Back to Journals » Journal of Inflammation Research » Volume 16
The Emerging Role of Ferroptosis in Various Chronic Liver Diseases: Opportunity or Challenge
Authors Zhu L , Luo S, Zhu Y, Tang S, Li C, Jin X, Wu F, Jiang H, Wu L, Xu Y
Received 12 August 2022
Accepted for publication 6 January 2023
Published 31 January 2023 Volume 2023:16 Pages 381—389
DOI https://doi.org/10.2147/JIR.S385977
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Lujian Zhu,1,* Shengnan Luo,1,* Yin Zhu,2 Shiyue Tang,1 Chenge Li,3 Xiaozhi Jin,4 Faling Wu,4 Huimian Jiang,4 Lina Wu,4 Yejin Xu1
1Department of Infectious Diseases, Jinhua Municipal Central Hospital, Jinhua, People’s Republic of China; 2Department of Infectious Diseases, Taizhou Enze Medical Center (Group), Enze Hospital, Taizhou, People’s Republic of China; 3College of Public Health and Management, Wenzhou Medical University, Wenzhou, People’s Republic of China; 4Department of Infectious Diseases, the First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yejin Xu, Department of Infectious Diseases, Jinhua Municipal Central Hospital, Jinhua, People’s Republic of China, Email [email protected]
Abstract: Ferroptosis is a recently identified iron-dependent form of intracellular lipid peroxide accumulation-mediated cell death. Different from other types of cell death mechanisms, it exhibits distinct biological and morphological features characterized by the loss of lipid peroxidase repair activity caused by glutathione peroxidase 4, the presence of redox-active iron, and the oxidation of phospholipids-containing polyunsaturated fatty acids. In recent years, studies have shown that ferroptosis plays a key role in various liver diseases such as alcoholic liver injury, non-alcoholic steatohepatitis, liver cirrhosis, and liver cancer. However, the mechanism of ferroptosis and its regulation on chronic liver disease are controversial among different types of cells in the liver. Herein, we summarize the current studies on mechanism of ferroptosis in chronic liver disease, aiming to outline the blueprint of ferroptosis as an effective option for chronic liver disease therapy.
Keywords: ferroptosis, iron metabolism, liver disease, oxidative stress, cell death
Introduction
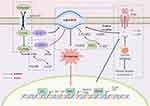
Ferroptosis is a type of programmed cell death caused by iron-dependent lipid peroxidation and overproduction of reactive oxygen species (ROS).1 When a disorder of intracellular iron metabolism increases the level of free iron, the latter catalyzes the Fenton reaction, which produces ROS that subsequently induces lipid peroxidation and leads to the accumulation of lipid peroxides.2 Above process not only depletes glutathione (GSH) but also reduces the activity of glutathione peroxidase 4 (GPX4), thereby further preventing lipid peroxide from being metabolized through the GPX4-catalyzed glutathione reductase reaction. Eventually, this disrupts the integrity of cell membranes, leading to ferroptosis.3 Ferroptosis shows specific characteristics such as the disappearance of mitochondrial cristae or even an increase in the membrane density of mitochondria without significant changes in nuclear morphology, with these morphological changes making ferroptosis distinct from other types of cell death.4 In terms of biochemical features, ferroptosis is thought to be characterized by a greater release of polyunsaturated fatty acids (PUFAs), the inhibition of GPX4 activity, the depletion of GSH, and an accumulation of ROS.5 Briefly, there are two main mechanisms involved in ferroptosis: lipid peroxidation6 and unstable iron and genes associated with iron metabolism7 (Figure 1). GPX4, a major neutralizing enzyme of peroxisomal phospholipids (PLOOH),8 is involved in ferroptosis induced by erastin/RSL3, thus inhibiting cystine transfer, promoting cell accumulation of more cysteine and promoting GSH production.9,10 Meanwhile, under certain pathological conditions, persistent parenchymal cell injury or even iron overload in the tissues and organs, an imbalance in iron homeostasis can occur.11 In the presence of excessive Fe2+, lipid peroxidation induces oxidative stress through the Fenton reaction catalyzed by free Fe2+, and this eventually increases cells’ sensitivity to ferroptosis.12
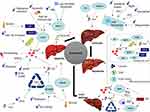
Chronic liver disease (CLD), such as hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease, is a global public health concern, causing considerable morbidity and mortality.13 With more and more attention on basic research and clinical research, the important regulatory status and regulatory mechanism of ferroptosis in CLD are gradually revealed.14–16 However, the role of ferroptosis appears to be varied, which may be caused by the different types and characteristics of cells (Figure 2). Therefore, mechanism involved in ferroptosis needs to be detailed and targeting ferroptosis in patients with AH may be a potential hepatoprotective strategy. An updated and concise review of the relevant literature on ferroptosis in CLD caused by different factors is presented here.
