Back to Journals » Journal of Inflammation Research » Volume 15
The Emerging Role of Immune Cells and Targeted Therapeutic Strategies in Diabetic Wounds Healing
Authors Song J, Hu L, Liu B, Jiang N, Huang H, Luo J, Wang L, Zeng J, Huang F, Huang M, Cai L, Tang L, Chen S, Chen Y, Wu A, Zheng S, Chen Q
Received 22 April 2022
Accepted for publication 7 July 2022
Published 20 July 2022 Volume 2022:15 Pages 4119—4138
DOI https://doi.org/10.2147/JIR.S371939
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jianying Song,1,2,* Lixin Hu,1,2,* Bo Liu,2 Nan Jiang,3 Houqiang Huang,4 JieSi Luo,3 Long Wang,3 Jing Zeng,3 Feihong Huang,3 Min Huang,5 Luyao Cai,2 Lingyu Tang,2 Shunli Chen,2 Yinyi Chen,2 Anguo Wu,3 Silin Zheng,4 Qi Chen1,2,6,7
1Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 2School of Nursing, Southwest Medical University, Luzhou, People’s Republic of China; 3Department of Pharmacology, School of Pharmacy, Southwest Medical University, Luzhou, People’s Republic of China; 4Department of Nursing, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 5Department of Respiratory and Critical Medicine, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 6Metabolic Vascular Disease Key Laboratory of Sichuan Province, Luzhou, People’s Republic of China; 7Cardiovascular and Metabolic Diseases Key Laboratory of Luzhou, Luzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Chen, Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China, Tel +0086-18383008873, Email [email protected] Silin Zheng, Department of Nursing, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China, Tel +86-13002866667, Email [email protected]
Abstract: Poor wound healing in individuals with diabetes has long plagued clinicians, and immune cells play key roles in the inflammation, proliferation and remodeling that occur in wound healing. When skin integrity is damaged, immune cells migrate to the wound bed through the actions of chemokines and jointly restore tissue homeostasis and barrier function by exerting their respective biological functions. An imbalance of immune cells often leads to ineffective and disordered inflammatory responses. Due to the maladjusted microenvironment, the wound is unable to smoothly transition to the proliferation and remodeling stage, causing it to develop into a chronic refractory wound. However, chronic refractory wounds consistently lead to negative outcomes, such as long treatment cycles, high hospitalization rates, high medical costs, high disability rates, high mortality rates, and many adverse consequences. Therefore, strategies that promote the rational distribution and coordinated development of immune cells during wound healing are very important for the treatment of diabetic wounds (DW). Here, we explored the following aspects by performing a literature review: 1) the current situation of DW and an introduction to the biological functions of immune cells; 2) the role of immune cells in DW; and 3) existing (or undeveloped) therapies targeting immune cells to promote wound healing to provide new ideas for basic research, clinical treatment and nursing of DW.
Keywords: diabetic wounds, wound healing, immune cells, inflammation, targeted therapy
Introduction
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia.1 In 2019, 463 million people had diabetes worldwide.2,3 Projections estimate an increase in the number of individuals with diabetes to 578 million by 2030 and as high as 700 million by 2045.3 DW is the main complication in patients with diabetes.4 The increasing number of patients with diabetes has caused a daily increase in the incidence of DW, substantially increasing the medical burden of wound repair worldwide.5 The global advanced wound care market is projected to reach $18.7 billion by 2027, increasing at a compound annual growth rate of 6.6% over the analysis period from 2020–2027.6 Studies have shown that the key link in difficult-to-heal DW is abnormal inflammation.7–9 Immune cells are an indispensable component regulating inflammation and maintaining body homeostasis. They are extremely sensitive to changes in signaling molecules in the body and respond quickly. The mechanism by which immune cells affect wound repair, especially difficult-to-heal wounds such as DW, has always been a hot and difficult topic in clinical research. At present, the main methods for the treatment of DW are mainly pressure offloading and ulcer protection, restoration of tissue perfusion, treatment of infection, and local ulcer care,10 but the desired results have not been achieved. Approaches targeting immune cells may provide new and efficient treatment strategies for DW repair. Therefore, in this article, we will use “immune cells” as the key point to discuss in detail the specific mechanisms of immune cells and DW repair. In addition, we also discuss methods to target the regulation of immune cells, promote the smooth transition of DW from inflammation to proliferation and remodeling, and finally promote the healing of DW. Our hope is to provide a new direction for DW treatment strategies.
Epidemiology, Adverse Consequences and Treatment of DW
DW occur in a specific population of patients with diabetes presenting impaired skin integrity due to various factors, such as abnormal blood glucose levels, an imbalanced immune response, peripheral neuropathy, and vascular damage. In recent years, the number of patients suffering from DW has increased. Between 19% and 34% of persons with diabetes are likely to be affected by diabetic foot ulcers (DFUs).11,12 The occurrence of DFUs substantially increases the risk of amputation.12 Worldwide, a person with major lower-extremity amputations (above the ankle) is estimated to have a lower 5-year survival rate (65.01%), followed by those with minor lower-extremity amputations (72.24%) and those without lower-extremity amputations (81.61%).13 The unpredictability of wound healing and the threat of amputation often leave patients in a state of guilt, depression, disappointment, worry and sadness. Moreover, the mean length of inpatient stay for ulcer-only, minor amputation, and major amputation was 13.3, 20.5, and 59.6 days, respectively.14 The average annual cost per patient was US $3368 (ulcer-only), US $10,468 (minor amputation), and US $30,131 (major amputation).14 DW has a long treatment cycle, high disability and fatality rates, high hospitalization rate and high medical expenses, which not only seriously affect the patient’s quality of life and physical and mental health but also impose a substantial economic burden on society.
The main treatment strategies for DW currently used in the clinic are described below.10,15 First, pressure offloading and ulcer protection should be considered. Second, tissue perfusion is restored through revascularization surgery. Third, necrotic tissue is debrided to remove dead tissue and prevent deterioration of the wound. Antibacterial and anti-inflammatory agents are applied through topical or systemic application of antibiotics. Fourth, glycemic control should be optimized, such as using metformin or insulin. Fifth, local care is employed to restore the normal immune microenvironment of the wound and accelerate angiogenesis and collagen deposition in the wound, eg, by choosing dressings according to the location, size, depth, exudate, presence of infection or necrosis, and the condition of the surrounding tissues. Although these measures consider both local and systemic treatment, the incidence, disability, and mortality rates of DW are still high. Therefore, more effective treatments mush be explored. The mechanism of refractory DW is complex and includes the abnormal inflammatory response theory,9,16 microvascular injury theory17 and neurotrophic factor starvation theory.18 However, an abnormal inflammatory response is the most important mechanism of refractory chronic DW.8 As mentioned above, immune cells are an indispensable and important component regulating inflammation. Therefore, an in-depth understanding of the specific relationship between DW and immune cells is necessary for treating DW.
Brief Primer on Immune Cell Biology
Neutrophils
Neutrophils, also known as polymorphonuclear neutrophils, are derived from bone myeloid lineage progenitor cells and enter the blood or tissues after the differentiation and development of the bone marrow. Neutrophils account for 50–70% of the total pool of peripheral blood leukocytes in humans (only 10–25% in mice).19,20 Neutrophils respond to and resist microbial invasion or tissue damage through phagocytosis, degranulation and the release of neutrophil extracellular traps (NETs). They are the first cells in the immune defense of the body.21 Abnormal neutrophils often lead to acute and suppurative infections, tissue damage, autoimmune diseases and the development of malignant tumors.
Macrophages
Macrophages are a key component of the immune system. They are traditionally considered a homogeneous population of phagocytic cells derived from circulating monocytes.22 The classification of macrophages is overly complicated. Different classifications have been developed based on activation cues, cell surface molecules or released proteins.23 The most basic and common classification method is to divide macrophages into M1 and M2 subtypes based on phenotypes. M1 macrophages are classically activated macrophages that attract neutrophils, immature dendritic cells, natural killer cells and activated T cells to the wound surface by secreting proinflammatory factors, proteolytic enzymes and a variety of chemokines to promote inflammation, remove foreign bodies and strengthen the body’s defense function. M2 macrophages mainly secrete anti-inflammatory and proangiogenic mediators, such as arginase-1 (Arg-1), IL-10, transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF), to stop the inflammatory response in a timely manner and promote angiogenesis. The dynamic balance of M1 and M2 macrophages is an indispensable condition for bodily health. However, researchers have not clearly determined whether this classification method is completely applicable to the description of macrophages in vivo.24 Expanded use of single-cell sequencing, transcriptomics and other techniques to analyze clinical samples is needed to mine and validate data.
Mast Cells
Mast cells (MCs) originate from hematopoietic stem cells in the bone marrow and are present in the connective and mucosal tissues throughout the body after transport through the blood circulation. Then, they differentiate and develop into mature MCs rich in heparin, 5-hydroxytryptamine, histamine, protease and other bioactive compounds.25 Based on their proteinase content, MCs have been divided into two subtypes: MCTC are rich in both tryptase and chymase, and MCT only express tryptase.26 High-affinity immunoglobulin E (IgE) receptors (also called FcεRI) on the MC surface are activated when the body is invaded by pathogens, triggering a series of cascade reactions that play an important role in many physiological and pathological processes, including allergic reactions, inflammation, wound healing, immune defense, angiogenesis, tumor occurrence and development.
