Back to Journals » Journal of Inflammation Research » Volume 14
The Emerging Clinical Application of m6A RNA Modification in Inflammatory Bowel Disease and Its Associated Colorectal Cancer
Authors Xu X, Huang J, Ocansey DKW , Xia Y, Zhao Z, Xu Z, Yan Y, Zhang X, Mao F
Received 16 May 2021
Accepted for publication 1 July 2021
Published 15 July 2021 Volume 2021:14 Pages 3289—3306
DOI https://doi.org/10.2147/JIR.S320449
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xinwei Xu,1,* Jintu Huang,2,* Dickson Kofi Wiredu Ocansey,1,3 Yuxuan Xia,1 Zihan Zhao,1 Zhiwei Xu,1 Yongmin Yan,1 Xu Zhang,1 Fei Mao1
1Department of Clinical Laboratory Diagnostics, Key Laboratory of Medical Science and Laboratory Medicine of Jiangsu Province, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, 212013, People’s Republic of China; 2Clinical Laboratory Department, The People’s Hospital of Danyang, Affiliated Danyang Hospital of Nantong University, Zhenjiang, Jiangsu, 212300, People’s Republic of China; 3Department of Clinical Laboratory Diagnostics, Directorate of University Health Services, University of Cape Coast, Cape Coast, Ghana
*These authors contributed equally to this work
Correspondence: Fei Mao
Key Laboratory of Medical Science and Laboratory Medicine of Jiangsu Province, School of Medicine, Jiangsu University, Zhenjiang, 212013, Jiangsu, People’s Republic of China
Tel/Fax +86 511 8503 8215
Email [email protected]
Abstract: Methylation, first proposed in DNAs, but later found in RNAs, serves as one of the most widespread epigenetic modifications in eukaryotes, where N6-methyladenosine (m6A) modification has been found to play an important role in a variety of cancers including colorectal cancer (CRC). Under the action of various enzymes and proteins, the regulatory role of m6A in RNAs and immune cells has also been gradually realized. This paper reviews the general biogenesis and effects of m6A, and its emerging crucial role in intestinal mucosal immunity via the regulation of RNAs and immune cells, and thus closely related to the occurrence and development of inflammatory bowel disease (IBD) and CRC. m6A-related genes and regulatory factors are expected to be potential predictive markers and therapeutic targets.
Keywords: N6-methyladenosine, inflammatory bowel disease, colorectal cancer, non-coding RNA, intestinal mucosal immunity
Introduction
IBD is a group of idiopathic chronic inflammatory diseases with abnormal immunity of intestinal mucosa caused by a combination of factors, and mainly include ulcerative colitis (UC) and Crohn’s disease (CD). Due to the abnormal autoimmune function in patients, other organs and tissues may be damaged. In addition, patients with IBD have a higher risk of colon cancer.1 Recent studies have shown that individual genetic susceptibility, environmental influence, intestinal microorganisms, and immune response are all involved and functionally integrated into the pathogenesis of IBD.2,3 Notwithstanding, the specific pathogenesis of IBD is still unclear and its clinical treatment mainly relies on surgery and traditional therapies that are unable to completely relieve the symptoms, and prone to adverse events that affect the quality of life of patients.4 Therefore, the urge to further explore the pathogenesis of IBD and find new alternative therapies is significantly necessitous.
Among the many (over 100) different chemical modifications, m6A has attracted wide attention due to its dynamic regulation and reversible post-transcriptional regulation. Its interaction with a variety of RNAs and signaling pathways makes it play an important role in the development of diseases. Common mRNA methylation modifications include m6A, N1-methyladenosine (m1A), and 5-methylcytosine (m5C). Methylation of adenosine N1 atoms to form 1-methyladenosine (m1A) has been identified at the nucleotide sites 9, 14, 22, 57, and 58 of different tRNAs. In some cases, these modifications have been shown to increase the stability of tRNA structures and induce correct tRNA folding.5 mRNA is locally modified by m5C at the DNA damage sites. The RNA methyltransferase, tRNA aspartic acid methyltransferase 1(tRDMT1), is recruited to the sites of DNA damage to promote m5C induction, suggesting that RNA post-transcriptional modification can also act as DNA damage code to regulate DNA repair.6 m6A was first found in mRNA in 19747 and is the most abundant internal RNA modification in eukaryotic cells, affecting many aspects of RNA metabolism, from RNA processing, nuclear output, RNA translation to degradation. Emerging evidence suggests that m6A methylation plays a key role in embryonic development, circadian rhythm, cell cycle, and tumorigenesis through a variety of mechanisms.8 Besides, m6A methylation provides more possibilities for the early diagnosis and treatment of a variety of cancers, such as gastric, liver, lung, bladder, and colon cancers.9–13
There is increasing evidence that m6A methylation, as an important post-transcriptional gene regulation mechanism, participates in IBD pathogenesis. For example, the deletion of RNA methylation writer methyltransferase like 14(METTL14), an RNA m6A methyltransferase component, in T cells triggers spontaneous colitis in mice, characterized by a Th1/Th17 phenotype. The development of colitis is due to dysfunctional regulatory T cells and is dependent on the gut microbiota.14 A similar study reports that mice with Foxp3-mediated deletion of methyltransferases like 3 (METTL3), an mRNA m6A methylation, in regulatory T cells develop a severe systemic autoimmune response,15 a characteristic of intestinal inflammatory conditions. This paper examines available literature on the major forms of m6A modification, explores their involvement in intestinal mucosal immunity, dendritic cell (DC) and T cell regulation, as well as the evolving clinical significance in IBD and CRC.
m6A RNA Modification
Biogenesis of m6A and Its Regulation of mRNA
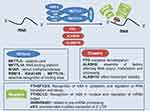
m6A is the most abundant internal modification in mRNA and lncRNA in eukaryotes.16 It is a reversible and dynamic methylated modification of adenosine in the sixth N of mRNA.17 It was not until 2012 that the modification map of m6A in the transcriptome (epitope transcriptome) was first mapped with the development of next-generation sequencing technology.18 The modification sites of m6A have a typical recognition sequence – DRACH motif (D stands for G/A/U, R stands for G/A, and H stands for A/C/U), and are enriched in the coding sequence (CDS), 3ʹ untranslated region (3 ‘UTRs) and 5ʹ untranslated region (5ʹ UTRs) of mRNAs,19 especially high m6A modification is common near the stop codon regions. The 3 ‘UTRs bind to many mRNA-binding proteins. The dynamic modification of m6A is mainly performed by three components, “writers”, “erasers” and “readers”. The writers refer to adenosine methyltransferase as the representative, erasers as the demethylases, and readers as the m6A binding proteins. Its dynamic and reversible modification process is mainly dependent on demethylase, while the methylation localization of a specific region depends on its main consensus motif recognition. Researches have shown that m6A modification can affect the stability of A: U base pairing.20
The methylation of mRNA is mainly dependent on the polyprotein adenosine methyltransferase complex, of which METTL3, METTL14, and Wilms tumor 1-associated protein (WTAP) are common complexes. METTL3 acts as the catalytic core, which is the S-adenosyl-L-methionine (SAM) binding component of the complex and has a catalytic function itself. METTL3 transfers methyl from SAM to the receptor adenine portion, while METTL14 acts as an RNA binding platform, facilitating RNA substrate binding and enhancing complex integrity. By intracellular localization of METTL3, it was found that METTL3 is expressed in both the nucleus and cytoplasm of the cell, which confirms that it could play a methylation function in different sites.21 Also, this multi-protein complex is essential in cells, and its removal leads to cell death. The loss of METTL3 in mice can lead to the death of early embryos,22 where METTL3 knockout in mouse embryonic stem cells (ESCs) significantly reduces the m6A peak, resulting in the loss of the self-renewal ability of the damaged ESCs and the differentiation of multiple lineages in vivo and in vitro.23
METTL14 is a homologous gene of METTL3, which can independently and specifically recognize the common motif GAC. The formation of a stable heterodimer core complex between the two makes the methylation component more active.24 The main function of WTAP is to recruit METTL3 and METTL14. Although the WTAP protein does not play a direct role in transmethylation, its homologous analogs Mum2 and FIP37 are related to METTL3 and are necessary components for effective methylation of mRNA. The interaction of WTAP with the METTL3-METTL14 complex can promote the translocation of the complex to nuclear spots. One study showed that methylation profiles defined by monitoring m6A levels at the depletion of WTAP could be divided into two categories: WTAP-dependent sites and non-WTAP dependent sites.25 Among them, WTAP dependent sites are located in the transcript, with a static topological structure and inversely proportional to mRNA stability, which can promote its degradation. A non-WTAP dependent site is formed at the base of the first transcription as part of the cap structure. Such sites exist in tens of millions of sites, resulting in unprecedented transcriptome complexity. It is worth mentioning that WTAP is not only a regulator required by m6A but also up-regulated in many tumors. A study has shown that both METTL3 knockdown and overexpression lead to up-regulation of WTAP protein,26 suggesting that METTL3 levels are critical to WTAP homeostasis and further suggesting that the oncogenic ability of WTAP may be closely related to the functional m6A methylation complex.
