Back to Journals » Journal of Inflammation Research » Volume 13
The Efficacy, Safety and Tolerability of Canakinumab in the Treatment of Familial Mediterranean Fever: A Systematic Review of the Literature
Authors Kacar M , Savic S , van der Hilst JCH
Received 14 January 2020
Accepted for publication 20 February 2020
Published 9 March 2020 Volume 2020:13 Pages 141—149
DOI https://doi.org/10.2147/JIR.S206204
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Mark Kacar,1 Sinisa Savic,1 Jeroen CH van der Hilst2,3
1Department of Clinical Immunology and Allergy, St James´s University Hospital, Leeds, UK; 2Department of Infectious Diseases and Immunity, Jessa Hospital, Hasselt, Belgium; 3BIOMED Research Institute, University of Hasselt, Hasselt, Belgium
Correspondence: Jeroen CH van der Hilst
Department of Infectious Diseases and Immunity, Jessa Hospital, Stadsomvaart 11, Hasselt 3500, Belgium
Tel +32 11309485
Email [email protected]
Abstract: Familial Mediterranean Fever (FMF) is the most prevalent genetic autoinflammatory disorder. In most patients, treatment with colchicine can prevent attacks of fever and inflammation. However, 5%– 10% of patients are resistant to colchicine treatment, while a similar percentage cannot tolerate colchicine in doses needed to prevent attacks. For these patients, Canakinumab, a full human antibody against IL-1β, has been approved recently by the FDA and EMA. In this article, we present a systematic review of the long-term efficacy, safety, and tolerability of Canakinumab in FMF patients who cannot tolerate colchicine or who are resistant to colchicine treatment.
Keywords: familial mediterranean fever, Canakinumab, anti-IL1 therapy
Introduction
Familial Mediterranean Fever (FMF) is the most prevalent of the genetic autoinflammatory syndromes. It is characterized by recurrent attacks of fever accompanied by signs of serosal inflammations such as peritonitis, arthritis, pericarditis and pleuritis.1 There are ~100.000 patients worldwide, with the highest prevalence of FMF found in people living in the countries bordering the Eastern and Southern Mediterranean and Armenia and in immigrant populations from these areas in Western Europe and the United States.2
A typical FMF patient suffers from short episodes of fever and inflammation lasting 12 h to 3 days, with symptom-free intervals between attacks. A subgroup of patients has a more severe phenotype with continuous inflammation and episodes of aggravation.3
Colchicine has been shown effective in preventing inflammatory attacks in most patients. However, an estimated 5–10% continue to experience inflammatory attacks despite maximal dose of colchicine.4,5 Patients with an attack frequency of >1 typical episode per 3 months and presence of amyloidosis or elevated acute phase reactants are considered to have colchicine-resistant FMF (crFMF).6 Furthermore, another 5% to 10% of patients experience serious side effects including severe diarrhea, neuropathy, rhabdomyolysis, and bone marrow suppression.7
Since inappropriate production of the pro-inflammatory cytokine interleukin 1β by cells of the innate immune system plays a central role in the pathogenesis of FMF, blocking of IL1 by biological drugs has been tried in colchicine-resistant FMF (crFMF) patients since the beginning of this century.8 Anakinra, a IL-1 receptor antagonist administered daily by subcutaneous injection, showed promising results in case reports and case series.9 In addition, Canakinumab, a full human antibody against IL-1β administered subcutaneously every 4–8 weeks, also appeared very effective in crFMF.10 It was approved by the FDA and EMA in 2016 for the treatment of colchicine-resistant FMF.
The long-term effectiveness, the rate and type of rare side effect, and adherence to therapy are hitherto unclear. Here we present a systematic review of the literature in order to fill the gap in knowledge about the safety, efficacy, and tolerability and of Canakinumab in crFMF patients.
Methods
We followed a protocol using the methodological approaches outlined in the Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews and applied the PRISMA Guidelines.11 The study was registered in the PROSPERO registry for systematic reviews (number CRD42019158100). The systematic literature review aimed to include all studies published until December 1, 2019 reporting on Canakinumab therapy in FMF patients. We searched Medscape, Web-of-Science, and SCOPUS using the following syntax: (ILARIS OR canakinumab) AND (familial mediterranean fever OR FMF) in all fields.