Dendritic Cells
Dendritic cells (DCs), which develop from bone marrow-derived hematopoietic stem cells,27,28 are a subset of antigen-presenting cells. They use pattern recognition receptors to initiate and regulate adaptive immune responses.29 Although DCs are less abundant in humans than other immune cells, such as neutrophils or macrophages, they control infections by activating cytotoxic T lymphocytes or helper T lymphocytes. Langerhans cells (LCs) are unique DCs in the skin that are mainly distributed in the middle and upper epidermis, namely, between epidermal spinous cells, and they are also found in the dermis. They form a sophisticated monitoring system on the epidermis, constantly measuring the possible dangerous situations around the epidermis. Abnormal DCs often disrupt the function of the immune system, leading to pathological changes such as inflammation, fibrosis, and tumor formation and deterioration.
T Lymphocytes
T lymphocytes are abbreviated as T cells, which are the main components of lymphocytes. They are derived from multipotent blood progenitors and reach the thymus through blood circulation. Early pro-T cells lack the expression of both CD4 and CD8 and are known as double-negative cells (DNs). DNs subsequently express CD4 and CD8 upon the induction of the thymus microenvironment to become double-positive cells (DPs).30 Then, DPs develop into single-positive T cells expressing the surface markers CD4 or CD8 through the action of a series of regulatory signals, such as protein arginine methyltransferase 531 and T cell factor 132 to coregulate the immune response. Therefore, T cells are often divided into CD4+ T cells or CD8+ T cells.33 The classification and naming principles of T cells are confusing due to their complex structures and constant differentiation and renewal. According to the expression of the T cell receptor, T cells have also been divided into two subgroups: αβT and γδT cells. γδT cells are widely present in epithelial tissue cells. γδT cells have invariant T cell receptors, and are known as dendritic epidermal T cells (DETCs). DETCs are important barrier cells in the skin that participate in skin homeostasis, regulating immunity, resisting pathogen invasion, wound healing, protecting tissues and other activities.34 DCs play an important role in maintaining the balance of the internal environment and promoting wound repair.
An introduction to the biology of these immune cells is provided in Table 1.
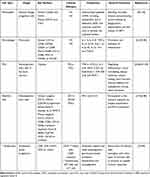 |
Table 1 Introduction to the Biology of Immune Cells |
Immune Cell Imbalance in the Process of DW
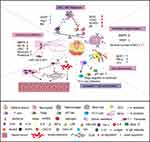
For wound healing, it is critical to coordinate the activities of immune cells accurately. The imbalance of immune cells will lead to the deterioration of immune microenvironment, and the wound will be stagnant in the inflammatory stage, which will further hinder the transition of wound healing from inflammation to proliferation and remodeling, and impair wound repair (Figure 1).
Persistent Neutrophil Infiltration Exacerbates the Abnormal Inflammatory Cascade in DW
Neutrophils are the first leukocytes to infiltrate the injured tissue.21 They are rarely present in healthy skin, but they populate the skin in inflammatory conditions and after wounding.50 When the integrity of the body is damaged for 4 to 6 hours, neutrophils are recruited to wounded tissues through the actions of bacterial products and chemokines, among other factors, and reach a peak number after 18 to 24 hours.35,51,52 Neutrophils, which are rapidly recruited to wounded tissues, form phagosomes by phagocytosing bacteria and tissue fragments. Then, a large amount of degranulation occurs, releasing matrix metalloproteinases (MMPs) and promoting the formation of an early proinflammatory microenvironment. Concurrently, NETs, which are composed of globulin/enzymes and nuclear material, destroy pathogens by trapping and removing the bacteria. In addition, neutrophil-derived cytokines and chemokines recruit monocytes to the wound site and then promote their differentiation into macrophages to jointly maintain the internal environmental balance of the body.53–55 After performing their functions, the production of specific proinflammatory defibrating mediators, such as resolvins and protectins, increases, which limits neutrophil infiltration. Apoptotic neutrophils or tissue fragments are continuously removed by macrophages. Only the timely formation of the early proinflammatory microenvironment and termination of the inflammatory reaction promote the smooth transition of the wound to the proliferative stage and finally accelerate wound healing.
However, in DW with a dysregulated microenvironment, RNA abnormalities in neutrophils and their function are impaired. For example, microRNAs (miRNAs), especially miR-129 family members, are abnormally expressed. The expression of miR-129-2-3p in DW tissues is significantly reduced, while miR-129-2-3p target genes are closely related to inflammation and apoptosis.56 However, researchers have not clearly determined whether this abnormality is related to the difficulty in healing DW or whether it is a key link in the difficult healing of DW, and further verification is needed. But there is no doubt that following the inflammatory phase, an intensive reduction in cellularity occurs by increased apoptotic cell death.57 The smooth progress of inflammation and apoptosis is conducive to the smooth start of the proliferation phase, which is very important for wound repair. In addition, the release of NETs in DW increases. In contrast to stable or healed wounds, neutrophil elastase, the prototypical NET marker, is present at 59% higher levels in worsening wounds.58 Lee YS and colleagues59 used immunohistochemistry, polymerase chain reaction and other methods to verify that the expression of NET proteins and mRNAs in DW specimens was also significantly increased. NETs overproduced in DW trigger NLRP3 inflammasome activation,60 which subsequently promotes the polarization of macrophages to the M1 subtype and intensifies the release of proinflammatory mediators, causing long-term abnormal inflammation. Therefore, in response to the overly inflammatory response, in addition to the targeted elimination of excessive inflammatory mediators by exogenous treatments such as drugs, timely termination of neutrophil infiltration, reduction of the generation and release of NETs, and elimination of overexpressed NETs are also very important for DW healing. Moreover, reactive oxygen species in patients with diabetes continue to increase in an uncontrolled manner because AGE (advanced glycation end product) formation increases when the body is continuously exposed to a high-glucose environment, activating the polyol pathway and PKC signaling.61,62 Abnormal activation of the PKC signaling pathway and increased production of nitric oxide increase the expression of endothelial cell adhesion molecules, thereby augmenting the ability of neutrophils to adhere to the wound.63,64 This process further increases the already excessive number of neutrophils in DW. As mentioned above, neutrophils are removed immediately after performing their functions to prevent excessive infiltration and inflammation. However, in DW, the synthesis of resolvins and protectins, which resolve inflammation by decreasing neutrophil infiltration and transmigration, increasing the phagocytic activity of macrophages and decreasing adipose tissue macrophages, is reduced.65,66 Increased production of leucine-rich-alpha-2-glycoprotein-1 (LRG1) also exacerbates inflammation.67 Therefore, proper regulation of the expression of resolvins, protectins and LRG1 in DW and termination of neutrophil infiltration in a timely manner are important for DW repair. However, the specific mechanisms underlying the effects of resolvins, protectins, and LRG1 on DW healing remain unclear, and further exploration is needed.
Neutrophils, the first group of cells arriving at the wound bed, release various inflammatory mediators and chemokines, which are prerequisites for guiding other immune cells to reach the wound bed to perform their respective biological functions. Neutrophil abnormalities often initiate and exacerbate a series of inflammatory cascades. As described above, neutrophils continuously infiltrate the wound bed. The excessive neutrophil-derived MMPs, neutrophil elastase, cathepsins and NETs also promote ECM cleavage by elastase and matrix metallopeptidase-9 (MMP-9), which destroys the tight junctions between cells.68 The abnormal neutrophils will shift DW repair into a vicious cycle.
The Abnormal Polarization of Macrophages Arrests DW Healing in the Inflammatory Stage
Macrophages are the core cells involved in the wound healing stage. In normal wound healing, M1 macrophages dominate the wound healing site from the time of injury to the third day and then begin to transition to M2 macrophages, with fewer M1 macrophages present. Most of the M1 and M2 macrophages disappear shortly after Day 10. In DW, M1 macrophages continue to be present in the wound bed, with few to no M2 macrophages present.69 At the early stage of wound repair, M1 macrophages eliminate pathogenic bacteria, cell debris and damaged matrix by promoting inflammation. When most pathogenic microorganisms are removed from the wound, apoptotic neutrophils release a large number of cytokines to promote macrophage phagocytosis and clearance, thus promoting macrophage polarization into M2 macrophages. M2 macrophages exert an anti-inflammatory effect to terminate the inflammatory response by releasing IL-4 and IL-10, among other proteins. In addition, M2 macrophages release VEGF and TGFβ1, among other proteins, to promote angiogenesis, collagen deposition and wound contraction. However, if a large number of foreign bodies or pathogens are present at the wound site, the number of M1 macrophages will increase continuously, these cells are not polarized to M2 macrophages, and the wound will stall at the inflammatory stage. During this prolonged period of inflammation, macrophages fuse and evolve into multinucleated giant cells,70 which limits the application of biomaterials in wound repair and makes wound healing more difficult.