In addition to the above enzymes which play an important role in the methylation process, in recent years, through proteomic screening and other high-throughput technologies, researchers have found that KIAA1429,25 RBM15,27 and METTL1628 may be other subunits of the methyltransferase complex, which can selectively identify binding sites to achieve accurate post-transcriptional regulation. In 2019, Methyltransferase like 5 (METTL5) was identified as the enzyme responsible for 18S rRNA m6A modification, and zinc finger CCHC-type containing 4 (ZCCHC4) was identified as a 28S rRNA modification enzyme, while METTL5 must form a heterodimer with the known methyltransferase activator tRNA methyltransferase subunit 11–2 (TRMT112) to achieve metabolic stability in cells.18,29
Although methyltransferases have been discovered since the 1970s, it was not until 2011 that the first demethylase – fat mass and obesity-associated protein (FTO) was discovered by Jia et al,30 which established the recognition of methylation reversibility. In vitro experiments have shown that FTO has effective oxidative demethylation activity targeting abundant m6A sites in RNA. Knocking down FTO with siRNA leads to an increase in m6A in mRNA, while overexpression of FTO leads to a decrease in m6A in human cells. Further results of nuclear spot co-localization confirmed that m6A is the main physiological substrate of FTO in nuclear RNA. Exogenous overexpression of FTO enables it to efficiently bind to RNA sites containing the m6A motif and specifically remove m6A modification in the GGACU and RRACU motifs in a concentration-dependent manner. This finding highlights the role of the dynamics of FTO in target selection, which is expected to promote the dynamic modification of m6A and the plasticity of FTO in biological function and disease.31 In 2013, Zheng et al found another enzyme ALKBH5 with a demethylation effect – mammalian RNA demethylation enzyme that affects RNA metabolism and mouse fertility,32 and its N terminal has an alanine-rich region and a unique coiled-coil structure. This demethylation activity of ALKBH5 significantly affected mRNA output, RNA metabolism, and the assembly of mRNA processing factors in nuclear spots. Recently, a new demethylase ALKB10B was discovered in Arabidopsis thaliana.33 The bidirectional modulation effect of Writers and Erasers deepened the clinical understanding of the disease, and it is believed that more m6A modification-related effects will be explored in future studies to guide clinical diagnosis and treatment.
m6A-modified mRNAs perform specific biological functions mainly through two pathways: while the first is to fine-tune the methylated transcript to block or induce protein–RNA interactions, the second is to require recognition by a specific RNA-binding protein (also known as methylated readers), to induce subsequent reactions. A variety of reading proteins have been identified by RNA pull-down assay, including proteins in the YTH domain, nuclear heterogeneous ribose protein (HNRNP), and eukaryotic initiation factor (eIF). The functions of these reading proteins include specific binding to the m6A methylation region, weakening the homologous binding of RNA-binding proteins, and altering the secondary structure of RNA to alter the protein-RNA interaction. Proteins with the YTH domain include YTHDC1,34,35 YTHDC2,36,37 YTHDF1,38 YTHDF2,39 and YTHDF3.40 While YTHDF1-3 specifically recognizes m6A-modified mRNA in the cytoplasm, YTHDC1-2 mainly acts in the nucleus. These proteins have the YTH domain at the C-terminal and can overlap with the m6A consensus motif to mediate RNA-specific binding, while the P/Q/N-rich domain is related to subcellular localization.
The eIF3 protein can bind to the m6A modified base on RNA 5 ‘UTRs to promote mRNA translation. The interaction between METTL3 and eukaryotic translation-initiator 3 subunit h (eIF3h) is necessary to promote translation and form densely packed multi-ribosomes and carcinogenic transformation. The findings of Choe et al reveal a mechanism for translation control based on the mRNA cycle and identify METTL3-eIF3h, as a potential therapeutic target for cancer patients.41 Moreover, HNRNPA2B1, a member of the hnRNP family of proteins, functions as a reading protein. Unlike YTHDC1, HNRNPA2B1 does not bind directly to the m6A modified base. In addition to activating the downstream pathway of miRNA primers (pri-miRNAs), HNRNPA2B1 is also involved in the processing of miRNA precursors (pre-miRNAs). Kwon et al showed that HNRNPA2B1 plays an important role in early embryogenesis by regulating transcription-related factors and determining cell fate transformation. HNRNPA2B1 is regulated by METTL3-dependent m6A RNA methylation.42 In summary, m6A can be deposited on the transcript during transcription and regulates biological processes such as cutting, output, translation, and degradation of mRNA by changing the structure of mRNA or depending on the specific recognition of readers, and thus may play an important role in the occurrence and development of a variety of diseases. Figure 1 summarizes the different roles played by methyltransferases, demethylases, and methylation binding proteins in the m6A modification of RNA.
Effects of m6A Modification on lncRNA
The role of lncRNAs in a variety of diseases has attracted increasing attention. ncRNAs modification may in its function adjustment, proteomic interactions, and downstream effector play an important role, and is very rich in m6A as eukaryotes lncRNA of interior decoration, by adjusting the cutting of ncRNAs itself, transshipment, stability, and degradation, affect the biological function of the cell to regulate cell proliferation and metastasis of cancer, stem cell differentiation, and homeostasis. Hence, they play an indispensable role in regulating the body’s physiological functions. A study described the effect of m6A modification of two chromatin-related lncRNAs MALAT1 and XIST on gene expression.43 MALAT1 is a highly abundant m6A modified transcript with metastasis-associated lung adenocarcinoma transcript 1, and its mutation is highly correlated with upregulation and cancer development and metastasis. However, XIST exists in the nucleus and acts as a major regulator of X chromosome inactivation.
Another study by Yang et al showed that the internal m6A modification of LINC1281 mediates a competitive endogenous RNA (ceRNA) model to regulate the differentiation of mouse ESC (mESCs).44 m6A is highly enriched in the LINC1281 transcript, which ensures the characterization of mESCs by isolating the pluripotency-related let-7 family of microRNAs (miRNAs), and this RNA–RNA interaction is dependent on m6A. After a comprehensive analysis of the m6A modification of lncRNA in CRC, Zuo et al found that the level of m6A of lncRNA in CRC tissues is higher than that in normal tissues of adjacent tumors. A total of 8332 m6A peaks were detected in 6690 lncRNAs in CRC tissues. Approximately 91% of the modified lncRNAs had a unique m6A modification peak. A total of 383 lncRNAs were differentially methylated in CRC, 48.24% of which were 1–1000 bp in length, and most of them were located on chromosomes 1, 2, 7, 11, 16, and 19. GO and KEGG analysis showed that genes close to differentially methylated or expressed lncRNAs were related to the occurrence and development of CRC. Methylation was positively correlated with lncRNA expression levels in CRC and normal tissues adjacent to tumors.45
Similarly, Liu et al showed that the internal m6A modification of lncRNA Thor modulates cancer cell proliferation in an m6A reader-dependent manner. Specific m6A readers YTHDF1 and YTHDF2 can read the highly enriched m6A motif on lncRNA Thor transcripts, regulate its stability, and thus maintain the carcinogenic effect.46 Interestingly, the two studies of He et al47 and Chen et al48 respectively confirmed that ALKBH5 played two opposite effects of promoting cancer and inhibiting cancer in different cancers by regulating different lncRNA levels through demethylation. ALKBH5 inhibits the progression of pancreatic cancer by demethylation of lncRNA KCNK15-AS1, while the up-regulation of lncRNA PVT1 mediated by ALKBH5 promotes the proliferation of osteosarcoma cells in vitro and tumor growth in vivo.