Study Selection
The selection was performed by two investigators (MK, JH). We included randomized controlled trials, non-randomized trials, retrospective analysis of these trials, cohort studies or cross-sectional studies, case reports, and case series. There were no language restrictions. We excluded in vitro and animal studies, review articles, and congress abstracts. We assessed all titles and abstracts identified by our search. Publications were considered eligible for the analysis if they contained data on Canakinumab in patients with FMF as defined by the Tel Hashomer criteria.12 Full-length articles were retrieved from all published papers.
Data Extraction
Using a standardized data extraction sheet, the following data were collected from the articles: lead author, publication year, study design, sample size, reason for Canakinumab use, MEFV-mutation, presence of type AA amyloidosis, the response to therapy, dosage, duration of therapy, previous Anakinra use, reasons for stopping Anakinra treatment, reported side effects. In addition, the following baseline characteristics were extracted: age of the patients, age at diagnosis and the number and proportion of male patients. Patients were considered to have a complete response if there was not a single attack whilst on Canakinumab.
Results
Study and Patient Characteristics
Among the 413 results provided by the database search, 192 unique studies were identified, of which 19 were eligible studies reporting on the effect of Canakinumab in FMF patients (Figure 1). These included 2 case reports, 6 case series, 7 cohort studies, 3 open-label prospective trials, and one randomised, placebo-controlled trial (Table 1).
 |
Table 1 Characteristics of Reviewed Studies and Participants |
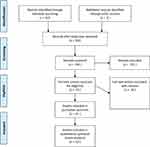 |
Figure 1 Search strategy and article selection. |
The 19 studies included data on 221 patients from 16 countries, 102 (46%) of whom were female.
The majority of patients (65.6%,) were homozygous for the M694V mutation. The pre-treatment attack frequency was reported in 12 studies and varied from 0 to 12 attacks per month. Seventy-five percent of patients with reported attack frequencies had 6 or more attacks per year before initiation of anti-IL1 therapy. The cumulative exposure to Canakinumab treatment was 265.4 patient-years.
Indications for Canakinumab
Colchicine resistance was reported in 89% of patients, with 12% having dose-limiting intolerance to the drug.
Data on previous immunosuppressive therapy were available for 100 patients.13–21 Forty-four patients used Anakinra prior to initiation of Canakinumab therapy. Of them, 26 had an intolerance to Anakinra and in 16 patients Anakinra was ineffective in preventing attacks. One patient had a good response to anakinra but was switched to Canakinumab due to ease of use,17 whereas the reason for the switch was not given in one.16
One hundred and seventy-four patients continued to use colchicine in association with Canakinumab. In 2 patients colchicine was stopped during treatment.14,22
Dosing of Canakinumab
The most common dose of Canakinumab was 2mg/kg in patients <40kg, or 150mg in those above.13,17,18,20–28 In case of inadequate disease control, an increase in dose to 3–4mg/kg in those below 40kg or 300mg in those over was used in 8 studies.10,14,15,19,23,29–31
In 8 studies,10,19,22,25–28,31 Canakinumab was given at 4-weekly intervals, in 3 studies18,21,24 it was given at 8-weekly intervals, whereas the remaining studies had dosing frequencies tailored to clinical needs and/or protocols, with the medication being given every 4, 6 or 8 weeks.
The study by Eren Akarcan et al23 included a reduction in frequency at 6 months with treatment discontinuation at 12 months. The patients experiencing flares (n=4) during follow-up were given Canakinumab at 12-weekly intervals.
Efficacy of Canakinumab
Response data were available for 189 patients, 2 of which did not meet the Tel-Hashomer criteria and will be reported separately. At the initial Canakinumab dose of 150mg or 2mg/kg in <40kg, 78.1% had a complete response, defined as the complete absence of attacks during Canakinumab treatment (Table 2). An additional 19.8% had a partial response with fewer attacks and/or reduced severity, with only 2.1% failing to attain any response at all.
 |
Table 2 Treatment Regimens, Responses and Safety Data |
The dose was increased to 300mg in 11 of the 37 partial responders and 3 of the 4 non-responders. In total, treatment with the optimal dosage of Canakinumab led to CR in 79.9% and PR in 19.3%, with only 1.1% of patients remaining non-responders.