Ferroptosis in Viral Hepatitis
Serum ferritin and iron metabolism-related indicators, such as hepcidin, are important indicators to evaluate the severity of chronic hepatitis B or chronic hepatitis C.17–20 Mitochondrial potential loss and lipid peroxidation were observed in hepatocytes infected with hepatitis A virus 3C protease,21 while reduction of ferritin was observed in hepatocytes infected with hepatitis C virus NS5A.22 Changes in hepcidin levels in hepatocytes, enterocytes, and macrophages in the presence and absence of HCV were observed, indicating that iron-assisted innate immune responses facilitated viral spreading.23 As an important process of iron metabolism, transferrin binding was decreased by prevention of TfR recycling.24 Previous experiments have also confirmed that SLC7A11 was inhibited by hepatitis B virus X protein.25 Eltrombopag, recommended as a first-line agent for chronic hepatitis B patients, mediated reactive oxygen species production by iron chelation, inhibiting the production of IFN-stimulated genes in monocytes in the antiviral response.26 However, the correlation between viral hepatitis and ferroptosis and the specific treatment strategies related to ferroptosis still remain to be further explored.
Ferroptosis in Drug-Induced Liver Injury
Fenton reactions and ROS accumulation cause ferroptosis in liver when ferrous iron (Fe2+) accumulates in the body.27 Prior research generally confirms that drugs may induce ferroptosis by mitochondrial homeostasis and lipid deposition. Cyproterone acetate, an antitumor drug, caused liver cirrhosis with iron overload.28 Efavirenz,29 rifampicin,30 and isoniazid31 are found to impair ATP biosynthesis by regulating ferritin or ferroptosis-related genes. High doses of auranofin can cause liver lipid peroxidation and ferroptosis by inhibiting the activity of thioredoxin reductase.32 In addition, upregulation of hepatic toxicity, lipid peroxidation, and iron death marker genes happened in the APAP-induced liver,33 which may be responsible for Obvious increasement in Fe2+.34
Ferroptosis in Alcoholic Fatty Liver Disease
Alcoholic liver disease (ALD) is a complex disease progression caused when alcohol is excessively consumed, and it includes conditions such as alcoholic cirrhosis, alcoholic liver fibrosis, alcoholic hepatitis, simple steatosis, or even liver cancer.35 Serum iron and ferritin levels are higher in patients with ALD,36,37 which may be a hallmark of ALD.38 Possible mechanisms include ethanol-enhanced reactive oxygen species accumulation and unstable mitochondrial iron pools39,40 in hepatocyte injury. In addition, alcohol-treated mice were even found to have a significantly lower level of NADPH, a coenzyme of GSH reductase, in their livers.41 In iron overload, the depletion of GSH, followed by the subsequent peroxidation of lipids and inflammation are thought to be characteristic of ferroptosis.42 Besides, deletion of hepatocyte-specific FNDC3B, the role of which in ferroptosis was verified by the administration of the ferroptosis inhibitor ferrostatin-1,43 increased liver steatosis induced by alcohol through inhibition of the AMP-activated protein kinase (AMPK). Taking into account the fact that in ALD, both lipid and iron metabolism are disrupted, ferroptosis is likely to be involved in its pathogenesis.
Ferroptosis in Metabolism-Related Fatty Liver Disease
Metabolism-related fatty liver disease (MAFLD), previously known as non-alcoholic fatty liver disease (NAFLD), is a commonly encountered chronic liver disease that encompasses different conditions ranging from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH) or even cirrhosis.43 A plethora of research work has explored the role and mechanism of iron metabolism, iron deposition, and ferroptosis in MAFLD, and researchers have reviewed and discussed this in considerable detail.44–46 Biopsies confirm poorer long-term outcomes in patients with NAFLD and iron overload,47 which may be due to elevated insulin resistance, excess hepatic lipid peroxidation, and iron overload contributing to the progression of liver fibrosis. GPX4-reduced ferroptosis is a crucial trigger for MAFLD progression to NASH.48 Using sodium selenite as a GPX4 activator attenuated RSL3-induced lipid peroxidation and cell death to reduce the severity of NASH.47 Pan et al analyzed single nucleotide polymorphism in Chinese patients with MAFLD, finding that MAFLD susceptibility may be influenced by the rs4434553 polymorphism in TFR2.49
Moreover, distant organ-liver axis is involved in ferroptosis in MAFLD. Iron is absorbed by duodenal intestinal epithelial cells and then entered into the blood, bound by transferrin, and arrived liver through hepatic portal circulation. Liu et al50 found that bromoacetic acid induced abnormal lipid metabolism. Further investigation using lipidomics and microbial metabolomics unveiled that gut microbiota metabolite glycochenodeoxycholate activated TFR-ACSL4-mediated ferroptosis. Mouth-liver axis was getting in on the act. After oral administration with Porphyromonas gingivalis, amino acid biosynthesis, TCA cycling, and fatty acid and lipid degradation were observed, which are metabolic pathways associated with ferroptosis.51
The above findings suggest that ferroptosis inhibition could be specifically targeted in the treatment of MAFLD.