Effects of m6A Modification on miRNA
m6A peaks are enriched at miRNA target sites at the 3ʹ end and 5ʹ end of the 3ʹUTR, suggesting a potential association between m6A modification and miRNA target sites. The relationship between the two is mainly reflected in miRNA biosynthesis, mRNA–miRNA interaction, and m6A target selection. Moreover, the abundance of m6A positively correlates with the Dicer enzyme mediating miRNA maturation.49,50 Research in mammals has shown that the m6A marker promotes the initiation of miRNA biogenesis as a key post-transcriptional modification. METTL3 can methylate pri-miRNAs and label them for recognition and processing by DGCR8. Similarly, METTL3 deletion reduces the binding of DGCR8 to pri-miRNAs, leading to an overall reduction in mature miRNAs, accompanied by the accumulation of unprocessed pri-miRNAs.51 Hao et al found that using RNA guide base editing technique of miRNA 675 m6A area of the base, substitution, and target mutations can induce the occurrence of apoptosis, and miR675 m6A modification sites upstream regions of mutations, resulting in a loss of the H19 gene expression in HEK293T cells and apoptosis induction. This shows that the methylation modification of miRNA plays an important role in cell.52 Furthermore, manipulation of miRNA expression or sequence can alter the modification level of m6A by regulating the binding of METTL3 methyltransferase to mRNAs containing miRNA targets. The increase of m6A content promotes the reprogramming of mouse embryonic fibroblasts (MEFs) into pluripotent stem cells. Conversely, the reduced m6A level impedes reprogramming53 and studies have shown the excellent potential of miRNA–mRNA interactions and methylation modifications in the field of cell reprogramming.
As described above, HNRNPA2B1 is involved in the processing of pri-miRNA. HNRNPA2B1 direct binding to a set of nuclear transcripts and produces a selective splicing effect similar to that of the m6A transcript METTL3. In addition, HNRNPA2B1 binds to the m6A marker in the subsets of primary miRNA transcripts and interacts with the miRNA microprocessor protein complex DGCR8 to promote primary miRNA processing. HNRNPA2B1 and METTL3 deletions also lead to similar processing defects in these pri-miRNA precursors. HNRNPA2B1 moderates the effect of this marker on primary miRNA processing and selective splicing to a certain extent.54 Moreover, another study showed that insulin-like growth factor 2 mRNA-binding protein 1(IGF2BP1, an mRNA binding protein) could promote the expression of SRF by impairing the mRNA degradation of miRNA-mediated transcription regulator SRF in an m6A-dependent manner, and thus regulates the expression of genes in cancer.55 This study identified the stabilizing role of miRNome- and m6A-dependent regulation of gene expression in cancer. Jin et al showed that m6A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity.56 In addition to being regulated by methylation, miRNA can also reverse regulate the methylation level of mRNA. Yang et al showed that miR-145 regulates the level of m6A by targeting the 3ʹ-UTR of YTHDF2 mRNA in HCC cells,57 which confirms the interaction between miRNA and m6A RNA. Moreover, the newly discovered methyltransferase complex component KIAA1429 can promote the progression of osteosarcoma by promoting stem cell properties and is regulated by miR-143-3p. Knocking down KIAA1429 or ectopically overexpressing miR-143-3p can inhibit stem cell properties and alleviate the disease.58 These studies have further clarified the effect of m6A modification on miRNA biosynthesis and their interaction on cell function, as well as the potential role it may play in cancer and other diseases.
Effects of m6A Modification on circRNA
CircRNAs, a type of ncRNAs, are widely present in eukaryotes and their abnormal expression is closely related to a variety of diseases. More researches have shown that circRNA regulates cell function in a variety of ways and can be used as an effective diagnostic marker for diseases. Zhou et al showed that the genome-wide mapping of m6A circRNA can identify broad and specific cell type methylation patterns that are distinct from mRNA.59 Methylation usually occurs in the exon region (but not in mRNA), suggesting that some m6A modifications to circRNAs may occur during or after circRNA formation. m6A modification of circRNA can affect the reverse splicing and translation process. Taking circ-ZNF609 as an example, the reverse splicing reaction of circRNA requires the participation and guidance of METTL3 and YTHDC1. Due to the translation capability of circ-ZNF609, further studies show that m6A-modified circ-ZNF609 regulates its translation through YTHDF3 and eIF4G2 recognition.60 A recent study showed that the m6A modification of human circRNA inhibits innate immunity.61 Exogenous circRNAs are effective adjuvants for inducing antigen-specific T cell activation, antibody production, and in vivo antitumor immunity. Unmodified circRNAs directly activate RNA pattern recognition receptor RIG-I in the presence of lysine-63-linked polyubiquitin chains, leading to filamentation of junction protein MAVs and activation of downstream transcription factor IRF3, while modification of m6A would inhibit its original activity. Findings by He et al suggest that circNSUN2 is a key biomarker for oncogenic circRNAs and liver metastasis from CRC.62 At present, due to the lack of understanding of the regulatory role of ncRNAs, its abundant chemical modification types present different challenges to be studied, with the potential of presenting significant progress of the mechanism, clinical diagnosis, and treatment of diseases.
In conclusion, m6A modification plays an important role in regulating gene expression, splicing, RNA editing, RNA stability, controlling mRNA lifetime and degradation, and mediating circRNA translation. m6A RNA methylation greatly affects RNA metabolism which is involved in the pathogenesis of a variety of diseases including cancer and gastrointestinal inflammation; hence, it is imperative to further study its regulatory mechanism.
Regulation of Intestinal Mucosal Immunity by m6A RNA Modification
m6A RNA Modification and Dendritic Cells
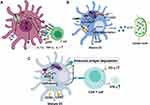
Dendritic cells (DCs), as important antigen-presenting cells, link innate and adaptive immune responses and are the core of coordinating host tolerance and host immunity in peripheral lymphoid tissues, as well as the first point of contact between intestinal flora and the immune system. Typically, immature DCs (imDCs) induce immune tolerance, and mature DCs (maDCs) stimulate and activate the immune response. The abnormal maturation or activation disorder of DCs at different stages can lead to abnormal responses of the host immune system, and even induce inflammation and autoimmune diseases.
As early as 2005, researchers found that DCs exposed to nucleoside modified RNA expressed significantly fewer cytokines and activation markers than DCs treated with unmodified RNA. In other words, nucleoside modification inhibits the potential of RNA to activate DCs.63 The study of Wang et al confirmed that METTL3-mediated mRNA m6A methylation promotes the activation and function of DCs.64 m6A modification of the METTL3-mediated CD40, CD80, and TLR4 signal adapter TIRAP transcription factors enhance its translation in DCs to stimulate T cell activation and enhance Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signal-induced cytokine production. However, the specific loss of METTL3 in DCs leads to impaired phenotype and functional maturation of DCs, decreased expression of co-stimulatory molecules CD40, CD80, and cytokine interleukin (IL)-12, which reduces its ability to stimulate T cell response in vivo and in vitro. Similarly, Wu et al found that METTL3-silences of DCs showed immature characteristics and prolonged allograft survival after constructing a mouse heart transplant model with METTL3 knockout,65 highlighting the regulatory role of m6A in DCs maturation and host immune response. Also, one study showed that C-C chemokine receptor 7 (CCR7) stimulation up-regulates lnc-Dpf3 by removing the m6A modification to prevent RNA degradation. The deletion of specific lnc-Dpf3 in DCs increases CCR7-mediated DC migration, leading to excessive adaptive immune response and inflammatory damage.66 In mechanism, CCR7 stimulation can activate the hypoxia-inducible factor 1α (HIF-1α) transcription factor pathway in DCs. lnc-Dpf3 directly binds to HIF-1α and inhibits the transcription of HIF-1α-dependent glycolytic gene LDHA, thereby inhibiting the glycolytic metabolism and migration of DCs.