The impaired phenotype and function of macrophages are important factors that make DW difficult to heal. Studies have reported differences in the expression of the chromatin-modifying enzyme Setdb2 in macrophages isolated from diabetic and nondiabetic wounds. The histone methyltransferase Setdb2 is an important regulator promoting the resolution of early-stage inflammatory wound macrophages into a reparative phenotype.71,72 In addition, the abnormal blood glucose level in individuals with DW will cause the activation of NLRP3 inflammasomes, and macrophages will subsequently polarize to the M1 subtype and secrete a large amount of proinflammatory mediators such as TNF-α, IL-6, and IL-1β.69 The persistent inflammation and increased numbers of M1 macrophages in these wounds lead to reduced proliferation and migration of the keratinocytes, fibroblasts, and endothelial cells necessary for wound repair.73 The polarization of M2 macrophages in DW is inhibited, which is also unfavorable for wound repair. And diabetes further disrupts macrophage function by impairing monocyte recruitment to the wound, reducing phagocytosis, and prohibiting macrophage polarization from the M1 to M2 phenotype.74 What’s more, S100A9, a Ca2+-binding protein of the S100 family, is overexpressed in DW and disturbs M2 macrophages differentiation via TLR4-NFκB signaling.75 Moreover, the level of insulin in patients with diabetes is lower than that in normal people, and insulin deficiency will also lead to an increase in nitric oxide production,76 which will aggravate the occurrence and development of oxidative stress. The release of a large number of proinflammatory mediators in DW, the activation of NLRP3 inflammasomes and the occurrence of oxidative stress lead to the imbalance of M1 and M2 macrophages, and thus DW exhibit a continuous inflammatory response, and the wound eventually becomes difficult to heal. Local use of insulin on diabetic foot wounds might accelerate wound healing, mainly because insulin promotes neutrophil apoptosis and subsequently triggers macrophage phenotypic polarization.77 At present, a main goal is to induce macrophage polarization from the M1 to M2 phenotype in DW to facilitate the smooth transition of the wound surface from inflammation to proliferation. Many therapeutic methods have begun to be developed. Exosomes derived from epidermal stem cells,78 the bioinspired design of mannose-decorated globular lysine dendrimers,79 and a pH-responsive hyaluronic acid hydrogelstem80 are good examples. However, because of the complexity of the immune system, the unpredictability of clinical trials, and ethical issues, these strategies have been validated only at the animal and cellular levels. Clinical research is particularly important.
The Ongoing Degranulation of MCs Hinders DW Healing
MCs, the inflammatory sentinels, are some of the earliest cells in the body that are activated to resolve trauma. They participate in all stages of wound healing, including hemostasis, inflammation, proliferation, and remodeling.81 After injury, MCs are recruited by the anaphylatoxins C3a and C5a that are produced through the effects of Hageman factor (XII) and stem cell factor secreted by keratinocytes.82 Activated MCs, likely through secretion of TNF-α, increase the expression of XIIIa, a potent fibrin-stabilizing factor that catalyzes the covalent cross-binding of fibrin fibrils in dermal dendrocytes to promote hemostasis and clot formation.83 MC-derived mediators, including CXCL2, TNF-α, MIP-2, and IL-8, recruit neutrophils to the wound bed and activate macrophages, accelerating the elimination of foreign pathogens.83 During the proliferation phase, shorter forms of perlecan are produced by MCs via proteolytic processing and alternative splicing to regulate angiogenesis and matrix conversion.84 MC-derived VEGF, CXCL16, endothelin-1, GM-CSF, and IL-8, and keratinocyte-derived monocyte chemotactic protein-1 (MCP-1) also jointly promote wound angiogenesis and accelerate wound healing.85 Weiskirchen R and colleagues documented that MCs proteases 4, 5, and 6 are mediators of the critical role of MCs in the proliferative phase of healing by promoting angiogenesis, fibrocyte proliferation, and collagen synthesis.86 Pistacia atlantica hull ointment increases the MCs distribution and infiltration, which in turn promotes neovascularization and ultimately promotes wound healing.87 During the remodeling phase, MC-derived mediators, such as histamine, proteases, SCF, TGF-β, VEGF and FGF-2, promote the proliferation and differentiation of fibroblasts.88 Some fibroblasts differentiate into myofibroblasts to promote wound contraction. Estevao LR et al increased the MCs concentration and promoted skin wound contraction in rats by applying an ointment containing 5% Brazilian pepper tree oil.89 MCs, which are important sensors and effector cells of the immune system,90 play a very important role in the process of wound healing. However, due to the significant difference in the contents of cytokines and proteolytic enzymes in mature MCs, the MCs phenotype is not fixed. Therefore, methods to promote the reasonable and stable expression of MCs conducive to wound healing are also directions that should be explored in the future.
A lack of MCs always promotes the development of severe insulitis and accelerates hyperglycemia, which in turn accelerates the occurrence and development of DM.91 However, when MCs persist, MC-derived mediators constantly recruit white blood cells to the wound bed, and inflammation continues to develop, leading to the failure of wound healing. At present, controversy exists regarding whether the total number of MCs in DW has changed. Some studies have reported no difference in the total number of MCs between DW and normal wounds,82 but others have reported an increase in the number of MCs in DW.92 This discrepancy may be due to the difference in modeling methods or the monitoring time of MCs in DW. Undoubtedly, in DW characterized by a high-glucose microenvironment, the degranulation of MCs increases, and the release of MC-derived VEGF is reduced. Georgios Theocharidis et al integrated skin transcriptomics and serum multiplex assays and verified this fact.16 The increase in MMP-9 levels accelerates the degradation of the extracellular matrix, making DW more difficult to heal. An indole-carboxamide-type MC stabilizer, MCS-01, was reported to be an effective inhibitor of MC degranulation in vitro. MCS-01 promotes DW healing by activating the NF-κB and STAT3 signaling pathways, inhibiting the production of MMP-9, and promoting the production of IFN-β, IFN-γ and VEGF-α.93 Targeted therapy of MCs that inhibits their degranulation and maintains stable functions has become a reliable and emerging method to promote DW healing. However, does the total number of MCs differ between chronic DW and normal wounds? If a difference exists, what role does this difference play in the healing process of DW? Clinical DW specimens should be collected ethically and with patient consent to better understand the role of MCs in DW. Then, more authoritative data can be obtained by combining emerging transcriptomics and single-cell sequencing to provide scientific evidence for DW treatment.
Abnormal Numbers and Impaired Migration of LCs Aggravate the Difficulty in DW Healing
In wound healing, the dendritic cells that play a major role are LCs. An increase in the number of LCs at the wound bed is related to the rapid healing of human DW. On the one hand, these cells homeostatically migrate to lymph nodes and present antigens to antigen-specific T cells94 to improve immune function and promote the “normalization” of the wound microenvironment. On the other hand, LCs contribute to the stability of the epidermal environment and promote keratinocyte proliferation.95 After the occurrence of trauma, many LCs are present on the epidermis around the wound,96 which facilitate keratinocyte proliferation. MCP-1 exerts a positive feedback effect on inducing LC recruitment to the skin.97 Keratinocytes and LCs work together to facilitate wound healing.
Studies have shown that healing DFUs contain a higher number of LCs than nonhealing DFUs.96 MCP-1, a chemokine produced by keratinocytes, functions to recruit LCs to the wound periphery. MCP-1−/− mice display significantly delayed wound re-epithelialization, and mice with diabetic chronic ulcers that eventually heal show normalization of keratinocyte production of MCP-1, whereas unhealed diabetic ulcers do not.98 The production of IFN-γ also exerts a regulatory effect on the migration of dendritic cells, but IL-13 and IFN-γ are inhibited in myeloid cells of patients with DM and DFUs, and the production of IFN-γ is deregulated. Dysregulated dendritic cell migration in DW also contributes to impaired healing.16 These results suggest that the reduced number of LCs and impaired migration together contribute to impaired healing of DW.
Imbalanced T Lymphocyte Homeostasis Worsens DW
When skin integrity is impaired, T cells migrate to the wound following the secretion of chemokines, such as IFN-γ. In the inflammatory phase, CD8+ T cells specifically recognize the MHC protein in abnormal cells, secrete perforin and granzyme, and directly kill infected or mutated cells, thus preventing the further deterioration of early wounds. CD4+ T cells are activated and differentiate into effector subsets, such as Th1, Th2, Th22 and Treg cells.99 Tregs modulate macrophage polarization through arginase signaling pathways and allow them to respond to different immune microenvironments.100 After the impaired cells are killed, some T cells transform into memory T cells, which renew in an antigen-independent manner. When T cells are exposed to the same antigen again, they immediately induce an immune response, effectively killing abnormal cells such as those carrying “bacteria or viruses”. In the proliferation stage, activated γδT cells produce effective cytokines or growth factors, such as KGF-1 (also called fibroblast growth factor-7, FGF-7), KGF-2 (FGF-10), and IGF-1, to promote the proliferation and differentiation of keratinocytes.101,102 In the remodeling stage, activated γδ T cells stimulate the proliferation of skin hair follicle stem cells to promote epidermal repair, ultimately accelerating wound healing.103
T cell abnormalities are an important pathogenic mechanism of diabetes. Type 1 diabetes mellitus (T1DM) is attributed to the death of insulin-producing β cells induced by CD8+ T cells.104 A tyrosine phosphatase PTPN2 deficiency in CD8+ T cells alone is sufficient to drive the destruction of pancreatic β cells and the onset of diabetes. T cell–specific PTPN2 deficiency is also accompanied by increased CD4+ T-helper type 1 differentiation and T follicular helper cell polarization and increases the abundance of β cells in pancreatic islets, as observed in humans with T1DM.105 In addition, the function and diversity of T cells in the islets of patients with diabetes are also significantly impaired.106 The systemic inflammation observed in patients with diabetes limits the migration of Tregs and increases the infiltration of Th17 cells in DW and thus represents one of the mechanisms underlying increased neutrophilic inflammation and a prolonged inflammatory phase.107 Topical application of retinoic acid induces plasticity of T cells and differentiation of Th17 cells toward Tregs, thereby promoting DW healing.107,108 T cell activation is increased in patients with type 2 diabetes mellitus (T2DM),109 and the frequency of one variable gene, TRBV7-8, is higher in individuals with T2DM.110 Defects in the functions of Tregs have been observed in patients with gestational DM, as evidenced by significantly decreased effector T cell production of interferon gamma and TNF-α through IL-10-mediated mechanisms.111 Structural abnormalities, dysfunctions and subgroup imbalances of T lymphocytes lead to the occurrence and development of diabetes.
