Back to Journals » Journal of Pain Research » Volume 12
The efficacy of pregabalin for the management of acute and chronic postoperative pain in thoracotomy: a meta-analysis with trial sequential analysis of randomized-controlled trials
Authors Yu Y , Liu N, Zeng Q, Duan J , Bao Q, Lei M , Zhao J, Xie J
Received 15 August 2018
Accepted for publication 21 November 2018
Published 28 December 2018 Volume 2019:12 Pages 159—170
DOI https://doi.org/10.2147/JPR.S183411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Yijin Yu,1 Nan Liu,2 Qingxin Zeng,3 Jing Duan,1 Qi Bao,1 Min Lei,1 Jinning Zhao,1 Junran Xie1
1Department of Anesthesiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Zhejiang, China; 2Department of Anesthesiology, Hangzhou Xiasha Hospital, Zhejiang, China; 3Department of Thoracic Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Zhejiang, China
Purpose: Pregabalin is commonly used as an analgesic for neuropathic pain. But pregabalin as an adjunct to a multimodal analgesic regimen – although standard clinical protocol in some settings – has remained controversial. This meta-analysis was conducted to identify the efficacy of pregabalin for management of postoperative pain in thoracotomy.
Materials and methods: Pubmed, Embase, Cochrane, Web of Science, Springer, and Clinical Trial Register database were searched for randomized controlled trials (RCTs) of pregabalin in preventing postoperative pain in thoracotomy. Review Manager 5.3 and STATA 12.0 were selected to conduct the meta-analysis. Trial sequential analysis was used to control random errors and calculate the required information size.
Results: Nine RCTs with 684 patients were included in our meta-analysis. Outcomes favoring pregabalin included less pain on a 0–10 scale on 1 day [mean difference (MD): –0.87; 95% CI: –1.55 to –0.19; P=0.01], 3 days (MD: –1.55; 95% CI: –1.93 to –1.18; P<0.00001), 1 month (MD: –1.58; 95% CI: –2.75 to –0.42; P=0.008), 3 months (MD: –1.69; 95% CI: –2.71 to –0.66; P=0.001) postoperatively, and less incidence of neuropathic pain (OR: 0.20; 95% CI: 0.05–0.91; P=0.04), less mean morphine consumption (MD: –5.03; 95% CI: –8.06 to –1.99; P=0.001), but more dizziness (OR: 3.33; 95% CI: 1.36–8.17; P=0.009), more drowsiness (OR: 8.61; 95% CI: 2.23–33.20; P=0.002), and less constipation (OR: 0.23; 95% CI: 0.09–0.59; P=0.002). There was no statistical differences in pain score on 7 days (MD:–0.77; 95% CI: –2.38 to 0.84; P=0.35), nausea (OR: 0.73; 95% CI: 0.42–1.26; P=0.26), and vomiting (OR: 0.83; 95% CI: 0.36–1.90; P=0.65).
Conclusion: Pregabalin can prevent postoperative pain in thoracotomy and decrease incidence of neuropathic pain and morphine consumption. Pregabalin may be a valuable asset in management of acute and persistent postoperative pain in thoracotomy.
Keywords: pregabalin, postoperative pain, thoracotomy, meta-analysis, neuropathic pain
Introduction
Patients commonly experience acute to chronic pain after thoracotomy. Bayman et al1 reported that a higher severity of pain at first postoperative 3 days may develop a higher rate of persistent pain. The incidence of chronic pain was 27% in thoracotomy and 8.2% were limited in their daily life. The incidence of chronic pain in video-assisted thoracoscopic surgery (VATS) was still not lower. Homma et al2 reported that incidence of chronic neuropathic pain was 25.9% in VATS, and 18.8% even in a year later.