In addition to the effects of m6A modification on the maturation and activation of DCs, DCs can also exert antitumor immunity through methylation-related modifications. Han et al established a mouse model of the m6A binding protein YTHDF1 deficiency and found that the defective mice showed an elevated antitumor response of antigen-specific CD8+ T cells.67 At present, most studies point to DCs maturation at different stages with s different effects on the body’s immune response, and m6A modification occurs in DCs by regulating the degradation and translation of RNA, which can be explored to restore the function of DCs required for normal stimulation of molecules and immune response, as shown in Figure 2. Current data still lack a complete and clear understanding of its detailed mechanism; therefore, further research on methylation modification in DCs needs to be carried out.
m6A RNA Modification and Its Regulation of T Cells
In recent years, with the continuous exploration of epigenetic modifications such as methylation, many papers have reported the crucial role of m6A in the regulation of T cell homeostasis and related pathways. Li et al showed that the loss of METTL3 in mouse T cells disrupts T cell homeostasis and differentiation.68 The mRNAs of suppressor of cytokine signaling (SOCS) family genes encoding signal transducer and activator of transcription 1 (STAT1), suppressor of cytokine signaling 3 (SOCS3), and cytokine-inducible SH2 containing protein (CISH) are all labeled with m6A. In METTL3-deficient naive T cells, the mRNA attenuation is slow and the protein expression level is increased, while the increased SOCS family activity further plays a role in inhibiting IL-7-mediated STAT5 activation and T cell homeostasis proliferation and differentiation. Another study confirmed a similar effect of m6A on T cells, in which m6A had control over transcription stability in unstimulated T cells. m6A affects all RNA kinetic rates during T cell differentiation mainly by 1) delaying induction of synthesis rate and simultaneously impair T cell, 2) regulation of processing rate and 3) continuous upregulation of degradation rate.69
Similarly, Zhu et al found that m6A is involved in regulating the development of follicular helper T cells (Tfh cells) through glycolysis. E3 ubiquitin ligase von Hippel–Lindau (VHL) is required for Tfh cells development and function during acute viral infection or antigen immunity. VHL actively regulates early Tfh cell initiation through HIF-1α dependent glycolysis pathway. However, once VHL is defective, its glycolytic activity is increased. Through RNA interference screening, it was found that the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) decreased the expression of inducible T cell costimulator (ICOS) through the modification of m6A, which is a key molecule in the development of Tfh cells.70 Tong et al found that m6A mRNA methylation maintains the inhibition function of Tregs and that m6A RNA modification could specifically target the same gene class, which encodes components of essential signaling pathways in different T cell subtypes, thereby controlling the differentiation of juvenile T cells and maintaining the inhibition function of Tregs.15 Interestingly, Sprent et al also demonstrated that inhibition of mRNA methylation by blocking the m6A writers’ action promotes T cell infantilization.71 In conclusion, m6A plays an important role in the normal cell biological functions of various T cells, regulating the state and maturity of cells, affecting the secretion and release of cytokines, and participating in the immune regulation process.
Advances in the Role of m6A in Intestinal Mucosal Immunity
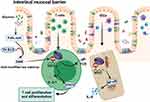
Under normal physiological conditions, intestinal mucosal tissue is an important barrier to isolate the body from foreign pathogens and maintain the immune homeostasis of the intestinal environment. Intestinal microecological imbalance harms the function of the intestinal mucosal barrier and permits pathogen invasion of the intestinal mucosa, leading to recruitment of intestinal inherent lymphocytes and mesenteric lymphatic tissue inflammation, where other immune cells are mobilized to the site to remove pathogen. Long-term chronic inflammatory pathological cases and excessive immune response are often accompanied by serious damage of intestinal mucosa, wherein pathogens damaged the mucous membrane layer to induce a continual immune response.
Wu et al showed that the dynamic epigenetic modification of intestinal tissue strongly mediates the crosstalk between intestinal microbes and the intestinal mucosal barrier. Lactobacillus and Bifidobacterium species in the intestinal symbiotic flora can synthesize folic acid to increase DNA methylation and mRNA m6A modification in the intestinal tract, thus ensuring the normal development of the intestinal tract.72 Similarly, Jabs et al found that the intestinal microbiota affects the epithelial transcriptome of m6A in the cecum of mice. Changes in intestinal microbiota are associated with m6A modification in the cecum and to a lesser extent, affect m6A modification in the liver, affecting pathways related to metabolism, inflammation, and antimicrobial responses.73 In addition to the involvement of intestinal microorganisms in m6A modification, Han et al confirmed the important role of m6A modification in intestinal stem cells (ISCs). The high expression of YTHDF1 in ISCS is detected to promote the translation of Wnt signal effectors including TCF7L2/TCF4, thus enhancing β-catenin activity. Gene ablation of YTHDF1 significantly blocks Wnt-driven regeneration and tumorigenesis and reduces the stem cell characteristics of ISCs. Targeting YTHDF1 in ISCs with established tumors shrinks the tumors and prolongs survival. These findings reveal that YTHDF1 is an amplifier of Wnt/β-catenin signaling at the translational level, which is necessary for maintaining ISCS regeneration and tumorigenesis and is essential for normal intestinal development in mice.74
DCs and T cells are also important participants in intestinal immunity, and the corresponding effects generated by their internal m6A modifications can control the excessive immune response, thus alleviating the persistent intestinal inflammation and excessive destruction of the mucosal barrier. In addition to these immune cells, intestinal mucosal epithelial cells (IECs), as the main component of the barrier, play an indispensable role, but when methylation occurs in IECs, it affects its normal barrier function. A recent study has shown that dietary gluten-induced RNA methylation changes regulate intestinal inflammation through allele-specific chromosome region maintenance protein 1 (XPO1, nuclear output receptor involved in key signaling pathways) translation in IECs.75 Individuals with celiac disease risk alleles have higher m6A methylation at 5ʹUTR of XPO1 RNA, which leads to higher XPO1 protein volume mediated by YTHDF1, resulting in downstream nuclear factor kappa B (NF-κB) activity, stimulating epithelial cells to produce large amounts of the pro-inflammatory cytokine IL-8, and mediating the subsequent inflammatory response. The results further support the possibility that the m6A-XPO1-NF-kB axis may respond to intestinal epithelial injury caused by other drugs, which may have direct significance for the treatment of a variety of gastrointestinal diseases.
In conclusion, all the m6A modifications involved in the composition of the intestinal mucosal barrier play an irreplaceable role in mucosal immunity. Surprisingly, a recent study by Gan et al showed that resveratrol and curcumin decrease m6A enrichment on intestinal tight junction protein transcripts and heme oxygenase-1, increase intestinal antioxidant capacity and tight junction protein mRNA expression, and ultimately improve intestinal mucosal integrity of weaned piglets.76 However, the current research on m6A and intestinal mucosal immunity are poorly understood. Therefore, future studies can carry out extensive exploration and discussions on the role of m6A in intestinal mucosal immunity, and further research the various effects of epigenetic modification. Figure 3 illustrates how m6A regulates intestinal immune components and participates in the process of intestinal mucosal immunity.
m6A RNA Methylation Modification and Inflammatory Bowel Disease
It is well known that IBD is accompanied by an inflammation of the intestinal mucosal barrier and an abnormally persistent immune response. A large number of previous studies have confirmed that m6A plays an important role in the regulation of immune cell state, normal physiological function, and intestinal mucosal immunity.77–79 Therefore, scholars speculate that m6A modification may play a role in the occurrence and development of IBD. An important question remains, whether clinical diagnosis and treatment be carried out by targeting m6A modification sites?