At present, research on T cells and DM mainly focuses on the occurrence and development of DM, and further research is needed to determine whether the expression, structure, function, and subpopulations of T cells in DW are abnormal. High glucose and advanced glycation end products significantly upregulate T cell expression and induce T mediated cell inflammation by upregulating ICOS/ICOSL.112 The high-glucose environment increases the production of the peptide hormone islet amyloid polypeptide, activating the NLRP3 inflammasome.113 Activation of the NLRP3 inflammasome prolongs inflammatory infiltration in DW. The activation of γδ T cells, which are an important component of human epidermal tissue, requires IL-1α and IL-7 secreted by keratinocytes.114 However, directional keratinocyte migration is defective and delayed in DW.115,116 The number of activated γδ T cells is reduced, which further hinders the transition of wound healing from inflammation to proliferation. Xu Peng et al also confirmed that patients with chronic DW have a lower abundance of skin-resident γδ T cells than patients with nondiabetic wounds.101 The single-cell transcriptomic landscape of DFUs also confirmed that compared with nonhealing DFUs, healing DFUs contain higher proportions of naive and early differentiated progenitor T lymphocytes, which activate various T cell subsets.117 In DW, the activation and IGF-1 production of DETCs are impaired. IL-15, a very important growth factor for the efficient activation of peripheral T cells, improves DW healing by enhancing the activation and IGF-1 production of DETCs.118 Therefore, an in-depth analysis of the specific mechanism of T cells in DW healing is needed to accelerate DW healing.
Targeted Immunotherapy for DW
Therapeutic Strategies Targeting Immune Cells Based on Drugs
Due to the disorder of immune cells in DW, wounds often fall into a vicious cycle of abnormal inflammation. According to the severity of inflammation, patients are often prescribed anti-inflammatory drugs, such as nonsteroidal anti-inflammatory drugs, to normalize the prolonged inflammation of DW. However, these drugs have many side effects. Therefore, a greater desire for effective targeted drugs has been noted. With the further study of immune cells in DW, several treatments targeting immune cells using drugs have been considered for wound healing. For example, clarithromycin restores the antibacterial activity of neutrophils and improves the wound healing capacity of fibroblasts through the upregulation of LL-37 on NET structures.119 Quercetin,120 astragaloside IV121 and docosahexaenoic acid122 also promote DW healing by restoring the balance of M1/M2 macrophages. Monika Vinish et al topically administered Fms-like tyrosine kinase-3 ligand to increase the number of dendritic cells in burn wounds, which in turn increased cellular proliferation and accelerated wound healing.123 However, the most common studies of drugs based on immune cells to promote DW healing are limited to single immune cells, potentially because the specific mechanism of immune cell cooperation in DW is not well understood. Alternately, when discussing the mechanism of drug action, only the pathway of interest was examined. Insulin promotes neutrophil apoptosis and subsequently triggers macrophage phenotypic polarization.77 However, we do not know whether the local application of insulin exerts an effect on other immune cells. In addition, these drugs act by targeting the immune system, and their toxicity and side effects are unclear. For patients with other immune system diseases, the selection of targeted drugs and delivery methods must be continuously verified.
Therapeutic Strategies Targeting Immune Cells Based on Natural Compounds
Natural compounds are a very rich drug library due to their advantages, such as a wide range of sources and low toxicity and side effects. Researchers have posited that up to 40% of all new molecular entities submitted to the Food and Drug Administration for approval are natural products, natural product-derived, or natural product-inspired (containing a natural product pharmacophore) compounds.124,125 As a global medical problem, DW has also been the subject of many studies examining natural products targeting immune cells to promote DW repair. However, most of them target neutrophils and macrophages, such as the yi medicine “yi bu a jie” extract,126 fermented papaya preparation,127 Quercus infectoria128 and Hsian-tsao.129 The focus on these cells is mainly because neutrophils and macrophages are the core cells involved in wound repair during inflammation, and the inhibition of their abnormal infiltration or the improvement of their functions is an important strategy to promote the normalization of the immune microenvironment. Among them, the yi medicine “yi bu a jie” extract promotes DW repair by inhibiting neutrophil infiltration.126 Fermented papaya preparation,127 Q. infectoria128 and Hsian-tsao129 all promote DW repair by inhibiting the abnormal infiltration of macrophages or improving macrophage function. Moreover, propolis not only inhibits the abnormal infiltration of neutrophils but also targets the regulation of macrophages to finally achieve gratifying results in the treatment of DW.130 Compared with studies on neutrophils and macrophages, studies on MCs, T cells and LCs in DW are relatively limited, and few studies have assessed natural products targeting MCs, T cells and LCs to promote DW healing. However, Gynura procumbens, an herb found in Southeast Asia, has been reported to accelerate wound healing by targeting the increase in MC accumulation or migration and inducing the expression of fibroblast growth factor, angiogenin, epidermal growth factor, transforming growth factor and VEGF.131 This result has emerged with the in-depth study of the pathophysiological mechanism of MCs in the process of DW healing. Therefore, with the identification of the pathophysiological mechanism of immune cells in DW, such as the study by Georgios Theocharidis et al using clinical specimens and single-cell RNA sequencing analysis to provide deep insights into cell function and disease pathophysiology, will facilitate the profiling of the transcriptome landscape of individual cells in DFU tissues.117 In the future, an increasing number of studies will be conducted on natural compounds targeting immune cells to promote DW repair.
Therapeutic Strategies Targeting Immune Cells Based on Physical Therapy
Physical therapy, an important auxiliary method, promotes wound repair by changing the physical environment of chronic wounds. As a form of validation, clinical treatment with hyperbaric oxygen promotes DW repair by inducing a significant reduction in the integrin-mediated adhesive properties of neutrophils through the engagement of both α4 and β2 integrins.132 Photobiomodulation significantly decreases the total number of MCs, which improves the healing process in diabetic animals.92 Different electrical stimulations also promote wound healing by regulating different immune cells.133 Holsapple JS134 et al recruited patients with treatment-resistant venous ulceration for two weeks of extracorporeal shockwave therapy (ESWT), and the results showed that ESWT significantly increased the uptake of apoptotic cells, healing-associated cytokine and growth factor gene expression and modulated the macrophage morphology to one suggestive of macrophage activation, all of which contributed to wound resolution. Moreover, ESWT decreased the number of total and degranulated MCs and alleviated pelvic pain in a rat model of prostatitis.135 Therefore, does ESWT promote DW repair by regulating macrophages and MCs in wound tissue? Although these physical therapies do not solve the root problem, they are safe, low cost, and stable when they are combined with other treatment strategies, they might promote wound repair more efficiently.
Therapeutic Strategies Targeting Immune Cells Based on Biomaterials
With the continuous improvement of medical technology and in-depth research on the immune system, many studies have explored the combination of biological materials and immune cell-targeted therapy to jointly promote DW repair. Although the application of biomaterials, such as alginate and foam dressings, has partially corrected the DW microenvironment, methods that better use increasingly sophisticated and diverse biomaterials to promote DW healing are still an important issue. To date, biomaterial research has mainly included hydrogels, intelligent dressings, and nanoparticles, among others. Effective bioadaptability and biocompatibility are essential for excellent biomaterials. Hydrogels have been widely used in the clinic due to their good biocompatibility, hydrophilicity, controlled drug release and smart drug delivery. HA-JK1 hydrogels are an ideal delivery scaffold for JK1 with a pH-dependent prolonged H2S release profile. At the stage of inflammation in wound healing, the acidic microenvironment accelerates H2S release, resulting in the polarization of macrophages from the M1 type to the M2 type, which terminates the abnormal inflammatory response over time. As the wound heals, the pH value gradually changes to a neutral or even slightly basic, leading to a gentle release of H2S from the HA-JK1 hydrogel to promote wound regeneration.136 The emergence of multifunctional injectable hydrogels makes full use of their advantages, such as better biodegradability and self-healing properties. Through a continuous supply of O2 and timed release of ExosM2@miR-223 and FGF-2, the HA@MnO2/FGF-2/Exos hydrogel creates an appropriate immune microenvironment to improve the healing of DW in vivo.137 Although the application of traditional biomaterials and wound dressings contributes to an effective wound healing outcome, DW repair is a dynamic and complex process. Excessive inhibition of wound inflammation is harmful. Fortunately, the emergence of intelligent dressings has provided a new solution to this problem. Fontenot KR138 et al used fluorescent elastase tripeptide or tetrapeptide biomolecules to prepare intelligent dressings, which have selectivity and affinity for human neutrophil elastase present in DW wound fluid. Intelligent dressings not only perform biological functions but also monitor changes in the DW microenvironment in real time, which provides instructions for the treatment of DW. In addition, nanoparticles also play a very important role in wound repair due to their characteristics, including a low cost. For example, KGM-modified SiO2 nanoparticles (KSiNPs), which are synthetic nanoparticles, do not contain any drugs and promote wound healing in individuals with diabetes by reprogramming mannose receptors on macrophages.7 Many types of these biomaterials have been developed, each with its own strengths. The combination of degradable polymer materials may facilitate the design of composite materials with more desirable properties to meet the needs of the complex DW microenvironment and different healing stages.