Thoracic epidural analgesia remained a controversy due to complications related to the catheterization procedure.3 Opioid-based patient-controlled analgesia has been widely used for its analgesic efficacy but with several adverse effects including respiratory depression, sedation, vomiting, and physical dependence, and it may not be effective for chronic neuropathic pain.4,5
Pregabalin is a structural analog of γ-aminobutyric acid that acts on the α2δ subunit of voltage-dependent calcium channels, which can reduce the release of neurotransmitters.6 It has been commonly used in treatment for neuropathic pain, but has remained a controversy in alleviating postoperative pain.7 There was no systematic review with direct-evidence meta-analysis of randomized-controlled trials (RCTs) for pregabalin used in postoperative pain of thoracotomy. This meta-analysis was sought to determine whether pregabalin used systematically can reduce postoperative pain.
Material and methods
The protocol for the meta-analysis is registered with PROSPERO (CRD42018100634).
Search strategy
This systematic review of RCTs was performed in accordance with the criteria of the PRISMA statement and the current recommendations of the Cochrane Collaboration.8,9 We searched the PubMed, Embase, Cochrane, Web of Science, Springer, and Clinical trials register databases for related articles published on or before April 30, 2018, using the terms “ thoracotomy pain” or “ thoracoscopic pain” and “pregabalin”.
Study selection
Inclusion criteria
- Settings and design: RCTs of pregabalin for prevention of postoperative pain in thoracotomy.
- Study subjects: Patients who suffered postoperative pain in thoracotomy.
- Interventions: The experimental group was administered pregabalin orally; the control group was administered conventional analgesia or placebo.
- Outcome indicators: Postoperative pain scores and incidence of neuropathic pain.
Exclusion criteria
- Combination with other antiepileptic drugs or anticonvulsive drugs.
- Incomplete data.
Trial selection, data extraction, and quality assessment
Two authors (Y-JY and NL) separately screened the articles, extracted data based on the inclusion and exclusion criteria, and evaluated the quality of each RCT using Cochrane Collaboration Risk of Bias Tool.10 Disagreements were resolved by consensus. The opinion of a third author (J-RX) was obtained when agreement could not be reached.
The extracted data included first author’s name, publication data, type of surgery and anesthesia, patient demographic, sample size, details regarding pregabalin medication (dose and duration), morphine consumption, pain scores, postoperative complications, and side effects (nausea, vomiting, dizziness, drowsiness, and constipation).
RCTs were assessed for various types of bias, including selection, performance, detection, attrition, and reporting. RCT quality scores were not a factor for trial exclusion.
Definitions and outcomes
The main outcomes included the numerical rating scale or the visual analog scale (VAS) pain scores, the incidence of neuropathic pain, mean morphine consumption, and incidence of nausea, vomiting, dizziness, drowsiness, and constipation postoperatively.
If studies did not show complete data, we e-mailed authors requesting the original data. If there was no reply, we use the software of plot digitizer 2.6.8.0 to measure the exact numbers in figures. Pain scores were transformed to a standardized 0–10 analog scale (0= no pain and 10= worst pain imaginable).11 Morphine consumption was the standard for opioid consumption. Fentanyl was converted to equi-analgesic morphine equivalent doses based on the following conversion scale: 100:1.12
Statistical methods
The data were analyzed by Review Manager Software 5.3. The effect size for continuous data is expressed as the mean difference and the 95% CI. The effect size for dichotomous data is expressed as the OR and the 95% CI. The χ2-test P-value and the I2-value were used to determine the level of heterogeneity. A random effect model was used in cases of heterogeneity (P<0.1 or I2≥50%), and a fixed effect model was used in cases of homogeneity (P≥0.1 or I2<50%).13 Publication bias using the Egger test and sensitivity analyses was conducted by STATA 12.0, where P>0.05 indicated no statistically significant publication bias.