Ferroptosis in Autoimmune Hepatitis
Autoimmune hepatitis (AIH), an inflammatory lesion of the liver, is characterized by hyperglobulinemia, multiple autoantibodies, and necrotic debris in the portal venous confluence area. It is mainly caused by injury mediated by the immune system and, in the absence of quick and effective treatment, it increases the risk for developing end-stage liver disease.52 The morphological features of autoimmune hepatitis are often variable, but the latter is known for its interface hepatitis on pathological sections as well as the absence of changes typical of other types of liver disease, such as hypergammaglobulinemia, which is more common in women.53 However, the pathogenesis of autoimmune hepatitis, especially the specific processes involved in the death of hepatocytes, are yet to be uncovered. Microarray also suggested the presence of ferroptosis in AIH mice.54 S100-induced autoimmune hepatitis in a mouse model showed that the knockdown of GPX4 promoted the S100-induced accumulation of the lipid peroxide MDA and Fe2+ in liver tissues, hence significantly exacerbating ferroptosis in AIH. Fer-1 is one of the most recognized and typical ferroptosis inhibitors known to reverse S100-induced AIH and subsequent ferroptosis to reduce liver injury.55 Intervention of another typical inhibitor of ferroptosis such as indoleamine 2,3-dioxygenase 1 (IDO1), an intracellular heme enzyme and immune-related modulator involved in the production of Fe2+, also attenuates immune-mediated ferroptosis in mice with liver injury.56 Studies57 have shown that gene knockout of caveolin-1 significantly promoted the massive accumulation of ROS and reactive nitrogen species induced by ConA while Fer-1, a ferroptosis inhibitor, could alleviate ConA-induced AIH.58 Those studies highlight the relevance of ferroptosis to immune-mediated hepatitis, its possible involvement in disease progression in autoimmune hepatitis as an initiator or intermediate mediator, as well as the potential of targeting ferroptosis for improving AIH treatment, thereby providing new perspectives for disease treatment.
Ferroptosis in Hepatic Ischemia-Reperfusion Injury
Hepatic ischemia-reperfusion injury (HIRI) is the result of temporary decrease in tissue blood supply due to vascular reconstruction.59 In 2014, Angeli et al60 proved that ferroptosis was a mechanism included in HIRI. Yamada et al61 analyzed clinical data from pediatric liver transplantation patients and found that iron overload was closely associated with poor prognosis, suggesting the potential effect of ferroptosis in HIRI. Further investigations suggested that nuclear factor erythroid 2-related factor 2 (Nrf2)62 and heme oxygenase 1(HO-1)63 were involved in. In addition, the HECT domain-containing ubiquitin E3 ligase was identified as a negative modulator through TfR in HIRI.64 High-iron diet in HIRI intensified macrophage extracellular trap (MET)65 and iron overload were alleviated after inhibiting METs. Tanshinone, functioning as a coenzyme of NAD(P)H: Quinone oxidoreductase 1 also detoxified lipid peroxy radicals and inhibited ferroptosis in HIRI.66 In liver surgery, the resection of liver lesions requires the blockage of liver blood flow, resulting in different degrees of IRI. The in-depth study of ferroptosis is of great significance for the diagnosis and treatment of IRI.
Ferroptosis in Liver Cirrhosis
For liver cirrhosis treatment, whether ferroptosis is to be inhibited or promoted is in dispute. Gao et al67 found that liver cells secreted iron through external vesicles leading to intracellular iron deficiency, and iron overload existed in hepatic stellate cells (HSC). After iron accumulation, HSC fibrogenic activated as a result of the overproduction of ROS. Yu et al68 also detected accumulation of non-transferrin binding iron in the liver and pathological features of hepatic fibrosis in liver Trf knockout mice. In this regard, possibility of inhibiting ferroptosis for hepatic fibrosis was put forward.