Therapeutic Strategies Targeting Immune Cells Based on RNA Interference
RNA interference (RNAi) is based on the interaction of a short RNA strand with messenger RNA due to sequence homology, resulting in translational inhibition.139 Gene expression is specifically knocked out or silenced by RNAi. Wound healing is promoted by regulating RNA expression, such as targeting Keap1,140 tumor protein p53,141 HIF-prolyl hydroxylase domain (PHD),142 and miR-31-5p.143 Localized and sustained delivery of an MMP-9 small interfering RNA (siRNA) promotes wound healing through effective silencing of the MMP-9 gene.144,145 However, because RNA is easily degraded and difficult to store, coupled with the complexity of the immune system and techniques such as single-cell sequencing and the high cost of transcriptomics, studies exploring the differences in immune cell-specific RNA expression in DW are still in their infancy. Undoubtedly, an in-depth study of immune cell-specific RNA expression in DW will be an important direction of future research. According to previous reports, miRNAs are abnormally expressed in neutrophils present in DW, particularly members of the miR-129 family.56 Moreover, the emergence of new gene carriers, such as bacterial cellulose-hyperbranched cationic polysaccharide derivatives,146 β-CD-(D3)7,147 and cationic star-shaped polymers,144 protects siRNAs from degradation by various enzymes. The development of new gene carriers and research progress in immune cell-specific abnormalities in RNA expression in DW provide new therapeutic insights into wound healing through targeted immune cell therapy based on RNAi.
Therapeutic Strategies Targeting Immune Cells Based on Stem Cells
Stem cells play very important roles in controlling tissue homeostasis and wound repair and are currently some of the most important, popular and cutting-edge directions in the world. For wound healing, the most common treatment strategy involves transplantation of cells and growth factors into the wound area.148 When transplanted into a wound, stem cells act in a direct and paracrine manner to promote cell recruitment, immunomodulation, extracellular matrix remodeling, and angiogenesis by secreting cytokines and growth factors.149,150 However, due to the harsh microenvironment in DW, treatment strategies often fail. Therefore, by focusing on the DW microenvironment, the medical community is investigating treatments targeting immune cells to promote the “normalization” of the trauma microenvironment and ultimately promote DW healing. Recent research has identified that a C–C motif chemokine receptor 2-engineered mesenchymal (CCR2-MSC) stromal cell infusion accelerates tissue repair in DW and reshapes local microenvironments by inhibiting monocyte infiltration, remodeling the inflammatory properties of macrophages, and promoting Treg cell accumulation.151 Mesenchymal stem cell (MSC)-derived exosomes also promote the polarization of macrophages to the M2 type via the PTEN/AKT signaling pathway.152 In addition, Guohu Di et al153 found that MSCs not only accelerate M2 macrophage polarization but also induce the activation of corneal epithelial stem/progenitor cells by secreting TSG-6 and further promote the healing of corneal epithelial wounds in diabetic mice. MSCs have provided new hope to accelerate the healing of DW and have laid the foundation for the discovery and application of other stem cells. Dental follicles,154 human dental pulp,155 adipose156 and umbilical cord-matrix stem cells157 also exert an anti-inflammatory effect by accelerating M2 macrophage polarization, but researchers have not determined whether they promote DW healing. However, exogenous stem cells are expensive and prone to immune rejection. Certain safety problems are encountered in their clinical applications. The emergence of induced pluripotent stem cells (iPSCs) solves the aforementioned problems to a comparable extent. iPSCs are derived from adult cells through the in vitro induction of pluripotency, obviating the ethical dilemmas surrounding the use of embryonic stem cells; they are harvested noninvasively and can be transplanted autologously, reducing immune rejection. Additionally, iPSCs are the only cell type capable of differentiating into all cell types present in healthy skin.149 iPSCs have also been explored in the microenvironment of immune cell disorders in DW. iPSC-derived fibroblasts are a good example.158 Treatments based on stem cells that target immune cells and are designed to restore the homeostasis of the internal environment and ultimately promote the healing of DW undoubtedly have great development prospects and application value. However, the immune rejection of stem cells is still one of the main obstacles of regenerative medicine.159 Although good progress has been achieved in diabetic stem cell intervention therapy to date, its long-term safety remains unclear.160 Researchers have not yet determined whether stem cells have the potential to differentiate into other cell lines or increase the risk of cancer in patients with long-term use of stem cell interventions.159 Coupled with issues such as ethics and economic costs, stem cell technology is still mainly in the basic research stage, and its clinical application is not yet mature.
Continuous development of targeted immunotherapy in DW has emerged and has achieved beneficial effects. Some examples are shown in Table 2.
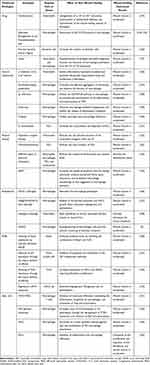 |
Table 2 Therapies Targeting Immune Cells in Wound Healing |
Conclusion and Perspective
Immune cells not only resist the invasion of foreign pathogens but also indirectly promote tissue repair through their bactericidal properties and by regulating the behavior of other cells and jointly promoting wound repair. However, DW stagnate in the inflammatory stage and are unable to smoothly transition to the stage of proliferation and remodeling, which is the key link in DW refractoriness. Moreover, the abnormal expression and distribution of immune cells in DW and the synergy between the cells are also an important basis for the development of wounds that tend to be difficult to heal or nonhealing wounds. With in-depth research on the pathophysiological mechanism of immune cells in DW repair, awareness of the importance of immune cell regulation for DW repair has increased. Therefore, targeted immune cell therapy based on drugs, natural compounds, physical therapy, biological materials, iRNA and stem cells is also increasing. However, the current research is mainly focused on a single treatment plan. The combined application of multiple methods may obtain better curative effects. For instance, local delivery of antimicrobial peptides using nanotechnologies shows great therapeutic potential due to their lower toxicity, prolonged stability, greater targeting ability, higher bioavailability and sustained activity.161 A flexible electrical stimulation device with a Chitosan-Vaseline® dressing is a combination of drugs (chitosan-Vaseline), biological materials (dressing) and physical therapy (high voltage monophasic pulsed current, HVMPC) designed to more effectively improve DW healing.162 In addition, we currently know little about the mechanism underlying the coordination between immune cells and their subgroups in DW. How are the major immune cell subgroups, such as proinflammatory, anti-inflammatory, and proangiogenic subgroups, distributed in DW? The regulation of environmental signals and the generation of specific signals suggesting what measures are needed for targeted intervention have not reached a consensus, necessitating further exploration.
Abbreviation
DM, diabetes mellitus; DW, diabetic wounds; DFUs, diabetic foot ulcers; NETs, neutrophil extracellular traps; Arg-1, arginase-1; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor; MCs, mast cells; IgE, immunoglobulin E; DNs, double-negative cells; DPs, double-positive cells; DETCs, dendritic epidermal T cells; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; MMPs, matrix metalloproteinases; RNAi, RNA interference; siRNA, small interfering RNA; PHD, HIF-prolyl hydroxylase domain; CCR2-MSCs, C–C motif chemokine receptor 2-engineered mesenchymal; MCP-1, keratinocyte-derived monocyte chemotactic protein-1; ESWT, extracorporeal shockwave therapy; KSiNPs, KGM-modified SiO2 nanoparticles; miRNAs, microRNAs; MSC, mesenchymal stem cell; iPSCs, induced pluripotent stem cells; ASCs, adipose stem cells.
Acknowledgments
We thank Prof. Shengmin Guo, Nursing Department, the Affiliated Hospital of Southwest Medical University for deep learning of diabetic wounds. We also thank Prof. Jianming Wu, Department of Pharmacology, for expansion of basic research related to wound healing.
Funding
This work was supported by the joint project of the Luzhou Municipal Government and Southwest Medical University (2020LZXNYDJ30), Doctoral Research Initiation Fund of Affiliated Hospital of Southwest Medical University (20009), scientific research project of Southwest Medical University (2020ZRQNA014, 2020ZSQN004).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Schmidt AM. Highlighting Diabetes Mellitus: the Epidemic Continues. Arterioscler Thromb Vasc Biol. 2018;38(1):e1–e8. doi:10.1161/ATVBAHA.117.310221
2. Chan JCN, Lim -L-L, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2020;396(10267):2019–2082.
3. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843.
4. Huang JJ, Xia CJ, Wei Y, et al. Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound Repair Regen. 2020;28(6):772–779.
5. Hamilton EJ, Davis WA, Siru R, Baba M, Norman PE, Davis TME. Temporal Trends in Incident Hospitalization for Diabetes-Related Foot Ulcer in Type 2 Diabetes: the Fremantle Diabetes Study. Diabetes Care. 2021;44(3):722–730.