The two patients who did not fulfil Tel-Hashomer criteria were both heterozygote for a disease-causing mutation.25,28 Complete response to Canakinumab could not be achieved in either case.
Safety and Tolerability of Canakinumab
Reporting thresholds for adverse events (AE) varied by study, with only serious AE being reported consistently. Safety data could be assessed for 265.4 patient-years. During this period, there were 8 cases of moderate to severe infections (three cases of pneumonia, two cases of pharyngotonsillitis, one case of pyelonephritis, one case of pelvic abscess and one of cellulitis). No deaths, mycobacterial or opportunistic infections were reported. For purposes of this review, non-SAE were assessed only in prospective studies (n=4) to avoid recall bias. Of those, only the prospective randomized, placebo-controlled trial by De Benedetti et al provided sufficient information about AE. The most common among the 332 reported AE were infections (23.8% of all AE), injection site reactions (6.0%), headache (3.9%) and abdominal pain (3.6%).10
None of the eleven reported unscheduled treatment discontinuations were due to SAE. The discontinuations occurred for the following reasons: progression of arthritis (n = 3), lack of effect (n = 3), pregnancy (n = 2), weight gain (n = 1), progression of inflammatory bowel disease necessitating alternative biological therapy (n =1) or patient decision not otherwise stated (n = 1).
Pregnancy
The articles by Gul et al and Babaoglu et al reported on one Canakinumab-exposed pregnancy each.14,19 The medication was discontinued in the peri-conception period and at 8 weeks’ gestation, respectively. There were no signs of congenital malformations or intra-uterine growth retardation, both infants were carried to term and no developmental abnormalities were reported.
Amyloidosis
Information about the presence of renal or systemic amyloidosis could be obtained from 121 patients in 11 of the assessed studies.14,17,24,26–28 Twenty-four patients had type AA amyloidosis at the start of treatment. Of those, six had proteinuria and reduced renal function which improved in 5 and remained stable in one patient after initiation of Canakinumab. Four patients had end-stage renal disease requiring dialysis when Canakinumab was started, of whom 2 experienced improvement in renal function, one discontinued Canakinumab due to disease progression, and one of them continued to renal transplantation without evidence of recurrence under Canakinumab treatment.17 A further 14 patients were renal allograft recipients following amyloid-induced end-stage renal failure.17,26,28 Of these, 7 had evidence of amyloid deposition in the allograft, leading to the introduction of Canakinumab,28 whilst poorly controlled inflammatory attacks were the indication for implementation of Canakinumab in the remaining patients. Renal function remained stable in all cases, with no improvement or exacerbation of proteinuria or significant changes to serum creatinine levels reported.
No patients developed new-onset systemic or renal amyloidosis whilst receiving Canakinumab.
Discussion
In this systematic review, we found that Canakinumab is highly effective and safe in patients with colchicine-resistant or colchicine-intolerant FMF. Nearly 80% of patients did not experience a single attack after the initiation of Canakinumab. Almost all the remaining patients had significantly reduced disease activity. These results are remarkable taking into consideration that most patients included in the studies had a particularly severe phenotype. The majority were homozygote for M694V, which is known to cause more severe presentation of FMF with higher rates of colchicine resistance.32 Where pre-Canakinumab attack frequencies could be assessed (n=72) 75.0% of patients had at least 6 attack per year, with the mean frequency exceeding 2.1 attacks per month and the maximum as high as 12 attacks per month. Since FMF severely affects the quality of life,33 efficacious therapy is crucial. Moreover, recurrent inflammation is directly related to the risk of type AA amyloidosis.34,35 If inflammation cannot be controlled, type AA amyloidosis develops in up to three quarters of patients during life.36 In the 97 patients without amyloidosis before initiation of therapy, none developed it during Canakinumab treatment. In addition, in patients with already established nephrotic syndrome caused by type AA amyloidosis, a significant reduction in proteinuria was consistently observed. Furthermore, in patients with renal transplantation, no recurrences of amyloidosis were seen when patients were on Canakinumab treatment.