A recent study showed that the deletion of METTL14, a component of RNA m6A methyltransferase, in T cells causes spontaneous colitis in mice. METTL14 deficiency in T cells causes spontaneous colitis in mice, and its development can be attributed to the dysfunction of Tregs. The decreased expression of Rorγ T in METTL14 deficient Tregs results in impaired induction from naive T cells to induce Tregs, while the adoptive transfer of wild-type Tregs weakens the colitis phenotype.14 Howell et al collected samples from 236 pediatric patients newly diagnosed with IBD with DNA methylation patterns and transcriptome of primary IECs, the results showed that compared with the control group, patients with CD and UC have obvious colon epithelium DNA methylation and transcription pattern, and DNA methylation changes with time are stable. Further in vitro analysis suggested a possible clinical application of distinguishing between IBD subtypes and prognosis judgment accordingly.80 In an experiment that investigated the regulatory effect of m6A on T cells, naive METTL3-deficient T cells were unable to expand their homeostatic balance in a mouse model of lymphatic adoptive transplantation and remained in a naive state for up to 12 weeks, thereby preventing the development of colitis.68
In addition, the novel m6A-XPO1-NF-kB pathway that is activated in CD patients also clearly explains the regulatory role of m6A in IBD.75 As the first demethylase known to humans, FTO plays an important role in protecting against adverse reactions caused by the treatment of IBD. Several studies have shown that FTO protects IBD patients from adverse reactions after thioprine treatment.81–83 However, whether it plays a role in IBD through demethylation is unknown, which is a very interesting area to investigate. One recent study of m6A associated-inflammatory pain in a complete Freund’s adjuvant in induced mice model revealed a significant rise in spinal cord m6A modification level with an increased spinal cord METTL3 expression. When expressed in uninfected mice’s spinal cord, METTL3 can lead to pain as observed in their behavior and neuron sensitization. This development of m6A modifications in pathological pain provides a new angle of view.84 Unfortunately, the current research on m6A and IBD is very limited, so the cognition of m6A is relatively limited. There is a need for relevant exploration to provide more possibilities for the pathogenesis, clinical diagnosis, and treatment application in IBD. Except in IBD, m6A also has an important function in IBD-associated CRC.
m6A RNA Methylation Modification and Colorectal Cancer
At present, a large number of studies have confirmed that m6A modification plays an important role in the occurrence and development of various cancers, including gastric cancer,11,85 lung cancer,13 breast cancer,9 liver cancer,10,62 and colon cancer.86,87 CRC, as the top three cancers in the world, has been studied by many scholars with regards to the mechanism of m6A modification. Zhang et al carried out methylated RNA immunoprecipitation sequencing (MeRIP-seq) on six pairs of CRC samples and normal tissues adjacent to the tumor, and found that compared with normal tissues adjacent to the tumor, CRC samples had 1343 out-of-balance m6A peaks, of which 625 were significantly up-regulated and 718 were significantly down-regulated. Genes involved in m6A peak changes play a key role in regulating glucose metabolism, RNA metabolism, and cancer stem cells.87 Liu et al used cancer genome atlas (TCGA), the gene expression omnibus (GEO), the human protein atlas (HPA) databases, and a tissue microarray (TMA) to verify the expression of m6A-related genes at mRNA and protein levels. It was found that most m6A-related genes were significantly up-regulated in tumor tissues, while METTL14, YTHDF3, and ALKBH5 were downregulated in CRC, and there was no significant difference in FTO,88 which suggests that the abnormal expression of m6A-related genes in CRC may play an important role in its progression. The latest research results of Wang et al further indicate that the poor prognosis of CRC may be closely related to the high expression of m6A and the high expression of METTL3, METTL16, and WTAP.89 Table 1 summarizes the different roles and mechanisms of m6A-related enzymes and proteins in IBD and CRC.
 |
Table 1 Functions of m6A Related Enzymes and Binding Proteins in IBD and CRC Progression |
In CRC resistant to immunotherapy, the deletion of METTL3 and METTL14 can inhibit m6A modification, and promote IFN-γ-Stat1-Irf1 signal transduction through YTHDF2 to stabilize STAT1 and Irf1 mRNA, thus enhancing the response to anti-PD-1 therapy, which provides METTL3 and METTL14 as potential therapeutic targets for anti-cancer immunotherapy.90 Li et al detected the expression of METTL3 in CRC patients and found that higher METTL3 expression is found in CRC metastatic tissues, which was related to poor prognosis. Furthermore, the target gene and downstream binding site of METTL3 were screened by bioinformatics. MeRIP-seq and showed that SRY (sex-determining region Y)-box 2 (SOX2) was the downstream gene of METTL3. Mechanically, methylated SOX2 transcripts, especially the CDS region, are bound by specific m6A binding protein insulin-like growth factor 2 mRNA binding protein 2 (IGF2BP2) to prevent SOX2 mRNA degradation.91 This research reveals a new mechanism that METTL3, as an oncogene, maintains SOX2 expression in CRC cells in an m6A-IGF2BP2-dependent manner, and provides a potential biomarker for predicting CRC prognosis.
Another clinical study found that METTL3 stabilizes the expression of HK2 and SLC2A1 (GLUT1) in CRC through an m6A-IGF2BP2/3-dependent mechanism, suggesting that targeting METTL3 and its pathway provide other reasonable therapeutic targets for CRC patients with high glucose metabolism.92 Similarly, Peng et al research revealed that the upregulated METTL3 is related to the abnormal modification of m6A in CRC, and positively correlates with tumor metastasis. METTL3 can methylate pri-miR-1246 and further promote the maturation of pri-miR-1246. Bioinformatics tools predict that the anti-cancer gene SPRED2 is the downstream target of miR-1246, and the downregulation of SPRED2 further reverses the inhibition of the MAPK pathway.93 The results suggest that the METTL3/miR-1246/SPRED2 axis plays an important role in tumor metastasis. Chen et al found that METTL3 induces GLUT1 translation in an m6A-dependent manner and promotes glucose uptake and lactic acid production, which leads to the activation of the mTORC1 signal and the development of CRC.94 In addition, METTL3 promotes CRC proliferation by stabilizing cyclin E1 (CCNE1) mRNA in an m6A-dependent manner.95 The above studies revealed that METTL3 plays a role in promoting CRC by interacting with various RNA and proteins and can be used as a potential biomarker and therapeutic target.
Interestingly, METTL14, as another important component of the methyltransferase complex, exerts an opposite effect on CRC progression. A clinical study has revealed that METTL14 deficiency is associated with poor prognosis in patients with CRC. Functional experiments show that the knockdown of METTL14 can greatly enhance the proliferation and invasion ability of CRC cells in vitro and promote tumorigenicity and metastasis in vivo. In terms of its mechanism, lncRNA XIST is identified as a downstream target of METTL14, and m6A methylated XIST is recognized by an m6A reader protein YTHDF2 to mediate the degradation of XIST. Once the METTL14 is knocked down, the m6A level of XIST is eliminated and XIST expression is enhanced.96 Therefore, preliminary studies have shown that METTL14 can inhibit the proliferation and metastasis of CRC by down-regulating the oncogenic lncRNA XIST.
Similarly, Chen et al also found that the knockout of METTL14 promotes CRC, but further screening of other targets of METTL14 indicated that METTL14 inhibits the growth of CRC cells through the miR-375/Yes-associated protein 1 (YAP1) pathway, and prevents the migration and invasion of CRC cells through the miR-375/SP1 pathway,97 which provides new insights into the mechanism of METTL14 in CRC. Another study showed that METTL14 knockout significantly inhibits the m6A modification of SRY-related high-mobility group box 4 (SOX4) mRNA and increases SOX4 mRNA expression, while METTL14-mediated degradation of SOX4 mRNA depends on the YTHDF2 pathway. Mechanically, METTL14 may partially inhibit the malignant process of CRC through the SOX4-mediated epithelial-mesenchymal transformation (EMT) process and the PI3K/Akt signal.98 The above studies reveal the mechanism of different pathways mediated by METTL4 in CRC.