Trial sequential analysis
Meta-analysis can result in type I errors (α) owing to repetitive testing of accumulated data, especially when the included studies have small sample sizes. Thus, we used trial sequential analysis (TSA) to examine the reliability and conclusiveness of our results. TSA depends on the quantification of the required information size. We calculated a diversity-adjusted (D2 required information size), since the heterogeneity adjustment with I2 underestimates the required information size and estimated the required information size using 0.05 for type 1 error and 0.20 for type 2 error. The relative risk reduction from the control group event rate from low-bias-risk trials included in the meta-analysis, according to the TSA user manual.14 TSA software version 0.9.5.10 Beta (http://www.ctu.dk/tsa) was used in this study. If the cumulative Z-curve crossed the trial sequential monitoring boundary or exceeded the required information size, a significant result had been reached and no further studies were needed. Otherwise, further studies were necessary to confirm the results.
Results
Study selection and characteristics
The literature search included 9 qualifying RCTs with 684 patients that met inclusion criteria (Figure 1).15–23 Quality assessment of RCTs was presented in Figures 2 and 3. The characteristics of patients and interventions are presented in Table 1. One was conducted in multicenters,15 and others were conducted in a single center. All RCTs applied randomized strategies. Three studies applied double-blind strategies. Three hundred forty-four patients were in the pregabalin group and 340 were in the control group.15,20,21 Two different dosages of pregabalin 150 mg16–19,21,22 or 300 mg15,20,23 were administered to patients orally every day. The administration period was from 1 to 90 days. Postoperative analgesia included epidural analgesia, celecoxib, morphine, and nonsteroidal anti-inflammatory drugs. Postoperative analgesia details in control group can be seen in Table 1.
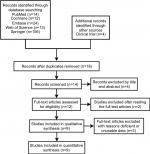  | Figure 1 Search results and selection procedure. |
  | Figure 2 Risk of bias graph. |
  | Figure 3 Risk of bias summary. |
Meta-analysis results
Postoperative pain scores
Six studies reported pain scores on postoperative 1 day. Pregabalin reduced scores by 0.87 points (n=385; 95% CI, –1.55 to –0.19, I2=63%, P=0.01). Four studies reported on postoperative 3 days. Pregabalin reduced scores by 1.55 points (n=275; 95% CI, –1.93 to –1.18, I2=0%, P<0.00001). Two studies reported on postoperative 1 month. Pregabalin reduced scores by 1.58 points (n=135; 95% CI, –2.75 to –0.42, I2=58%, P=0.008). Four studies reported on postoperative 3 months. Pregabalin reduced scores by 1.69 points (n=235; 95% CI, –2.71 to –0.66, I2=83%, P=0.001). There was no difference on postoperative 7 days (n=184; 95% CI: –2.38–0.84; I2=92%; P=0.35; Figure 4).
The Egger test for publication bias (P=0.601 on postoperative 1 day; P=0.778 on postoperative 3 days; P=0.761 on postoperative 7 days; and P=0.167 on postoperative 3 months); and sensitivity analysis did not significantly alter the summarized results. TSA results demonstrated that the cumulative Z-score of VAS on 1 day, 3 days, 1 month, and 3 months crossed its monitoring boundaries and reliable conclusions had been drawn. But the sample size of VAS on 7 days did not reach the required sample size (Figure 5).
Incidence of neuropathic pain postoperatively
Three studies (n=217) investigated the incidence of postoperative neuropathic pain. The incidence of neuropathic pain was 80% lower with pregabalin (95% CI, 0.05–0.91, I2=62%, P=0.04). The Egger test for publication bias (P=0.296) and sensitivity analysis did not significantly alter the summarized results. And TSA indicated that the sample size in the meta-analysis did not reach the required sample size (Figure 6).
Morphine consumption postoperatively
Results describing postoperative morphine consumption were available from two studies (n=160). Pregabalin was effective in reducing postoperative morphine consumption by –5.03 (95% CI: –8.06 to –1.99; I2=0%; P=0.001). Sensitivity analysis did not significantly alter the summarized results, and TSA indicated that crossed its monitoring boundaries and reliable conclusions had been drawn (Figure 7).