Colchicine remains the mainstay of therapy in FMF. It has been shown to prevent type AA amyloidosis.37 Two patients stopped colchicine therapy whilst on anti-IL1 treatment. An additional 11 patients were unable to tolerate colchicine. Since type AA amyloidosis only develops in the presence of the acute phase reactant serum amyloid A (SAA), it is unclear if patients under anti-IL1 treatment with a complete response clinical and biochemical response benefit from additional colchicine.34 The current guideline still advice to continue colchicine during anti-IL1 treatment.38
Canakinumab was not only highly efficacious, it was also safe and well-tolerated. Based on the 265.4 patient-years of cumulative exposure described in the reviewed studies, only 8 severe infections were reported in 7 patients. There were no reports of opportunistic infections.
Of the 221 patients treated with Canakinumab, only three stopped due to lack of efficacy, two of which did not fulfil the Tel-Hashomer criteria. No patients discontinued treatment due to side effects, a marked contrast to the 59% of Anakinra discontinuations due to side effects reported in the reviewed patients who had received anakinra before. Canakinumab seems to be better tolerated than Anakinra, the other anti-IL1 that is used for the treatment of crFMF. In general, between 6.5% and 30% of patients using Anakinra therapy withdraw due to adverse events.39,40 The main reason for stopping Anakinra therapy is severe injection site reaction. This was not reported in any of the patients on Canakinumab. Although the efficacy of Anakinra seems similar to Canakinumab,41 Canakinumab was initiated in 16 patients because of insufficient response to Anakinra. Response to Canakinumab among that subset could be assessed in 14 patients; CR was achieved in 64.3%. However, the lack of high-quality evidence precludes judgment about the superiority of one the anti-IL1 treatments over another.
The current literature does not allow us to make a statement about optimal dosing. Although the majority of patients were treated with 150 mg every 4 weeks, there was considerable variation in the dosing interval between studies. A lower dose interval did not seem to give high numbers of treatment failures. This suggests that at least in some patients receiving 150mg, an interval length exceeding 4 weeks may be feasible. This would be desirable considering the high cost of therapy and potential side effects.
Conclusion
Cumulative evidence shows that Canakinumab is very effective in preventing attacks in almost all patients with colchicine resistant or colchicine-intolerant FMF. It may prevent the development of type AA amyloidosis and it appears safe and well-tolerated. Future research is needed to asses the optimal dosing strategy.
Disclosure
Prof. Dr. Sinisa Savic reports grants, personal fees from Novartis and SOBI, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Ozen S, Bilginer Y. A clinical guide to autoinflammatory diseases: familial Mediterranean fever and next-of-kin. Nat Rev Rheumatol. 2014;10(3):135–147. doi:10.1038/nrrheum.2013.174
2. Savic S, Dickie LJ, Battellino M, McDermott MF. Familial Mediterranean fever and related periodic fever syndromes/autoinflammatory diseases. Curr Opin Rheumatol. 2012;24(1):103–112. doi:10.1097/BOR.0b013e32834dd2d5
3. Gershoni-Baruch R, Brik R, Shinawi M, Livneh A. The differential contribution of MEFV mutant alleles to the clinical profile of familial Mediterranean fever. Eur J Hum Genet. 2002;10(2):145–149. doi:10.1038/sj.ejhg.5200776
4. Lidar M, Scherrmann J-M, Shinar Y, et al. Colchicine nonresponsiveness in familial Mediterranean fever: clinical, genetic, pharmacokinetic, and socioeconomic characterization. Semin Arthritis Rheum. 2004;33(4):273–282. doi:10.1053/S0049-0172(03)00137-9
5. Ozen S, Kone-Paut I, Gül A. Colchicine resistance and intolerance in familial mediterranean fever: definition, causes, and alternative treatments. Semin Arthritis Rheum. 2017;47(1):115–120. doi:10.1016/j.semarthrit.2017.03.006
6. Erden A, Batu ED, Sarı A, et al. Which definition should be used to determine colchicine resistance among patients with familial Mediterranean fever? Clin Exp Rheumatol. 2018;36;6Suppl 115:97–102.