Moreover, the regulatory factor of m6A mRNA methylation also plays an important role in CRC, and Kuai et al identified four m6A genes (YTHDF1, IGF2BP1, IGF2BP3, and eIF3b) as potential biomarkers of CRC and adenoma.99 In addition to a methyltransferase, which plays an important role in CRC, m6A binding protein is also indispensable as the core factor of methylation modification. Knocking down YTHDF1 expression significantly inhibits tumorigenicity of CRC cells in vitro and xenograft tumor growth in mice. YTHDF1 silencing inhibits the ability to form colon cancer in vitro. The silencing of YTHDF1 significantly inhibits the activity of the Wnt/β-catenin pathway in CRC cells, revealing a positive regulatory role of YTHDF1 in CRC.100
LncRNAs play an important role in the epigenetic regulation of cancer cells. In recent years, researches on the regulatory role of m6A modification of lncRNA in a variety of cancers have gained attention. Currently, several studies have reported the key role of m6A modification of lncRNA in the progression of CRC. A study conducted by Zuo et al detected the correlation between the modification of lncRNA m6A in CRC and adjacent normal tissues and found that lncRNA m6A levels in CRC tissues are higher than those in adjacent normal tissues. A total of 8332 m6A peaks were detected in 6690 lncRNAs in CRC tissues. About 91% of the modified lncRNAs had a unique m6A modification peak, and a total of 383 lncRNAs were differentially methylated in CRC.101 Ni et al found that lncRNA GAS5 expression negatively correlates with YAP and YTHDF3 protein levels in tumors from patients with CRC, and YAP activation is an important participant in a variety of cancers including CRC. The researchers found that the lncRNA GAS5 modified by m6A is negatively regulated by m6A “readers” YTHDF3, and further inhibits the progress of CRC by interacting with YAP to trigger its phosphorylation and degradation process.12 This experiment reveals the negative regulatory loop of the lncRNA GAS5-YAP-YTHDF3 axis and expands a new idea for the treatment of CRC.
Wang et al screened the highly expressed lncRNAs in human CRC samples and found that lncRNA LINRIS is upregulated in CRC tissues with poor overall survival, and inhibition of LINRIS prevents tumor proliferation in both the in-situ tumor model and patient-derived xenotransplantation (PDX) model. LINRIS knockout weakens the downstream effects of m6A “readers” IGF2BP2, especially the Myc-mediated glycolysis of CRC cells.102 LINRIS can be used as an independent prognostic marker of CRC and the LINRIS-IGF2BP2-MYC axis promotes the progression of CRC, which is a potential clinical therapeutic target. The methylation modification of RP11/hnRNPA2B1 mRNA compound accelerates the two E3 ligase Siah1 and Fbxo45 mRNA degradation stops the Zeb1 ectomesenchymal transformation (a kind of epithelial related transcription factor) of the proteasome degradation, and increases RP11 nuclear accumulation, thus promote the CRC cell migration, invasion, and EMT. Therefore, RP11 has the potential as a predictive biomarker or therapeutic target for CRC.103 Similarly, LINC00460 enhances the stability of HMGA1 mRNA by interacting with IGF2BP2 and DHX9, and binding to m6A-modified HMGA1 mRNA, thereby inducing the EMT process, promoting the proliferation, migration, and invasion of tumor cells in vitro, and tumor growth and metastasis in vivo.104 Therefore, LINC00460 can be used as a promising predictive biomarker for the diagnosis and prognosis of patients with CRC.
In CRC liver metastasis, circNSUN2 plays a promoting role. In circNSUN2 exists an m6A base sequence (GGACU), one IGF2BP2 combined with base sequence (CAUCAU), and one HMGA2 mRNA binding sites (AAACA), so the tag has m6A motif circNSUN2 through combination with IGF2BP2 recognition and forming circNSUN2/IGF2BP2/HMGA2 complex, wherein HMGA2 mRNA is stable from degradation, thus promoting the settlement of colorectal liver metastasis.62 This suggests that circNSUN2 may represent a key prognostic marker or therapeutic target for this disease. Table 2 lists the recent research advances of the m6A-modified-RNA in the development of colitis and CRC.
 |
Table 2 Advances in the Mechanism of m6A Modification-Related RNA in the Development of Colitis and CRC |
Conclusion
Extensive modification is a general code of RNA and the basis of its structure and catalytic function but remains largely uncharted territory. Silencing of m6A methyltransferase can significantly affect gene expression and other splicing patterns, leading to regulation of the p53 signaling pathway and apoptosis.18 Large numbers of studies have confirmed that m6A-related genes and different regulatory factors are positively or negatively regulated in the progression of CRC through a variety of mechanisms, which can be used as promising biomarkers for early diagnosis and prognosis as well as therapeutic targets. On the other hand, there has been very little research on IBD, so the general understanding of its relationship with m6A methyltransferase remains limited. However, given that m6A has been found to play an important role in intestinal mucosal immunity and colitis, future studies should pay more attention to the role of m6A in IBD, to lay a solid theoretical foundation for clinical diagnosis and possible cure for IBD, and to reduce the risk of CRC. The study of epigenetic modification represented by m6A in influencing RNA function and metabolism, cell physiological function, and the important role of abnormal modification in a variety of diseases should also receive extensive attention and exploration.
Acknowledgments
The National Natural Science Foundation of China (Grant no. 32000903), the project of Zhenjiang key research and development plan (social development) (Grant no. SH2019025), Scientific Research Project of Health Commission of Jiangsu Province (Grant no. Z2019036) and Innovation Training Program of Jiangsu University Students in 2020 (Grant no. 202010299704X; Grant no. 202010299837X). All authors approved the final version of the article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bernstein CN, Benchimol EI, Bitton A, et al. The impact of inflammatory bowel disease in Canada 2018: extra-intestinal diseases in IBD. J Can Assoc Gastroenterol. 2019;2(Suppl 1):S73–s80. doi:10.1093/jcag/gwy053
2. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91–99. doi:10.3748/wjg.v20.i1.91
3. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi:10.1038/nrgastro.2015.150
4. Wu Y, Qiu W, Xu X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res. 2018;10(7):2026–2036.
5. Oerum S, Degut C, Barraud P, Tisne C. m1A post-transcriptional modification in tRNAs. Biomolecules. 2017;7(1):20. doi:10.3390/biom7010020
6. Chen H, Yang H, Zhu X, et al. m(5)C modification of mRNA serves a DNA damage code to promote homologous recombination. Nat Commun. 2020;11(1):2834. doi:10.1038/s41467-020-16722-7
7. Reichel M, Koster T, Staiger D. Marking RNA: m6A writers, readers, and functions in arabidopsis. J Mol Cell Biol. 2019;11(10):899–910. doi:10.1093/jmcb/mjz085
8. Zhang L, Hou C, Chen C, et al. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol Cancer. 2020;19(1):105. doi:10.1186/s12943-020-01224-3
9. Chen M, Nie ZY, Wen XH, Gao YH, Cao H, Zhang SF. m6A RNA methylation regulators can contribute to malignant progression and impact the prognosis of bladder cancer. Biosci Rep. 2019;39(12):12. doi:10.1042/BSR20192892
10. Chen M, Wong CM. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol Cancer. 2020;19(1):44. doi:10.1186/s12943-020-01172-y
11. Hu BB, Wang XY, Gu XY, et al. N(6)-methyladenosine (m(6)A) RNA modification in gastrointestinal tract cancers: roles, mechanisms, and applications. Mol Cancer. 2019;18(1):178. doi:10.1186/s12943-019-1099-7
12. Ni W, Yao S, Zhou Y, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18(1):143. doi:10.1186/s12943-019-1079-y
13. Zhang Y, Liu X, Liu L, Li J, Hu Q, Sun R. Expression and prognostic significance of m6a-related genes in lung adenocarcinoma. Med Sci Mon Int Med J Exp Clin Res. 2020;26:e919644.
