asymptomatic Bacteriuria in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322(12):1188–1194. doi:10.1001/jama.2019.13069
3. Azami M, Jaafari Z, Masoumi M, et al. The etiology and prevalence of urinary tract infection and asymptomatic bacteriuria in pregnant women in Iran: a systematic review and Meta-analysis. BMC Urol. 2019;19(1):43. doi:10.1186/s12894-019-0454-8
4. Mokube MN, Atashili J, Halle-Ekane GE, Ikomey GM, Ndumbe PM. Bacteriuria amongst pregnant women in the Buea Health District, Cameroon: prevalence, predictors, antibiotic susceptibility patterns and diagnosis. PLoS One. 2013;8(8):e71086. doi:10.1371/journal.pone.0071086
5. Edae M, Teklemariam Z, Weldegebreal F, Abate D. Asymptomatic Bacteriuria among Pregnant Women Attending Antenatal Care at Hiwot Fana Specialized University Hospital, Harar, Eastern Ethiopia: magnitude, Associated Factors, and Antimicrobial Susceptibility Pattern. Int J Microbiol. 2020;2020:1763931. doi:10.1155/2020/1763931
6. Abu D, Abula T, Zewdu T, Berhanu M, Sahilu T. Asymptomatic Bacteriuria, antimicrobial susceptibility pattern and associated risk factors among pregnant women attending antenatal care in Assosa General Hospital, Western Ethiopia. BMC Microbiol. 2021;21(1):348. doi:10.1186/s12866-021-02417-6
7. Lee AC, Mullany LC, Koffi AK, et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth. 2019;20(1):1. doi:10.1186/s12884-019-2665-0
8. Nteziyaremye J, Iramiot SJ, Nekaka R, et al. Asymptomatic bacteriuria among pregnant women attending antenatal care at Mbale Hospital, Eastern Uganda. PLoS One. 2020;15(3):e0230523. doi:10.1371/journal.pone.0230523
9. Puopolo KM, Lynfield R. Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134(1):1.
10. StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2017.
11. Wabe YA, Reda DY, Abreham ET, Gobene DB, Ali MM. Prevalence of Asymptomatic Bacteriuria, Associated Factors and Antimicrobial Susceptibility Profile of Bacteria Among Pregnant Women Attending Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. Ther Clin Risk Manag. 2020;16:923–932. doi:10.2147/TCRM.S267101
12. Oli AN, Okafor CI, Ibezim EC, Akujiobi CN, Onwunzo MC. The prevalence and bacteriology of asymptomatic bacteriuria among antenatal patients in Nnamdi Azikiwe University Teaching Hospital Nnewi; South Eastern Nigeria. Niger J Clin Pract. 2010;13(4):409–412.
13. Agarwal A, Pandey S, Maheshwari U, Singh MP, Srivastava J, Bose S. Prevalence of Asymptomatic Bacteriuria and Antimicrobial Resistance Profile among Pregnant Females in a Tertiary Care Hospital. Indian J Community Med. 2021;46(3):469–473.
14. Abdel-Aziz Elzayat M, Barnett-Vanes A, Dabour MF, Cheng F. Prevalence of undiagnosed asymptomatic bacteriuria and associated risk factors during pregnancy: a cross-sectional study at two tertiary centres in Cairo, Egypt. BMJ open. 2017;7(3):e013198. doi:10.1136/bmjopen-2016-013198
15. Abdullah AA, Al-Moslih MI. Prevalence of asymptomatic bacteriuria in pregnant women in Sharjah, United Arab Emirates. Eastern Mediterranean Health j. 2005;11(5–6):1045–1052.
16. Ahmad S. Asymptomatic group B streptococcal bacteriuria among pregnant women in Saudi Arabia. Br J Biomed Sci. 2015;72(3):135–139. doi:10.1080/09674845.2015.11666810
17. Musleh J, Al Qahtani N. Group B Streptococcus Colonization among Saudi Women During Labor. Saudi j med med sci. 2018;6(1):18–22. doi:10.4103/sjmms.sjmms_175_16
18. Schneeberger C, Kazemier BM, Geerlings SE. Asymptomatic bacteriuria and urinary tract infections in special patient groups: women with diabetes mellitus and pregnant women. Curr Opin Infect Dis. 2014;27(1):108–114. doi:10.1097/QCO.0000000000000028
19. Rosenberger KD, Seibert A, Hormig S. Asymptomatic GBS bacteriuria during antenatal visits: to treat or not to treat? Nurse Pract. 2020;45(7):18–25. doi:10.1097/01.NPR.0000669112.69022.aa
20. Tevdorashvili G, Tevdorashvili D, Andghuladze M, Tevdorashvili M. Prevention and treatment strategy in pregnant women with group B streptococcal infection. Georgian Med News. 2015;1(241):15–23.
21. Al-Bash MR, Mathew M, Al-Kharusi LA, Abu-Heija AT. Symptomatic Urinary Tract Infection in Diabetic Pregnant Women, Effect of the Type of Diabetes and Glycemic Control. Saudi j med med sci. 2016;4(2):104–107. doi:10.4103/1658-631X.178327
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