7. Putterman C, Ben-Chetrit E, Caraco Y, Levy M. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum. 1991;21(3):143–155. doi:10.1016/0049-0172(91)90003-I
8. Mistry A, Savic S, van der Hilst JCH. Interleukin-1 blockade: an update on emerging indications. BioDrugs. 2017;31(3):207–221. doi:10.1007/s40259-017-0224-7
9. van der Hilst J, Moutschen M, Messiaen P, Lauwerys B, Vanderschueren S. Efficacy of anti-IL-1 treatment in familial Mediterranean fever: a systematic review of the literature. Biol Targets Ther. 2016;10:75. doi:10.2147/BTT.S102954
10. De Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378(20):1908–1919. doi:10.1056/NEJMoa1706314
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21_1):b2535. doi:10.1136/bmj.b2535
12. Livneh A, Langevitz P, Zemer D, et al. Criteria for the diagnosis of familial mediterranean fever. Arthritis Rheum. 1997;40(10):1879–1885. doi:10.1002/art.1780401023
13. Başaran Ö, Kavuncu S, Güven A, Uncu N, Acar-Çelikel B, Çakar N. Familial mediterranean fever associated with optic neuritis, successfully treated with anti-interleukin 1 agents. Turk J Pediatr. 2016;58(3):327–330. doi:10.24953/turkjped.2016.03.018
14. Babaoglu H, Varan O, Kucuk H, et al. Effectiveness of canakinumab in colchicine- and anakinra-resistant or -intolerant adult familial mediterranean fever patients. JCR J Clin Rheumatol. 2018:1. doi:10.1097/RHU.0000000000000873
15. Gülez N, Makay B, Sözeri B. Long-term effectiveness and safety of canakinumab in pediatric familial Medıterranean fever patıents. Mod Rheumatol. 2018;1–13. doi:10.1080/14397595.2018.1559488
16. Kisla Ekinci RM, Balci S, Dogruel D, et al. Canakinumab in children with familial Mediterranean fever: a single-center, retrospective analysis. Pediatr Drugs. 2019;21(5):389–395. doi:10.1007/s40272-019-00354-6
17. Sendogan DO, Saritas H, Kumru G, et al. Outcomes of canakinumab treatment in recipients of kidney transplant with familial mediterranean fever: a Case Series. Transplant Proc. 2019;51(7):2292–2294. doi:10.1016/j.transproceed.2019.03.049
18. Alpa M, Roccatello D. Canakinumab as rescue therapy in familial mediterranean fever refractory to conventional treatment. Drug Des Devel Ther. 2015;9:1983–1987. doi:10.2147/DDDT.S69117
19. Gül A, Ozdogan H, Erer B, et al. Efficacy and safety of canakinumab in adolescents and adults with colchicine-resistant familial Mediterranean fever. Arthritis Res Ther. 2015;17(1):243. doi:10.1186/s13075-015-0765-4
20. Laskari K, Boura P, Dalekos GN, et al. Longterm beneficial effect of canakinumab in colchicine-resistant familial mediterranean fever. J Rheumatol. 2017;44(1):102–109. doi:10.3899/jrheum.160518
21. Meinzer U, Quartier P, Alexandra J-F, Hentgen V, Retornaz F, Koné-Paut I. Interleukin-1 targeting drugs in familial mediterranean fever: a case series and a review of the literature. Semin Arthritis Rheum. 2011;41(2):265–271. doi:10.1016/j.semarthrit.2010.11.003
22. Yasuda R, Mizuochi T, Kitamura M, Migita K, Yamashita Y. Canakinumab eliminates resistant familial mediterranean fever in a Japanese girl. Pediatr Int. 2019;61(11):1173–1174. doi:10.1111/ped.13968
23. Eren Akarcan S, Dogantan S, Edeer Karaca N, Aksu G, Kutukculer N. Successful management of colchicine resistant familial mediterranean fever patients with a standardized canakinumab treatment protocol: a case series and literature review. Rheumatol Int. 2019;0123456789. doi:10.1007/s00296-019-04366-w