14. Lu TX, Zheng Z, Zhang L, et al. A new model of spontaneous colitis in mice induced by deletion of an RNA m(6)A methyltransferase component METTL14 in T cells. Cell Mol Gastroenterol. 2020;10(4):747–761. doi:10.1016/j.jcmgh.2020.07.001
15. Tong J, Cao G, Zhang T, et al. m(6)A mRNA methylation sustains treg suppressive functions. Cell Res. 2018;28(2):253–256. doi:10.1038/cr.2018.7
16. Liu ZX, Li LM, Sun HL, Liu SM. Link between m6A modification and cancers. Front Bioeng Biotechnol. 2018;6:89. doi:10.3389/fbioe.2018.00089
17. Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37(3):270–288. doi:10.1016/j.ccell.2020.02.004
18. Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi:10.1038/nature11112
19. Meyer KD, Patil DP, Zhou J, et al. 5ʹ UTR m(6)A promotes cap-independent translation. Cell. 2015;163(4):999–1010. doi:10.1016/j.cell.2015.10.012
20. Kierzek E, Kierzek R. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 2003;31(15):4472–4480. doi:10.1093/nar/gkg633
21. Schwartz S, Agarwala SD, Mumbach MR, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell. 2013;155(6):1409–1421. doi:10.1016/j.cell.2013.10.047
22. Geula S, Moshitch-Moshkovitz S, Dominissini D, et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347(6225):1002–1006. doi:10.1126/science.1261417
23. Batista PJ, Molinie B, Wang J, et al. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15(6):707–719. doi:10.1016/j.stem.2014.09.019
24. Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi:10.1038/nchembio.1432
25. Schwartz S, Mumbach MR, Jovanovic M, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5ʹ sites. Cell Rep. 2014;8(1):284–296. doi:10.1016/j.celrep.2014.05.048
26. Sorci M, Ianniello Z, Cruciani S, et al. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9(8):796. doi:10.1038/s41419-018-0843-z
27. Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–373. doi:10.1038/nature19342
28. Pendleton KE, Chen B, Liu K, et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169(5):824–835.e814. doi:10.1016/j.cell.2017.05.003
29. van Tran N, Ernst FGM, Hawley BR, et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019;47(15):7719–7733. doi:10.1093/nar/gkz619
30. Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi:10.1038/nchembio.687
31. Li Y, Wu K, Quan W, et al. The dynamics of FTO binding and demethylation from the m6A motifs. RNA Biol. 2019;16(9):1179–1189. doi:10.1080/15476286.2019.1621120
32. Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi:10.1016/j.molcel.2012.10.015
33. Duan HC, Wei LH, Zhang C, et al. ALKBH10B is an RNA N(6)-methyladenosine demethylase affecting Arabidopsis floral transition. Plant Cell. 2017;29(12):2995–3011. doi:10.1105/tpc.16.00912
34. Roundtree IA, Luo GZ, Zhang Z. et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. eLife. 2017:6. doi:10.7554/eLife.31311
35. Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–519. doi:10.1016/j.molcel.2016.01.012
36. Kretschmer J, Rao H, Hackert P, Sloan KE, Höbartner C, Bohnsack MT. The m(6)A reader protein YTHDC2 interacts with the small ribosomal subunit and the 5ʹ-3ʹ exoribonuclease XRN1. RNA. 2018;24(10):1339–1350. doi:10.1261/rna.064238.117
37. Mao Y, Dong L, Liu XM, et al. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun. 2019;10(1):5332. doi:10.1038/s41467-019-13317-9
38. Ye J, Wang Z, Chen X, et al. YTHDF1-enhanced iron metabolism depends on TFRC m(6)A methylation. Theranostics. 2020;10(26):12072–12089. doi:10.7150/thno.51231
39. Fei Q, Zou Z, Roundtree IA, Sun HL, He C. YTHDF2 promotes mitotic entry and is regulated by cell cycle mediators. PLoS Biol. 2020;18(4):e3000664. doi:10.1371/journal.pbio.3000664
40. Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi:10.1038/cr.2017.15
41. Choe J, Lin S, Zhang W, et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561(7724):556–560. doi:10.1038/s41586-018-0538-8
42. Kwon J, Jo YJ, Namgoong S, Kim NH. Functional roles of hnRNPA2/B1 regulated by METTL3 in mammalian embryonic development. Sci Rep. 2019;9(1):8640. doi:10.1038/s41598-019-44714-1
43. Coker H, Wei G, Brockdorff N. m6A modification of non-coding RNA and the control of mammalian gene expression. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):310–318. doi:10.1016/j.bbagrm.2018.12.002
44. Yang D, Qiao J, Wang G, et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46(8):3906–3920. doi:10.1093/nar/gky130
45. Zuo L, Su H, Zhang Q, et al. Comprehensive analysis of lncRNAs N(6)-methyladenosine modification in colorectal cancer. Aging. 2021;12.
46. Liu H, Xu Y, Yao B, Sui T, Lai L, Li Z. A novel N6-methyladenosine (m6A)-dependent fate decision for the lncRNA THOR. Cell Death Dis. 2020;11(8):613. doi:10.1038/s41419-020-02833-y
47. He Y, Hu H, Wang Y, et al. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell Physiol Biochem. 2018;48(2):838–846. doi:10.1159/000491915
48. Chen S, Zhou L, Wang Y. ALKBH5-mediated m(6)A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Cancer Cell Int. 2020;20:34. doi:10.1186/s12935-020-1105-6
49. Erson-Bensan AE, Begik O. m6A Modification and Implications for microRNAs. Microrna. 2017;6(2):97–101.
50. Cao G, Li HB, Yin Z, Flavell RA. Recent advances in dynamic m6A RNA modification. Open Biol. 2016;6(4):160003. doi:10.1098/rsob.160003
51. Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–485. doi:10.1038/nature14281
52. Hao J, Li C, Lin C, et al. Targeted point mutations of the m6A modification in miR675 using RNA-guided base editing induce cell apoptosis. Biosci Rep. 2020;40(5). doi:10.1042/BSR20192933
53. Chen T, Hao YJ, Zhang Y, et al. m(6)A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16(3):289–301. doi:10.1016/j.stem.2015.01.016
54. Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6) A-dependent nuclear RNA processing events. Cell. 2015;162(6):1299–1308. doi:10.1016/j.cell.2015.08.011
55. Müller S, Glaß M, Singh AK, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019;47(1):375–390. doi:10.1093/nar/gky1012
56. Jin D, Guo J, Wu Y, et al. m(6)A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR-107/LATS2-mediated YAP activity in NSCLC. Mol Cancer. 2020;19(1):40. doi:10.1186/s12943-020-01161-1
57. Yang Z, Li J, Feng G, et al. MicroRNA-145 modulates N(6)-methyladenosine levels by targeting the 3ʹ-untranslated mRNA region of the N(6)-methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292(9):3614–3623. doi:10.1074/jbc.M116.749689
58. Han Q, Yang J, Yang H, Li C, Li J, Cao Y. KIAA1429 promotes osteosarcoma progression by promoting stem cell properties and is regulated by miR-143-3p. Cell Cycle. 2020;19(10):1172–1185. doi:10.1080/15384101.2020.1749465
59. Zhou C, Molinie B, Daneshvar K, et al. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20(9):2262–2276. doi:10.1016/j.celrep.2017.08.027
60. Di Timoteo G, Dattilo D, Centrón-Broco A, et al. Modulation of circRNA metabolism by m(6)A modification. Cell Rep. 2020;31(6):107641. doi:10.1016/j.celrep.2020.107641
61. Chen YG, Chen R, Ahmad S, et al. N6-methyladenosine modification controls circular RNA immunity. Mol Cell. 2019;76(1):96–109.e109. doi:10.1016/j.molcel.2019.07.016
62. He RZ, Jiang J, Luo DX. M6A modification of circNSUN2 promotes colorectal liver metastasis. Genes Dis. 2021;8(1):6–7. doi:10.1016/j.gendis.2019.12.002
63. Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi:10.1016/j.immuni.2005.06.008