Side effects
Six studies investigated the incidence of dizziness, and with pregabalin the incidence was higher (n=184; 95% CI: 1.36–8.17; I2=0%; P=0.009). The Egger test for publication bias (P=0.082). Four studies investigated the incidence of drowsiness, and with pregabalin the incidence was higher (n=278; 95% CI: 2.23–33.20; I2=0%; P=0.002). The Egger test for publication bias (P=0.826). Three studies investigated the incidence of constipation, and with pregabalin the incidence was lower (n=208; 95% CI: 0.09–0.59; I2=0%; P=0.002). The Egger test for publication bias (P=0.710). Six studies investigated the incidence of nausea and two studies investigated the incidence of vomiting. There was no statistically significant difference in nausea (n=500; 95% CI: 0.42–1.26; I2=40%; P=0.26; the Egger test for publication bias, P=0.867) and vomiting (n=200; 95% CI: 0.36–1.90; I2=0%; P=0.65; Figure 8). Sensitivity analysis did not significantly alter the summarized results. TSA indicated that the sample size in the meta-analysis did not reach the required sample size (Figure 9).
  | Figure 9 TSA: adverse effects in patients receiving pregabalin. Notes: (A) Nausea. (B) Vomiting. (C) Dizziness. (D) Drowsiness. (E) Constipation. Abbreviation: TSA, trial sequential analysis. |
Discussion
This is the first meta-analysis to evaluate the analgesic efficacy of pregabalin as an adjuvant to a perioperative multimodal analgesic regimen in thoracotomy. After analyzing the combined results of 9 RCTs, we found that pregabalin significantly reduced pain scores on 1 and 3 days and 1 and 3 months, reduced incidence of postoperative neuropathic pain, and reduced morphine consumption. However, the clinical significance of results may be limited by the heterogeneity in the included studies.
Our results are consistent with a meta-analysis that assess efficacy of pregabalin in acute postoperative pain under different surgical categories.24 The results showed that pregabalin reduced the pain score at rest 2 hours after surgery in the cardiothoracic procedure. But there was not a definite conclusion for persistent pain because of insufficient data. In other articles of meta-analysis, the efficacy of pregabalin in the specific surgical style of thoracotomy was not mentioned. The efficacy of perioperative pregabalin treatment for preventing chronic pain remains a matter of debate. In a recent meta-analysis of pregabalin, it especially evaluates the incidence of chronic postsurgical pain (CPSP) in 3, 6, and 12 months and the incidence of chronic postsurgical neuropathic pain at the same time point, including all published and unpublished articles.25 The conclusion is that the available data do not support with a moderate level of evidence for a systematic prevention of CPSP with pregabalin. Interestingly, it is shown that almost all of the overall effect comes from unpublished data being reverse of what one may expect and what has been published. None of unpublished trials reported pregabalin to be effective for preventing CPSP at any time (3, 6, and 12 months). In our study, pregabalin made a reduction in pain scores in 1 and 3 months and the incidence of postsurgical neuropathic pain, and it may be attributed to our study including all published articles under a single style surgery of thoracotomy. A higher incidence of chronic pain especially chronic neuropathic pain in the surgical style of thoracotomy, and pregabalin was used for chronic pain especially chronic neuropathic pain better than acute pain. It may be the reason why pregabalin is effective in thoracotomy.1,2 But the number of included articles was small. Therefore, more studies are needed to report the incidence of CPSP in thoracotomy, even when their results are not consistent with the earlier articles.