A key characteristic of liver cirrhosis is the excessive deposition of extracellular matrix (ECM) due to the activation of hepatic stellate cell (HSC).69 There was evidence that some drugs could alleviate cirrhosis by promoting HSC ferroptosis. Sorafenib and erastin could promote the expression of autophagy-related gene 16L1 protein, induce the binding of nuclear receptor coactivator 4 to ferritin heavy chain 1, target the degradation of ferritin, and excessive release of iron ions in activated HSC.70 Heme oxygenase 1-induced iron death of HSC is required for magnesium isoglycyrrhizinate to improve liver fibrosis.71 Wogonoside72 and Artemether73 could eventually induce ferroptosis in HSC through P53 pathway.
Ferroptosis in Hepatocellular Carcinoma (HCC)
During tumorigenesis, ferroptosis plays a dual role in promoting and inhibiting tumor,74 which is dependent on the release of damage-associated molecular patterns in the tumor microenvironment and activation of immune responses triggered by iron death injury.
Targeted induction of ferroptosis in HCC cells is a treatment for liver cancer. The first type of drug used to treat advanced hepatocellular carcinoma is usually the multikinase inhibitor Sorafenib, with Louandre et al75 demonstrating that the drug could induce ferroptosis. Since then, the role of ferroptosis in the occurrence and development of HCC has attracted the attention of scholars. The p62-Keap1-NRF2 antioxidant signaling pathway is a key negative regulator involved in the transcriptional activation of ROS and iron-death related genes in HCC cells.76 The results showed that p62-mediated Keap1 degradation led to the activation of Nrf2, and Nrf2 regulatory genes NQO1, HO-1 and FTH1 are inhibited. Jiang et al77 reported that p53 can inhibit the transcription of SLC7A11 and enhanced ferroptosis through the p53 reaction element at 5’ end. Louandre et al78 confirmed that the expression level of Rb was significantly decreased in HCC cells after being treated with sorafenib. Sorafenib could induce ferroptosis by producing mitochondrial ROS, while the inactivation of Rb will increase mitochondrial ROS and enhance oxidative stress. Yuan et al79 treated HepG2 and Hep3B with Alastin, a classical iron death inducer, and found that it could promote the expression of CDGSH iron sulfur domain 1 (CISD1) in an iron-dependent manner. In contrast, the treatment of HCC cells with pioglitazone, an iron-sulfur cluster stabilizer of CISD1, showed that pioglitazone inhibited mitochondrial iron uptake and lipid peroxidation. Alpha-enolase 1 (ENO1) acts as an RNA-binding protein to degrade mRNA, indicating that ENO1 binds and degrades the mRNA of iron regulatory protein 1 gene, thereby regulating the metabolic homeostasis of iron in cells. These results gradually shed light on the mechanism of ferroptosis in liver cancer. The research and development of ferroptosis inducers for tumor targeted therapy, such as arginine-modified vesicular manganese silicate nanobubbles76 and nanobubble-based oxygen-boosted sonodynamic therapy,80 can cause tumor cell death by targeting different ferroptosis-related pathways, which is expected to open up a new picture of precision therapy.
Tumor microenvironment is involved in the process of tumor ferroptosis. The work of Zhao et al81 further revealed the regulatory role of oncometabolite lactate in the tumor microenvironment in the process of ferroptosis in tumor cells. It was confirmed that lactic acid can induce monounsaturated fatty acid formation, which can help HCC cells resist lipid peroxidation induced by oxidative stress. Innate immune cells, including macrophages and neutrophils, are the main components of the tumor microenvironment and promote the dysregulation of iron metabolism in tumor cells.82 On the other hand, ferroptosis of immune cells promotes tumor development. The latest study conducted by Kim et al83 showed that ferroptosis of pathologically activated neutrophils-termed myeloid-derived suppressor cells (PMN-MDSC) in immunologically normal hosts promoted tumor growth by limiting anti-tumor immunity and inducing ferritin hypertrophy promoted tumor growth. Ferritin deposition is a unique immunosuppressant mechanism of PMN-MDSCs in the tumor microenvironment, which can be regulated by targeted drugs to limit tumor development. APOC1 inhibits the development of HCC cancer cells by reversing the M2 phenotype to the M1 phenotype via the iron death pathway of tumor-associated macrophage cells.