24. Cetin P, Sari I, Sozeri B, et al. Efficacy of interleukin-1 targeting treatments in patients with familial mediterranean fever. Inflammation. 2015;38(1):27–31. doi:10.1007/s10753-014-0004-1
25. Kucuksahin O, Yildizgoren MT, Ilgen U, et al. Anti-interleukin-1 treatment in 26 patients with refractory familial mediterranean fever. Mod Rheumatol. 2017;27(2):350–355. doi:10.1080/14397595.2016.1194510
26. Yildirim T, Yilmaz R, Uzerk Kibar M, Erdem Y. Canakinumab treatment in renal transplant recipients with familial Mediterranean fever. J Nephrol. 2018;31(3):453–455. doi:10.1007/s40620-018-0475-5
27. Yazılıtaş F, Aydoğ Ö, Özlü SG, et al. Canakinumab treatment in children with familial mediterranean fever: report from a single center. Rheumatol Int. 2018;38(5):879–885. doi:10.1007/s00296-018-3993-5
28. Trabulus S, Korkmaz M, Kaya E, Seyahi N. Canakinumab treatment in kidney transplant recipients with AA amyloidosis due to familial mediterranean fever. Clin Transplant. 2018;32(8):e13345. doi:10.1111/ctr.13345
29. Ekinci RMK, Balci S, Bisgin A, et al. Renal amyloidosis in deficiency of adenosine deaminase 2: successful experience with canakinumab. Pediatrics. 2018;142:5. doi:10.1542/peds.2018-0948
30. Berdeli A, Senol O, Talay G. Treatment of familial mediterranean fever with canakinumab in patients who are unresponsive to colchicine. Eur J Rheumatol. 2019;6(2):82–85. doi:10.5152/eurjrheum.2019.18190
31. Brik R, Butbul-Aviel Y, Lubin S, et al. Canakinumab for the treatment of children with colchicine-resistant familial mediterranean fever: a 6-month open-label, single-arm pilot study. Arthritis Rheumatol (Hoboken, NJ). 2014;66(11):3241–3243. doi:10.1002/art.38777
32. Grossman C, Kassel Y, Livneh A, Ben-Zvi I. Familial mediterranean fever (FMF) phenotype in patients homozygous to the MEFV M694V mutation. Eur J Med Genet. 2019;62(6):103532. doi:10.1016/j.ejmg.2018.08.013
33. Buskila D, Zaks N, Neumann L, et al. Quality of life of patients with familial mediterranean fever. Clin Exp Rheumatol. 1997;15(4):355–360.
34. van der Hilst JCH. Recent insights into the pathogenesis of type AA amyloidosis. ScientificWorldJournal. 2011;11:641–650. doi:10.1100/tsw.2011.64
35. Lachmann HJ, Goodman HJB, Gilbertson JA, et al. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356(23):2361–2371. doi:10.1056/NEJMoa070265
36. van der Hilst JCH, Simon A, Drenth JPH. Hereditary periodic fever and reactive amyloidosis. Clin Exp Med. 2005;5(3):87–98. doi:10.1007/s10238-005-0071-6
37. Lachmann HJ. Periodic fever syndromes. Best Pract Res Clin Rheumatol. 2017;31(4):596–609. doi:10.1016/j.berh.2017.12.001
38. Ozen S, Demirkaya E, Erer B, et al. EULAR recommendations for the management of familial mediterranean fever: table 1. Ann Rheum Dis. 2016;75(4):644–651. doi:10.1136/annrheumdis-2015-208690
39. Thaler K, Chandiramani DV, Hansen RA, Gartlehner G. Efficacy and safety of anakinra for the treatment of rheumatoid arthritis: an update of the Oregon drug effectiveness review project. Biologics. 2009;3:485–498. doi:10.2147/btt.2009.3755
40. Fleischmann RM, Tesser J, Schiff MH, et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65:1006–1012. doi:10.1136/ard.2005.048371
41. van der Hilst JC, Moutschen M, Messiaen PE, Lauwerys BR, Vanderschueren S. Efficacy of anti-IL-1 treatment in familial mediterranean fever: a systematic review of the literature. Biologics. 2016;10:75–80. doi:10.2147/BTT.S102954
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