6. Sen CK. Human Wound and Its Burden: updated 2020 Compendium of Estimates. Adv Wound Care. 2021;10(5):281–292.
7. Gan J, Liu C, Li H, et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials. 2019;219.
8. Wu Y, Quan Y, Liu Y, et al. Hyperglycaemia inhibits REG3A expression to exacerbate TLR3-mediated skin inflammation in diabetes. Nat Commun. 2016;7(1):87.
9. Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun. 2020;11(1):4678.
10. Schaper NC, van Netten JJ, Apelqvist J, et al. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3266.
11. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Oct. 2016;388(10053):1545–1602.
12. Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376(24):2367–2375.
13. Lin CH, Armstrong DG, Liu PH, Lin CW, Huang CH, Huang YY. Survival of Patients Following First Diagnosis of Diabetic Foot Complications: a Nationwide 15-Year Longitudinal Analysis. Front Endocrinol (Lausanne). 2021;12:801324.
14. Lo ZJ, Surendra NK, Saxena A, Car J. Clinical and economic burden of diabetic foot ulcers: a 5‐year longitudinal multi‐ethnic cohort study from the tropics. Int Wound J. 2021;18(3):375–386.
15. Bus SA, Armstrong DG, Gooday C, et al. Guidelines on offloading foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36(Suppl 1):e3274.
16. Theocharidis G, Baltzis D, Roustit M, et al. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes. 2020;69(10):2157–2169.
17. Hao M, Li S, Sun C, et al. Amelioration effects of berberine on diabetic microendothelial injury model by the combination of high glucose and advanced glycation end products in vitro. Eur J Pharmacol. 2011;654(3):320–325.
18. Li R, Li Y, Wu Y, et al. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials. 2018;168:24–37.
19. Metzemaekers M, Gouwy M, Proost P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol. 2020;17(5):433–450.
20. Hidalgo A, Chilvers ER, Summers C, Koenderman L. The Neutrophil Life Cycle. Trends Immunol. 2019;40(7):584–597.
21. Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the Culprits: macrophages-Versatile Regulators of Wound Healing. Adv Wound Care. 2013;2(7):357–368.
22. Rehman A, Pacher P, Haskó G. Role of Macrophages in the Endocrine System. Trends Endocrinol Metab. 2021;32(4):238–256.
23. Rehak L, Giurato L, Meloni M, Panunzi A, Manti GM, Uccioli L. The Immune-Centric Revolution in the Diabetic Foot: monocytes and Lymphocytes Role in Wound Healing and Tissue Regeneration-A Narrative Review. J Clin Med. 2022;11(3):5488.
24. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
25. Weiskirchen R, Meurer SK, Liedtke C, Huber M. Mast Cells in Liver Fibrogenesis. Cells. 2019;8(11):54.
26. Bian G, Gu Y, Xu C, et al. Early development and functional properties of tryptase/chymase double-positive mast cells from human pluripotent stem cells. J Mol Cell Biol. 2021;13(2):104–115.
27. Lin J, Wang H, Liu C, et al. Dendritic Cells: versatile Players in Renal Transplantation. Front Immunol. 2021;12.
28. Guermonprez P, Gerber-Ferder Y, Vaivode K, Bourdely P, Helft J. Origin and development of classical dendritic cells. Int Rev Cell Mol Biol. 2019;349:1–54.
29. Strbo N, Yin N, Stojadinovic O. Innate and Adaptive Immune Responses in Wound Epithelialization. Adv Wound Care. 2014;3(7):492–501.
30. Hosokawa H, Rothenberg EV. How transcription factors drive choice of the T cell fate. Nat Rev Immunol. 2020;21(3):162–176.
31. Sengupta S, Kennemer A, Patrick K, Tsichlis P, Guerau-de-Arellano M. Protein Arginine Methyltransferase 5 in T Lymphocyte Biology. Trends Immunol. 2020;41(10):918–931.
32. Jung S, Baek JH. The Potential of T Cell Factor 1 in Sustaining CD8(+) T Lymphocyte-Directed Anti-Tumor Immunity. Cancers. 2021;13(3):214.
33. Zheng B, Zhang J, Chen H, et al. T Lymphocyte-Mediated Liver Immunopathology of Schistosomiasis. Front Immunol. 2020;11:548.
34. Deng Z, Wang H, Chen Z, Wang T. Bibliometric Analysis of Dendritic Epidermal T Cell (DETC) Research From 1983 to 2019. Front Immunol. 2020;11:47.
35. Brazil JC, Quiros M, Nusrat A, Parkos CA. Innate immune cell–epithelial crosstalk during wound repair. J Clin Invest. 2019;129(8):2983–2993.
36. Engblom C, Pfirschke C, Zilionis R, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science. 2017;358(6367):548.
37. Li Y-W, Qiu S-J, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497–505.
38. Ahmad JN, Holubova J, Benada O, et al. Bordetella Adenylate Cyclase Toxin Inhibits Monocyte-to-Macrophage Transition and Dedifferentiates Human Alveolar Macrophages into Monocyte-like Cells. mBio. 2019;10(5):548.
39. Liu J, Tang J, Li X, Yan Q, Ma J, Jiang Z. Curdlan (Alcaligenes faecalis) (1→3)-β-d-Glucan Oligosaccharides Drive M1 Phenotype Polarization in Murine Bone Marrow-Derived Macrophages via Activation of MAPKs and NF-κB Pathways. Molecules. 2019;24(23):548.
40. Bailey KN, Furman BD, Zeitlin J, et al. Intra-articular depletion of macrophages increases acute synovitis and alters macrophage polarity in the injured mouse knee. Osteoarthritis and Cartilage. 2020;28(5):626–638.
41. Kallionpaa RA, Ahramo K, Martikkala E, et al. Mast Cells in Human Cutaneous Neurofibromas: density, Subtypes, and Association with Clinical Features in Neurofibromatosis 1. Dermatology. 2021;8:1–11.
42. Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK. Mast Cells Initiate the Recruitment of Neutrophils Following Ocular Surface Injury. Invest Ophthalmol Vis Sci. 2018;59(5):1732–1740.
43. Mohajeri M, Kovanen PT, Bianconi V, Pirro M, Cicero AFG, Sahebkar A. Mast cell tryptase – marker and maker of cardiovascular diseases. Pharmacol Ther. 2019;199:91–110.
44. Ravindran A, Rönnberg E, Dahlin JS, et al. An Optimized Protocol for the Isolation and Functional Analysis of Human Lung Mast Cells. Front Immunol. 2018;9:65.
45. Andersson CK, Andersson-Sjoland A, Mori M, et al. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respir Res. 2011;12:139.
46. Verna G, Liso M, Cavalcanti E, et al. Quercetin Administration Suppresses the Cytokine Storm in Myeloid and Plasmacytoid Dendritic Cells. Int J Mol Sci. 2021;22:15.
47. Mosayebi G, Moazzeni SM. Isolation and phenotyping of normal mouse liver dendritic cells by an improved method. Iran J Basic Med Sci. 2011;14(4):354–360.
48. Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234(1):120–141.
49. Miyan M, Schmidt-Mende J, Kiessling R, Poschke I, de Boniface J. Differential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancer. J Transl Med. 2016;14:1.
50. Nguyen AV, Soulika AM. The Dynamics of the Skin’s Immune System. Int J Mol Sci. 2019;20(8):548.
51. Kim M-H, Liu W, Borjesson DL, et al. Dynamics of Neutrophil Infiltration during Cutaneous Wound Healing and Infection Using Fluorescence Imaging. J Investigative Dermatol. 2008;128(7):1812–1820.
52. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175.
53. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol Sep. 2018;233(9):6425–6440.
54. Recalcati S, Gammella E, Buratti P, et al. Macrophage ferroportin is essential for stromal cell proliferation in wound healing. Haematologica. 2019;104(1):47–58.
55. Yu T, Gao M, Yang P, et al. Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-gamma signaling during diabetic wound healing. J Cell Physiol. 2019;234(4):4217–4231.
56. Umehara T, Mori R, Mace KA, et al. Identification of Specific miRNAs in Neutrophils of Type 2 Diabetic Mice: overexpression ofmiRNA-129-2-3pAccelerates Diabetic Wound Healing. Diabetes. 2019;68(3):617–630.
57. Dardmah F, Farahpour MR. Quercus infectoria gall extract aids wound healing in a streptozocin-induced diabetic mouse model. J Wound Care. 2021;30(8):618–625.
58. Fadini GP, Menegazzo L, Rigato M, et al. NETosis Delays Diabetic Wound Healing in Mice and Humans. Diabetes. 2016;65(4):1061–1071.
59. Lee YS, Kang SU, Lee MH, et al. GnRH impairs diabetic wound healing through enhanced NETosis. Cell Mol Immunol. 2020;17(8):856–864.
60. Liu D, Yang P, Gao M, et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci. 2019;133(4):565–582.
61. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070.
62. Zhang W, Chen L, Xiong Y, et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front Bioeng Biotechnol. 2021;9:707479.
63. Takeuchi Y, Okayama N, Imaeda K, et al. Effects of histamine 2 receptor antagonists on endothelial-neutrophil adhesion and surface expression of endothelial adhesion molecules induced by high glucose levels. J Diabetes Complications. 2007;21(1):50–55.
64. Omi H, Okayama N, Shimizu M, et al. Participation of high glucose concentrations in neutrophil adhesion and surface expression of adhesion molecules on cultured human endothelial cells: effect of antidiabetic medicines. J Diabetes Complications. 2002;16(3):201–208.