Conclusion and Future Research Perspectives
As one of the main organs responsible for iron processing, liver is the first to suffer once iron homeostasis is disrupted. As studies on the mechanism of ferroptosis in the pathogenesis of CLD gradually emerged, diagnosis and treatment strategies of CLD based on ferroptosis are on the agenda.
Ferroptosis is a threat to the survival of any cell, including hepatocytes, stellate cells, Kupffer cells, and cancer cells. However, various properties and roles of different cells in CLD make the clinical feasibility of treating CLD by inhibiting ferroptosis become questionable. Targeted inhibition of ferroptosis was reported to be important for preventing lipid peroxidation-mediated liver injury, inflammatory infiltration, and immune disorders in the development of these diseases, while resistance to targeted therapy needs to be reversed by inducing ferroptosis. There are still many problems worth discussing. What role ferroptosis plays in the evolution from hepatitis to cirrhosis or HCC is still required for further basic and clinical research breakthrough. In addition, how iron transport between intracellular and extracellular compartments affects liver status remains mysterious, due to the constant variation of iron between reduced and oxidized forms.
In conclusion, with the ongoing development of new projects dedicated to basic research on ferroptosis, a full picture of intracellular interactions after ferroptosis is expected, and the development of new diagnostic system and therapeutic strategies based on ferroptosis will become a hot spot of liver disease research in the future.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by a grant from Zhejiang Province Public Welfare Fund Research Project (LGD22H030007), and Open Fund of Zhejiang Provincial Key Laboratory of Precision Diagnosis, Treatment and Translation of Chronic Liver Diseases (No.2020E10014-003 and No.2020E10014-007), and the Wenzhou Science and Technology Bureau basic medical and health science and technology projects (No. Y20210147 and No. 2021Y1691).
Disclosure
The authors declare that they have no competing interests.
References
1. Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–143. doi:10.1016/j.freeradbiomed.2018.09.043
2. Finn AV, Nakano M, Polavarapu R, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59(2):166–177. doi:10.1016/j.jacc.2011.10.852
3. Zhou N, Bao J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database. 2020;2020. doi:10.1093/database/baaa021
4. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi:10.1038/s41422-020-00441-1
5. Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020;9(6):1505. doi:10.3390/cells9061505
6. Kose T, Vera-Aviles M, Sharp PA, Latunde-Dada GO. Curcumin and (-)- epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals. 2019;12(1). doi:10.3390/ph12010026
7. Song X, Xie Y, Kang R, et al. FANCD2 protects against bone marrow injury from ferroptosis. Biochem Biophys Res Commun. 2016;480(3):443–449. doi:10.1016/j.bbrc.2016.10.068
8. Wang W, Green M, Choi JE, et al. CD8 T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi:10.1038/s41586-019-1170-y
9. Tang HM, Tang HL. Cell recovery by reversal of ferroptosis. Biol Open. 2019;8(6). doi:10.1242/bio.043182
10. Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–2081. doi:10.1080/15548627.2020.1810918
11. Wu J, Eckard J, Chen H, Costa M, Frenkel K, Huang X. Altered iron homeostasis involvement in arsenite-mediated cell transformation. Free Radic Biol Med. 2006;40(3):444–452. doi:10.1016/j.freeradbiomed.2005.08.035
12. Canli Ã, Alankuåÿ YB, Grootjans S, et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127(1):139–148. doi:10.1182/blood-2015-06-654194
13. Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. doi:10.1016/j.jhep.2018.05.011
14. Gao W, Zhao J, Gao Z, Li H. Synergistic interaction of light alcohol administration in the presence of mild iron overload in a mouse model of liver injury: involvement of triosephosphate isomerase nitration and inactivation. PLoS One. 2017;12(1):e0170350. doi:10.1371/journal.pone.0170350
15. Qi J, Kim J-W, Zhou Z, Lim C-W, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. Am J Pathol. 2020;190(1):68–81. doi:10.1016/j.ajpath.2019.09.011
16. Yamane D, Hayashi Y, Matsumoto M, et al. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem Biol. 2022;29(5):799–810.e4. doi:10.1016/j.chembiol.2021.07.022
17. Wang R, Zhao S, Du J, Qiao J, Li W, Nan Y. A correlation analysis of the serum hepcidin concentrations and viral loads in HCV-infected patients. Am J Transl Res. 2021;13(6):6297–6304.