64. Wang H, Hu X, Huang M, et al. Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun. 2019;10(1):1898. doi:10.1038/s41467-019-09903-6
65. Wu H, Xu Z, Wang Z, Ren Z, Li L, Ruan Y. Dendritic cells with METTL3 gene knockdown exhibit immature properties and prolong allograft survival. Genes Immun. 2020;21(3):193–202. doi:10.1038/s41435-020-0099-3
66. Liu J, Zhang X, Chen K, et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity. 2019;50(3):600–615.e615. doi:10.1016/j.immuni.2019.01.021
67. Han D, Liu J, Chen C, et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566(7743):270–274. doi:10.1038/s41586-019-0916-x
68. Li HB, Tong J, Zhu S, et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548(7667):338–342. doi:10.1038/nature23450
69. Furlan M, Galeota E, de Pretis S, Caselle M, Pelizzola M. m6A-dependent RNA dynamics in T cell differentiation. Genes. 2019;10(1):28. doi:10.3390/genes10010028
70. Zhu Y, Zhao Y, Zou L, Zhang D, Aki D, Liu YC. The E3 ligase VHL promotes follicular helper T cell differentiation via glycolytic-epigenetic control. J Exp Med. 2019;216(7):1664–1681. doi:10.1084/jem.20190337
71. Sprent J, Surh CD. Writer’s block: preventing m(6)A mRNA methylation promotes T cell naivety. Immunol Cell Biol. 2017;95(10):855–856. doi:10.1038/icb.2017.67
72. Wu J, Zhao Y, Wang X. et al. Dietary nutrients shape gut microbes and intestinal mucosa via epigenetic modifications. Crit Rev Food Sci Nutr. 2020:1–15. doi:10.1080/10408398.2020.1828813
73. Jabs S, Biton A, Bécavin C, et al. Impact of the gut microbiota on the m(6)A epitranscriptome of mouse cecum and liver. Nat Commun. 2020;11(1):1344. doi:10.1038/s41467-020-15126-x
74. Han B, Yan S, Wei S, et al. YTHDF1-mediated translation amplifies Wnt-driven intestinal stemness. EMBO Rep. 2020;21(4):e49229. doi:10.15252/embr.201949229
75. Olazagoitia-Garmendia A, Zhang L, Mera P, et al. Gluten-induced RNA methylation changes regulate intestinal inflammation via allele-specific XPO1 translation in epithelial cells. Gut. 2021. doi:10.1136/gutjnl-2020-322566
76. Gan Z, Wei W, Wu J, et al. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m(6)A RNA methylation in the intestine of weaning piglets. ACS Omega. 2019;4(17):17438–17446. doi:10.1021/acsomega.9b02236
77. Song H, Feng X, Zhang H, et al. METTL3 and ALKBH5 oppositely regulate m(6)A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation-treated cardiomyocytes. Autophagy. 2019;15(8):1419–1437. doi:10.1080/15548627.2019.1586246
78. Song P, Feng L, Li J, et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol Cancer. 2020;19(1):129.
79. Yue C, Chen J, Li Z, Li L, Chen J, Guo Y. microRNA-96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2-FTO-m6A/MYC axis. J Exp Clin Cancer Res. 2020;39(1):240. doi:10.1186/s13046-020-01731-7
80. Howell KJ, Kraiczy J, Nayak KM, et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology. 2018;154(3):585–598. doi:10.1053/j.gastro.2017.10.007
81. Chen S, Tan WZ, Sutiman N, et al. An intronic FTO variant rs16952570 confers protection against thiopurine-induced myelotoxicities in multiethnic Asian IBD patients. Pharmacogenomics J. 2020;20(3):505–515. doi:10.1038/s41397-019-0126-9
82. Kim HS, Cheon JH, Jung ES, et al. A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD. Gut. 2017;66(11):1926–1935.
83. Sato T, Takagawa T, Kakuta Y, et al. NUDT15, FTO, and RUNX1 genetic variants and thiopurine intolerance among Japanese patients with inflammatory bowel diseases. Intest Res. 2017;15(3):328–337. doi:10.5217/ir.2017.15.3.328
84. Zhang C, Wang Y, Peng Y, Xu H, Zhou X. METTL3 regulates inflammatory pain by modulating m(6) A-dependent pri-miR-365-3p processing. FASEB J. 2020;34(1):122–132. doi:10.1096/fj.201901555R
85. Zhang C, Zhang M, Ge S, et al. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. 2019;8(10):4766–4781. doi:10.1002/cam4.2360
86. Zhang J, Cheng X, Wang J, Huang Y, Yuan J, Guo D. Gene signature and prognostic merit of M6a regulators in colorectal cancer. Exp Biol Med (Maywood). 2020;245(15):1344–1354. doi:10.1177/1535370220936145
87. Zhang Z, Wang Q, Zhang M. et al. Comprehensive analysis of the transcriptome-wide m6A methylome in colorectal cancer by MeRIP sequencing. Epigenetics. 2020:1–11. doi:10.1080/15592294.2020.1861170
88. Liu X, Liu L, Dong Z, et al. Expression patterns and prognostic value of m(6) A-related genes in colorectal cancer. Am J Transl Res. 2019;11(7):3972–3991.
89. Wang S, Fan X, Zhu J, et al. The differentiation of colorectal cancer is closely relevant to m6A modification. Biochem Biophys Res Commun. 2021;546:65–73. doi:10.1016/j.bbrc.2021.02.001
90. Wang L, Hui H, Agrawal K, et al. m(6) A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. EMBO J. 2020;39(20):e104514. doi:10.15252/embj.2020104514
91. Li T, Hu PS, Zuo Z, et al. METTL3 facilitates tumor progression via an m(6) A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18(1):112. doi:10.1186/s12943-019-1038-7
92. Shen C, Xuan B, Yan T, et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19(1):72. doi:10.1186/s12943-020-01190-w
93. Peng W, Li J, Chen R, et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res. 2019;38(1):393. doi:10.1186/s13046-019-1408-4
94. Chen H, Gao S, Liu W, et al. RNA N(6)-methyladenosine methyltransferase METTL3 facilitates colorectal cancer by activating the m(6) A-GLUT1-mTORC1Axis and is a therapeutic target. Gastroenterology. 2020.
95. Zhu W, Si Y, Xu J, et al. Methyltransferase like 3 promotes colorectal cancer proliferation by stabilizing CCNE1 mRNA in an m6A-dependent manner. J Cell Mol Med. 2020;24(6):3521–3533. doi:10.1111/jcmm.15042
96. Yang X, Zhang S, He C, et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19(1):46. doi:10.1186/s12943-020-1146-4
97. Chen X, Xu M, Xu X, et al. METTL14 suppresses CRC progression via regulating N6-methyladenosine-dependent primary miR-375 processing. Mol Ther. 2020;28(2):599–612. doi:10.1016/j.ymthe.2019.11.016
98. Chen X, Xu M, Xu X, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19(1):106. doi:10.1186/s12943-020-01220-7
99. Kuai D, Zhu S, Shi H, et al. Aberrant expression of m(6)A mRNA methylation regulators in colorectal adenoma and adenocarcinoma. Life Sci. 2021;273:119258. doi:10.1016/j.lfs.2021.119258
100. Bai Y, Yang C, Wu R, et al. YTHDF1 regulates tumorigenicity and cancer stem cell-like activity in human colorectal carcinoma. Front Oncol. 2019;9:332. doi:10.3389/fonc.2019.00332
101. Zuo L, Su H, Zhang Q, et al. Comprehensive analysis of lncRNAs N(6)-methyladenosine modification in colorectal cancer. Aging. 2021;13(3):4182–4198. doi:10.18632/aging.202383
102. Wang Y, Lu JH, Wu QN, et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18(1):174. doi:10.1186/s12943-019-1105-0
103. Wu Y, Yang X, Chen Z, et al. m(6)A-induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18(1):87. doi:10.1186/s12943-019-1014-2
104. Hou P, Meng S, Li M, et al. LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. J Exp Clin Cancer Res. 2021;40(1):52. doi:10.1186/s13046-021-01857-2
105. Sun L, Wan A, Zhou Z, et al. RNA-binding protein RALY reprogrammes mitochondrial metabolism via mediating miRNA processing in colorectal cancer. Gut. 2020. doi:10.1136/gutjnl-2020-320652
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.