65. Shofler D, Rai V, Mansager S, Cramer K, Agrawal DK. Impact of resolvin mediators in the immunopathology of diabetes and wound healing. Expert Rev Clin Immunol Apr. 2021;22:1–10.
66. Menon R, Krzyszczyk P, Berthiaume F. Pro-Resolution Potency of Resolvins D1, D2 and E1 on Neutrophil Migration and in Dermal Wound Healing. Nano Life. 2017;7(1):89.
67. Liu C, Teo MHY, Pek SLT, et al. A Multifunctional Role of Leucine-Rich alpha-2-Glycoprotein 1 in Cutaneous Wound Healing Under Normal and Diabetic Conditions. Diabetes. 2020;69(11):2467–2480.
68. Zhu Y, Huang Y, Ji Q, et al. Interplay between Extracellular Matrix and Neutrophils in Diseases. J Immunol Res. 2021;2021:8243378.
69. Louiselle AE, Niemiec SM, Zgheib C, Liechty KW. Macrophage polarization and diabetic wound healing. Translational Res. 2021;236:109–116.
70. Arya RK, Goswami R, Rahaman SO. Mechanotransduction via a TRPV4-Rac1 signaling axis plays a role in multinucleated giant cell formation. J Biol Chem. 2021;296:100129.
71. Knipper JA, Ding X, Eming SA. Diabetes Impedes the Epigenetic Switch of Macrophages into Repair Mode. Immunity. 2019;51(2):199–201.
72. Kimball AS, Davis FM, denDekker A, et al. The Histone Methyltransferase Setdb2 Modulates Macrophage Phenotype and Uric Acid Production in Diabetic Wound Repair. Immunity. 2019;51(2):258–271.e5.
73. Aitcheson SM, Frentiu FD, Hurn SE, Edwards K, Murray RZ. Skin Wound Healing: normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules. 2021;26:16.
74. den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Translational Res. 2019;204:39–50.
75. Franz S, Ertel A, Engel KM, Simon JC, Saalbach A. Overexpression of S100A9 in obesity impairs macrophage differentiation via TLR4-NFkB-signaling worsening inflammation and wound healing. Theranostics. 2022;12(4):1659–1682.
76. Sun C, Sun L, Ma H, et al. The phenotype and functional alterations of macrophages in mice with hyperglycemia for long term. J Cell Physiol. 2012;227(4):1670–1679.
77. Yang P, Wang X, Wang D, et al. Topical insulin application accelerates diabetic wound healing by promoting anti-inflammatory macrophage polarization. J Cell Sci. 2020;133(19):548.
78. Wang P, Theocharidis G, Vlachos IS, et al. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J Invest Dermatol. 2022.
79. Jiang Y, Zhao W, Xu S, et al. Bioinspired design of mannose-decorated globular lysine dendrimers promotes diabetic wound healing by orchestrating appropriate macrophage polarization. Biomaterials. 2022;280:121323.
80. Jia Y, Zhang X, Yang W, et al. A pH-responsive hyaluronic acid hydrogel for regulating the inflammation and remodeling of the ECM in diabetic wounds. J Mater Chem B. 2022;1:548.
81. Abebayehu D, Spence AJ, McClure MJ, Haque TT, Rivera KO, Ryan JJ. Polymer scaffold architecture is a key determinant in mast cell inflammatory and angiogenic responses. J Biomed Mater Res A. 2019;107(4):884–892.
82. Dong J, Chen L, Zhang Y, et al. Mast Cells in Diabetes and Diabetic Wound Healing. Adv Ther. 2020;37(11):4519–4537.
83. Komi DEA, Khomtchouk K, Santa Maria PL. A Review of the Contribution of Mast Cells in Wound Healing: involved Molecular and Cellular Mechanisms. Clin Rev Allergy Immunol. 2019;58(3):298–312.
84. Jung M, Lord MS, Cheng B, et al. Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: a role in angiogenesis and wound healing. J Biol Chem. 2013;288(5):3289–3304.
85. McHale C, Mohammed Z, Gomez G. Human Skin-Derived Mast Cells Spontaneously Secrete Several Angiogenesis-Related Factors. Front Immunol. 2019;10:129.
86. Succar J, Douaiher J, Lancerotto L, et al. The role of mouse mast cell proteases in the proliferative phase of wound healing in microdeformational wound therapy. Plast Reconstr Surg. 2014;134(3):459–467.
87. Farahpour MR, Mirzakhani N, Doostmohammadi J, Ebrahimzadeh M. Hydroethanolic Pistacia atlantica hulls extract improved wound healing process; evidence for mast cells infiltration, angiogenesis and RNA stability. Int J Surg. 2015;17:88–98.
88. Landolina N, Gangwar RS, Levi-Schaffer F. Mast cells’ integrated actions with eosinophils and fibroblasts in allergic inflammation: implications for therapy. Adv Immunol. 2015;125:41–85.
89. Estevao LR, Medeiros JP, Simoes RS, et al. Mast cell concentration and skin wound contraction in rats treated with Brazilian pepper essential oil (Schinus terebinthifolius Raddi). Acta Cir Bras. 2015;30(4):289–295.
90. Kroner J, Kovtun A, Kemmler J, et al. Mast Cells Are Critical Regulators of Bone Fracture-Induced Inflammation and Osteoclast Formation and Activity. J Bone Miner Res. 2017;32(12):2431–2444.
91. Carlos D, Yaochite JN, Rocha FA, et al. Mast cells control insulitis and increase Treg cells to confer protection against STZ-induced type 1 diabetes in mice. Eur J Immunol. 2015;45(10):2873–2885.
92. Soleimani H, Amini A, Abdollahifar MA, et al. Combined effects of photobiomodulation and curcumin on mast cells and wound strength in wound healing of streptozotocin-induced diabetes in rats. Lasers Med Sci. 2021;36(2):375–386.
93. Tellechea A, Bai S, Dangwal S, et al. Topical Application of a Mast Cell Stabilizer Improves Impaired Diabetic Wound Healing. J Invest Dermatol. 2020;140(4):901–911 e11.
94. Doebel T, Voisin B, Nagao K. Langerhans Cells – the Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017;38(11):817–828.
95. Lewis JM, Bürgler CD, Freudzon M, et al. Langerhans Cells Facilitate UVB-Induced Epidermal Carcinogenesis. J Investigative Dermatol. 2015;135(11):2824–2833.
96. Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic-Canic M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol Res. 2013;57(1–3):222–228.
97. Nakamura K, Williams IR, Kupper TS. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J Invest Dermatol. 1995;105(5):635–643.
98. Low QEH, Drugea IA, Duffner LA, et al. Wound Healing in MIP-1α−/− and MCP-1−/− Mice. Am J Pathol. 2001;159(2):457–463.
99. Chien M-W, Lin M-H, Huang S-H, et al. Glucosamine Modulates T Cell Differentiation through Down-regulating N-Linked Glycosylation of CD25. J Biol Chem. 2015;290(49):29329–29344.
100. Hu X, Liu G, Hou Y, et al. Induction of M2-like macrophages in recipient NOD-scid mice by allogeneic donor CD4(+)CD25(+) regulatory T cells. Cell Mol Immunol. 2012;9(6):464–472.
101. Xu P, Fu X, Xiao N, et al. Involvements of gammadeltaT Lymphocytes in Acute and Chronic Skin Wound Repair. Inflammation. 2017;40(4):1416–1427.
102. Daemi A, Lotfi M, Farahpour MR, Oryan A, Ghayour SJ, Sonboli A. Topical application of Cinnamomum hydroethanolic extract improves wound healing by enhancing re-epithelialization and keratin biosynthesis in streptozotocin-induced diabetic mice. Pharm Biol. 2019;57(1):799–806.
103. Lee P, Gund R, Dutta A, et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of gammadeltaT-cells. Elife Dec. 2017;4:6.
104. Garyu JW, Uduman M, Stewart A, et al. Characterization of Diabetogenic CD8+ T Cells. J Biol Chem. 2016;291(21):11230–11240.
105. Wiede F, Brodnicki TC, Goh PK, et al. T-Cell-Specific PTPN2 Deficiency in NOD Mice Accelerates the Development of Type 1 Diabetes and Autoimmune Comorbidities. Diabetes. 2019;68(6):1251–1266.
106. Tong Y, Li Z, Zhang H, et al. T Cell Repertoire Diversity Is Decreased in Type 1 Diabetes Patients. Genomics Proteomics Bioinformatics. 2016;14(6):338–348.
107. Holl J, Kowalewski C, Zimek Z, et al. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells. 2021;10(3):58.
108. Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans Retinoic Acid Inhibits Type 1 Diabetes by T Regulatory (Treg)-Dependent Suppression of Interferon- -Producing T-cells Without Affecting Th17 Cells. Diabetes. 2008;58(1):146–155.
109. Hoseini-Aghdam M, Sheikh V, Eftekharian MM, Rezaeepoor M, Behzad M. Enhanced expression of TIGIT but not neuropilin-1 in patients with type 2 diabetes mellitus. Immunol Lett. 2020;225:1–8.
110. Frankl JA, Thearle MS, Desmarais C, Bogardus C, Krakoff J. T-cell receptor repertoire variation may be associated with type 2 diabetes mellitus in humans. Diabetes Metab Res Rev. 2016;32(3):297–307.