18. Chang M-L, Hu J-H, Yen C-H, et al. Evolution of ferritin levels in hepatitis C patients treated with antivirals. Sci Rep. 2020;10(1):19744. doi:10.1038/s41598-020-76871-z
19. Bian Z, Hann H-W, Ye Z, et al. Ferritin level prospectively predicts hepatocarcinogenesis in patients with chronic hepatitis B virus infection. Oncol Lett. 2018;16(3):3499–3508. doi:10.3892/ol.2018.9099
20. Luo J, Liang X, Xin J, et al. Serum ferritin diagnosis and prediction of hepatitis B virus-related acute-on-chronic liver failure. J Med Virol. 2022;95:e28183.
21. Komissarov AA, Karaseva MA, Roschina MP, et al. Individual expression of hepatitis A virus 3C protease induces ferroptosis in human cells in vitro. Int J Mol Sci. 2021;22(15):7906. doi:10.3390/ijms22157906
22. Dimitriadis A, Foka P, Kyratzopoulou E, et al. The Hepatitis C virus NS5A and core proteins exert antagonistic effects on HAMP gene expression: the hidden interplay with the MTF-1/MRE pathway. FEBS Open Bio. 2021;11(1):237–250. doi:10.1002/2211-5463.13048
23. Foka P, Dimitriadis A, Karamichali E, et al. HCV-induced immunometabolic crosstalk in a triple-cell co-culture model capable of simulating systemic iron homeostasis. Cells. 2021;10(9):2251. doi:10.3390/cells10092251
24. Haberger V, Elgner F, Roos J, Bender D, Hildt E. Regulation of the transferrin receptor recycling in hepatitis C virus-replicating cells. Front Cell Dev Biol. 2020;8:44. doi:10.3389/fcell.2020.00044
25. Liu G-Z, Xu X-W, Tao S-H, Gao M-J, Hou Z-H. HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J Biomed Sci. 2021;28(1):67. doi:10.1186/s12929-021-00762-2
26. Ma S, Liu A, Hu X, et al. Eltrombopag inhibits Type I interferon-mediated antiviral signaling by decreasing cellular iron. Biochem Pharmacol. 2021;186:114436. doi:10.1016/j.bcp.2021.114436
27. Xing G, Meng L, Cao S, et al. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022;23(8):e52280. doi:10.15252/embr.202052280
28. Bessone F, Lucena MI, Roma MG, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver Int. 2016;36(2):302–310. doi:10.1111/liv.12899
29. Imaizumi N, Kwang Lee K, Zhang C, Boelsterli UA. Mechanisms of cell death pathway activation following drug-induced inhibition of mitochondrial complex I. Redox Biol. 2015;4:279–288. doi:10.1016/j.redox.2015.01.005
30. Zhou J, Tan Y, Hu L, et al. Inhibition of HSPA8 by rifampicin contributes to ferroptosis via enhancing autophagy. Liver Int. 2022;42(12):2889–2899. doi:10.1111/liv.15459
31. Pan Y, Tang P, Cao J, et al. Lipid peroxidation aggravates anti-tuberculosis drug-induced liver injury: evidence of ferroptosis induction. Biochem Biophys Res Commun. 2020;533(4):1512–1518. doi:10.1016/j.bbrc.2020.09.140
32. Yang L, Wang H, Yang X, et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct Target Ther. 2020;5(1):138. doi:10.1038/s41392-020-00253-0
33. Niu B, Lei X, Xu Q, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in Acetaminophen-induced acute liver injury. Cell Biol Toxicol. 2022;38(3):505–530. doi:10.1007/s10565-021-09624-x
34. Wu L, Ding Q, Wang X, et al. Visualization of dynamic changes in labile iron(II) pools in endoplasmic reticulum stress-mediated drug-induced liver injury. Anal Chem. 2020;92(1):1245–1251. doi:10.1021/acs.analchem.9b04411
35. Martin SR, Alvarez F, Anand R, Song C, Yin W. Outcomes in children who underwent transplantation for autoimmune hepatitis. Liver Transpl. 2011;17(4):393–401. doi:10.1002/lt.22244
36. Suzuki Y, Saito H, Suzuki M, et al. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26(8Suppl):26S–31S. doi:10.1111/j.1530-0277.2002.tb02698.x
37. Dostalikova-Cimburova M, Balusikova K, Kratka K, et al. Role of duodenal iron transporters and hepcidin in patients with alcoholic liver disease. J Cell Mol Med. 2014;18(9):1840–1850. doi:10.1111/jcmm.12310
38. Li L-X, Guo -F-F, Liu H, Zeng T. Iron overload in alcoholic liver disease: underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell Mol Life Sci. 2022;79(4):201. doi:10.1007/s00018-022-04239-9
39. Liu J, He H, Wang J, et al. Oxidative stress-dependent frataxin inhibition mediated alcoholic hepatocytotoxicity through ferroptosis. Toxicology. 2020;445:152584. doi:10.1016/j.tox.2020.152584
40. Zhang Y, Zhao S, Fu Y, et al. Computational repositioning of dimethyl fumarate for treating alcoholic liver disease. Cell Death Dis. 2020;11(8):641. doi:10.1038/s41419-020-02890-3
41. Xu M-J, Cai Y, Wang H, et al. Fat-specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology. 2015;149(4):1030–1041.e6. doi:10.1053/j.gastro.2015.06.009
42. Zhou Z, Ye TJ, Bonavita G, et al. Adipose-specific lipin-1 overexpression renders hepatic ferroptosis and exacerbates alcoholic steatohepatitis in mice. Hepatol Commun. 2019;3(5):656–669. doi:10.1002/hep4.1333
43. You Y, Liu C, Liu T, et al. FNDC3B protects steatosis and ferroptosis via the AMPK pathway in alcoholic fatty liver disease. Free Radic Biol Med. 2022;193:808–819. doi:10.1016/j.freeradbiomed.2022.10.322
44. Ma C, Han L, Zhu Z, Heng Pang C, Pan G. Mineral metabolism and ferroptosis in non-alcoholic fatty liver diseases. Biochem Pharmacol. 2022;205:115242. doi:10.1016/j.bcp.2022.115242
45. Wang S, Liu Z, Geng J, Li L, Feng X. An overview of ferroptosis in non-alcoholic fatty liver disease. Biomed Pharmacother. 2022;153:113374. doi:10.1016/j.biopha.2022.113374
46. Feng G, Byrne CD, Targher G, Wang F, Zheng M-H. Ferroptosis and metabolic dysfunction-associated fatty liver disease: is there a link? Liver Int. 2022;42(7):1496–1502. doi:10.1111/liv.15163
47. Eder SK, Feldman A, Strebinger G, et al. Mesenchymal iron deposition is associated with adverse long-term outcome in non-alcoholic fatty liver disease. Liver Int. 2020;40(8):1872–1882. doi:10.1111/liv.14503
48. Tsurusaki S, Tsuchiya Y, Koumura T, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10(6):449. doi:10.1038/s41419-019-1678-y
49. Pan X, Peng H, Zhang J, Wu Y, Hu Z, Peng X-E. Genetic variants in promoter region of is associated with the risk of non-alcoholic fatty liver disease in a Chinese Han population: a case-control study. Gastroenterol Rep. 2022;10:goac060. doi:10.1093/gastro/goac060
50. Liu S, Gao Z, He W, et al. The gut microbiota metabolite glycochenodeoxycholate activates TFR-ACSL4-mediated ferroptosis to promote the development of environmental toxin-linked MAFLD. Free Radic Biol Med. 2022;193(Pt 1):213–226. doi:10.1016/j.freeradbiomed.2022.10.270
51. Yao C, Lan D, Li X, Wang Y, Qi S, Liu Y. Porphyromonas gingivalis is a risk factor for the development of nonalcoholic fatty liver disease via ferroptosis. Microbes Infect. 2022;25:105040. doi:10.1016/j.micinf.2022.105040
52. Montano-Loza AJ, Ronca V, Ebadi M, et al. Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J Hepatol. 2022;77(1):84–97. doi:10.1016/j.jhep.2022.01.022
53. Hajoui O, Martin S, Alvarez F. Study of antigenic sites on the asialoglycoprotein receptor recognized by autoantibodies. Clin Exp Immunol. 1998;113(3):339–345. doi:10.1046/j.1365-2249.1998.00673.x
54. Liu Y, Chen H, Hao J, Li Z, Hou T, Hao H. Characterization and functional prediction of the microRNAs differentially expressed in a mouse model of concanavalin A-induced autoimmune hepatitis. Int J Med Sci. 2020;17(15):2312–2327. doi:10.7150/ijms.47766
55. Zhu L, Chen D, Zhu Y, et al. GPX4-regulated ferroptosis mediates S100-induced experimental autoimmune hepatitis associated with the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2021;2021:6551069. doi:10.1155/2021/6551069
56. Zeng T, Deng G, Zhong W, et al. Indoleamine 2, 3-dioxygenase 1enhanceshepatocytes ferroptosis in acute immune hepatitis associated with excess nitrative stress. Free Radic Biol Med. 2020;152:668–679. doi:10.1016/j.freeradbiomed.2020.01.009
57. Selmi C, Generali E, Gershwin ME. Rheumatic manifestations in autoimmune liver disease. Rheum Dis Clin North Am. 2018;44(1):65–87. doi:10.1016/j.rdc.2017.09.008
58. Deng G, Li Y, Ma S, et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic Biol Med. 2020;148:151–161. doi:10.1016/j.freeradbiomed.2019.12.026