111. Yang Y, Liu I, Liu B, et al. Functional Defects of Regulatory T Cell Through Interleukin 10 Mediated Mechanism in the Induction of Gestational Diabetes Mellitus. DNA Cell Biol. 2018;37(3):278–285.
112. Zhang HY, Ruan LB, Li Y, et al. ICOS/ICOSL upregulation mediates inflammatory response and endothelial dysfunction in type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2018;22(24):8898–8908.
113. Haneklaus M, O’Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62.
114. Lee P, Gund R, Dutta A, et al. Stimulation of hair follicle stem cell proliferation through an IL-1 dependent activation of γδT-cells. eLife. 2017;6.
115. Arya AK, Tripathi K, Das P. Promising Role of ANGPTL4 Gene in Diabetic Wound Healing. Int J Low Extrem Wounds. 2014;13(1):58–63.
116. Lan -C-CE, Wu C-S, Huang S-M, et al. High-Glucose Environment Inhibits p38MAPK Signaling and Reduces Human β-3 Expression in Keratinocytes. Mol Med. 2011;17(7–8):771–779.
117. Theocharidis G, Thomas BE, Sarkar D, et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. 2022;13(1):181.
118. Wang Y, Bai Y, Li Y, et al. IL-15 Enhances Activation and IGF-1 Production of Dendritic Epidermal T Cells to Promote Wound Healing in Diabetic Mice. Front Immunol. 2017;8:1557.
119. Arampatzioglou A, Papazoglou D, Konstantinidis T, et al. Clarithromycin Enhances the Antibacterial Activity and Wound Healing Capacity in Type 2 Diabetes Mellitus by Increasing LL-37 Load on Neutrophil Extracellular Traps. Front Immunol. 2018;9.
120. Fu J, Huang J, Lin M, Xie T, You T. Quercetin Promotes Diabetic Wound Healing via Switching Macrophages From M1 to M2 Polarization. J Surgical Res. 2020;246:213–223.
121. Luo X, Huang P, Yuan B, et al. Astragaloside IV enhances diabetic wound healing involving upregulation of alternatively activated macrophages. Int Immunopharmacol. 2016;35:22–28.
122. Jia Y-C, Qiu S, Xu J, Kang Q-L, Chai Y-M. Docosahexaenoic Acid Improves Diabetic Wound Healing in a Rat Model by Restoring Impaired Plasticity of Macrophage Progenitor Cells. Plast Reconstr Surg. 2020;145(5):942e–950e.
123. Vinish M, Cui W, Stafford E, et al. Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regeneration. 2016;24(1):6–13.
124. Zhang DD, Chapman E. The role of natural products in revealing NRF2 function. Nat Prod Rep. 2020;37(6):797–826.
125. Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661.
126. Lu LL, Wan P, Li LZ, Zhao MJ, Hu JY, Zhao YF. Experimental study on topical treatment of diabetic skin ulcers with yi medicine “yi bu a jie” extract. Chin J Integr Med Jun. 2013;19(6):464–467.
127. Collard E, Roy S. Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid Redox Signal. 2010;13(5):599–606.
128. Chokpaisarn J, Urao N, Voravuthikunchai SP, Koh TJ. Quercus infectoria inhibits Set7/NF-kappaB inflammatory pathway in macrophages exposed to a diabetic environment. Cytokine. 2017;94:29–36.
129. Fan SL, Lin JA, Chen SY, et al. Effects of Hsian-tsao (Mesona procumbens Hemsl.). Cell Physiol Biochem. 2021;12(1):119–132.
130. McLennan SV, Bonner J, Milne S, et al. The anti-inflammatory agent Propolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen. 2008;16(5):706–713.
131. Sutthammikorn N, Supajatura V, Yue H, et al. Topical Gynura procumbens as a Novel Therapeutic Improves Wound Healing in Diabetic Mice. Plants. 2021;10:Jun.
132. Aykin-Burns N, Baiula M, Greco R, et al. Integrin-mediated adhesive properties of neutrophils are reduced by hyperbaric oxygen therapy in patients with chronic non-healing wound. PLoS One. 2020;15:8.
133. Luo R, Dai J, Zhang J, Li Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv Healthcare Mater. 2021;10:16.
134. Holsapple JS, Cooper B, Berry SH, et al. Low Intensity Shockwave Treatment Modulates Macrophage Functions Beneficial to Healing Chronic Wounds. Int J Mol Sci. 2021;22:15.
135. Song Z, Jin C, Bian Z, Liang C. Extracorporeal shock wave therapy decreases the number of total and degranulated mast cells and alleviates pelvic pain in a rat model of prostatitis. Mol Cell Biochem. 2021;476(4):1905–1913.
136. Wu J, Chen A, Zhou Y, et al. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials. 2019;222:119398.
137. Xiong Y, Chen L, Liu P, et al. All‐in‐One: multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed‐Release of Exosome and Fibroblast Growth Factor. Small. 2021.
138. Fontenot KR, Edwards JV, Haldane D, et al. Designing cellulosic and nanocellulosic sensors for interface with a protease sequestrant wound-dressing prototype: implications of material selection for dressing and protease sensor design. J Biomater Appl. 2017;32(5):622–637.
139. Ofek P, Tiram G, Satchi-Fainaro R. Angiogenesis regulation by nanocarriers bearing RNA interference. Adv Drug Deliv Rev. 2017;119:3–19.
140. Rabbani PS, Zhou A, Borab ZM, et al. Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials. 2017;132:1–15.
141. Layliev J, Wilson S, Warren SM, Saadeh PB. Improving Wound Healing with Topical Gene Therapy. Adv Wound Care. 2012;1(5):218–223.
142. Mezey E, Zhang X, Yan X, et al. Wound Healing Improvement with PHD-2 Silenced Fibroblasts in Diabetic Mice. PLoS One. 2013;8(12):548.
143. Huang J, Yu M, Yin W, et al. Development of a novel RNAi therapy: engineered miR-31 exosomes promoted the healing of diabetic wounds. Bioactive Materials. 2021;6(9):2841–2853.
144. Li Y, Luo H-C, Zhang L-M, et al. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int J Nanomedicine. 2014;1:84.
145. Lan B, Zhang L, Yang L, et al. Sustained delivery of MMP-9 siRNA via thermosensitive hydrogel accelerates diabetic wound healing. J Nanobiotechnology. 2021;19:1.
146. Lan B, Wu J, Li N, et al. Hyperbranched cationic polysaccharide derivatives for efficient siRNA delivery and diabetic wound healing enhancement. Int J Biol Macromol. 2020;154:855–865.
147. Li N, Luo HC, Ren M, et al. Efficiency and Safety of beta-CD-(D3)7 as siRNA Carrier for Decreasing Matrix Metalloproteinase-9 Expression and Improving Wound Healing in Diabetic Rats. ACS Appl Mater Interfaces. 2017;9(20):17417–17426.
148. Choudhury S, Surendran N, Das A. Recent advances in the induced pluripotent stem cell‐based skin regeneration. Wound Repair Regeneration. 2021.
149. Gorecka J, Kostiuk V, Fereydooni A, et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther. 2019;10:1.
150. Cao Y, Gang X, Sun C, Wang G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J Diabetes Res. 2017;2017:1–10.
151. Kuang S, He F, Liu G, et al. CCR2-engineered mesenchymal stromal cells accelerate diabetic wound healing by restoring immunological homeostasis. Biomaterials. 2021;1:275.
152. Liu W, Yu M, Xie D, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11:1.
153. Di G, Du X, Qi X, et al. Mesenchymal Stem Cells Promote Diabetic Corneal Epithelial Wound Healing Through TSG-6–Dependent Stem Cell Activation and Macrophage Switch. Investigative Opthalmology Visual Science. 2017;58:10.
154. Chen X, Yang B, Tian J, et al. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol Biochem. 2018;51(5):2290–2308.
155. Li Y, Zhang D, Xu L, et al. Cell–cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16(12):908–920.
156. Cho K-S, Kang SA, Kim S-D, Mun S-J, Yu HS, Roh H-J. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res. 2019;4:39.
157. Zhang S, Chen L, Zhang G, Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11:1.
158. Kashpur O, Smith A, Gerami-Naini B, et al. Differentiation of diabetic foot ulcer-derived induced pluripotent stem cells reveals distinct cellular and tissue phenotypes. FASEB J. 2019;33(1):1262–1277.
159. Tootee A, Nikbin B, Ghahary A, et al. Immunopathology of Type 1 Diabetes and Immunomodulatory Effects of Stem Cells: a Narrative Review of the Literature. Endocrine, Metabolic & Immune Disorders. Drug Targets. 2021;4:21.
160. Helman A, Melton DA, Stem Cell A. Approach to Cure Type 1 Diabetes. Cold Spring Harb Perspect Biol. 2021;13:1.
161. Gera S, Kankuri E, Kogermann K. Antimicrobial peptides – unleashing their therapeutic potential using nanotechnology. Pharmacol Ther. 2021;25.
162. Wang X-F, Li M-L, Fang -Q-Q, et al. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioactive Materials. 2021;6(1):230–243.
163. Sun Y, Song L, Zhang Y, Wang H, Dong X. Adipose stem cells from type 2 diabetic mice exhibit therapeutic potential in wound healing. Stem Cell Res Ther. 2020;11(1):548.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.