59. Gracia-Sancho J, Casillas-RamÃ-rez A, Peralta C. Molecular pathways in protecting the liver from ischaemia/reperfusion injury: a 2015 update. Clin Sci. 2015;129(4):345–362. doi:10.1042/CS20150223
60. Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi:10.1038/ncb3064
61. Yamada N, Karasawa T, Wakiya T, et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am J Transplant. 2020;20(6):1606–1618. doi:10.1111/ajt.15773
62. Ye J, Peng J, Liu K, Zhang T, Huang W. MCTR1 inhibits ferroptosis by promoting NRF2 expression to attenuate hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2022;323(3):G283–G293. doi:10.1152/ajpgi.00354.2021
63. Li X, Wu L, Tian X, et al. miR-29a-3p in exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells alleviates steatotic liver ischemia-reperfusion injury in rats by suppressing ferroptosis via iron responsive element binding protein 2. Oxid Med Cell Longev. 2022;2022:6520789. doi:10.1155/2022/6520789
64. Wu Y, Jiao H, Yue Y, et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 2022;29(9):1705–1718. doi:10.1038/s41418-022-00957-6
65. Wu S, Yang J, Sun G, et al. Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br J Pharmacol. 2021;178(18):3783–3796. doi:10.1111/bph.15518
66. Wang T-X, Duan K-L, Huang Z-X, et al. Tanshinone functions as a coenzyme that confers gain of function of NQO1 to suppress ferroptosis. Life Sci Alliance. 2023;6(1):e202201667. doi:10.26508/lsa.202201667
67. Gao H, Jin Z, Bandyopadhyay G, et al. Aberrant iron distribution via hepatocyte-stellate cell axis drives liver lipogenesis and fibrosis. Cell Metab. 2022;34(8):1201–1213.e5. doi:10.1016/j.cmet.2022.07.006
68. Yu Y, Jiang L, Wang H, et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136(6):726–739. doi:10.1182/blood.2019002907
69. Yang YM, Noureddin M, Liu C, et al. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci Transl Med. 2019;11:496. doi:10.1126/scitranslmed.aat9284
70. Zhang Z, Guo M, Li Y, et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16(8):1482–1505. doi:10.1080/15548627.2019.1687985
71. Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125–133. doi:10.1016/j.biopha.2018.06.060
72. Liu G, Wei C, Yuan S, et al. Wogonoside attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis through SOCS1/P53/SLC7A11 pathway. Phytother Res. 2022;36(11):4230–4243. doi:10.1002/ptr.7558
73. Wang L, Zhang Z, Li M, et al. P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life. 2019;71(1):45–56. doi:10.1002/iub.1895
74. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–296. doi:10.1038/s41571-020-00462-0
75. Louandre C, Ezzoukhry Z, Godin C, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133(7):1732–1742. doi:10.1002/ijc.28159
76. Wang S, Li F, Qiao R, et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS Nano. 2018;12(12):12380–12392. doi:10.1021/acsnano.8b06399
77. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi:10.1038/nature14344
78. Louandre C, Marcq I, Bouhlal H, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356(2Pt B):971–977. doi:10.1016/j.canlet.2014.11.014
79. Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478(2):838–844. doi:10.1016/j.bbrc.2016.08.034
80. Chen Y, Shang H, Wang C, et al. RNA-seq explores the mechanism of oxygen-boosted sonodynamic therapy based on all-in-one nanobubbles to enhance ferroptosis for the treatment of HCC. Int J Nanomedicine. 2022;17:105–123. doi:10.2147/IJN.S343361
81. Zhao Y, Li M, Yao X, et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. 2020;33(10):108487. doi:10.1016/j.celrep.2020.108487
82. Liang W, Ferrara N. Iron metabolism in the tumor microenvironment: contributions of innate immune cells. Front Immunol. 2020;11:626812. doi:10.3389/fimmu.2020.626812
83. Kim R, Hashimoto A, Markosyan N, et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi:10.1038/s41586-022-05443-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.