In addition, we found the morphine consumption was lower in the pregabalin group, although there were only two studies included. In a recent meta-analysis, it is shown that pregabalin may be a beneficial but small effect in postoperative pain management with minimal clinical relevant effect of morphine 5 mg in 24 hours of opioid consumption, this result was consistent with us.26 We converted opioid use to morphine equivalents in studies because different opioid drugs and units were used to record opioid consumption. It was important to reduce opioid consumption because it has some side effects such as addiction, nausea, vomiting, constipation, and so on. In our study, there was a lower incidence of constipation, which may be the result of lower morphine consumption.27
We also found that there was a higher incidence of dizziness and drowsiness. Dizziness and drowsiness were most common adverse effects of pregabalin. Griffin reported that dizziness, fatigue, and somnolence were among the most common adverse effects of pregabalin.28 There was no significant difference of nausea and vomiting between the pregabalin group and the control group. In another study, postoperative administration of pregabalin has been shown to reduce nausea and vomiting for the reason of reduction in opioid consumption.29,30 In our study, the phenomenon that pregabalin did not reduce nausea and vomiting may result from the additional analgesic drugs with nonopioids instead of opioids drugs in most included studies.
This meta-analysis has several limitations that should be considered. First, the standard trials were limited, and the sample size was relatively insufficient, these factors could make the power test insufficient. Second, differences existed among trials, such as the doses of administration and outcome indicators of included RCTs. These factors may affect the meta-analysis and conclusion. Therefore, more high-quality studies are needed to reduce the effect of bias on study results.
Conclusion
In conclusion, our meta-analysis indicated that pregabalin could improve acute and chronic pain control, and reduce opioids consumption. However, future studies regarding doses and pregabalin medication are required.
Acknowledgments
This study was supported by The Medical Science Research Foundation of Zhejiang Province (2019314366), The Natural Science Fund of Zhejiang Province (LY17H090011) and The Science and Technology Department of Zhejiang Province Public Welfare Project (2016C33G2010165).
Disclosure
The authors report no conflicts of interest in this work.
References
Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126(5):938–951. | ||
Homma T, Doki Y, Yamamoto Y, et al. Risk factors of neuropathic pain after thoracic surgery. J Thorac Dis. 2018;10(5):2898–2907. | ||
Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107(3):1026–1040. | ||
Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048–1056. | ||
Yekkirala AS, Roberson DP, Bean BP. Breaking barriers to novel analgesic drug development. Anesthesiology. 2017;16(11):810. | ||
Li Z, Taylor CP, Weber M, et al. Pregabalin is a potent and selective ligand for α(2)δ-1 and α(2)δ-2 calcium channel subunits. Eur J Pharmacol. 2011;667(1–3):80–90. | ||
Gray P. Pregabalin in the management of central neuropathic pain. Expert Opin Pharmacother. 2007;8(17):3035–3041. | ||
Higgins J, Green S. Guide to the contents of a Cochrane protocol and review. In: Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0. Chapter 4. The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org. Accessed December 1, 2016. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(7):b2700. | ||
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–872. | ||
Brogi E, Kazan R, Cyr S, Giunta F, Hemmerling TM. Transversus abdominal plane block for postoperative analgesia: a systematic review and meta-analysis of randomized-controlled trials. Can J Anaesth. 2016;63(10):1184–1196. | ||
Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–687. | ||
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. | ||
Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research; 2011;1–115. | ||
Konstantatos AH, Howard W, Story D, Mok LY, Boyd D, Chan MT. A randomised controlled trial of peri-operative pregabalin vs. placebo for video-assisted thoracoscopic surgery. Anaesthesia. 2016;71(2):192–197. | ||
Yoshimura N, Iida H, Takenaka M, et al. Effect of postoperative administration of pregabalin for post-thoracotomy pain: a randomized study. J Cardiothorac Vasc Anesth. 2015;29(6):1567–1572. | ||
Miyazaki T, Sakai T, Sato S, et al. Is early postoperative administration of pregabalin beneficial for patients with lung cancer? Randomized control trial. J Thorac Dis. 2016;8(12):3572–3579. | ||
Matsutani N, Dejima H, Takahashi Y, Kawamura M. Pregabalin reduces post-surgical pain after thoracotomy: a prospective, randomized, controlled trial. Surg Today. 2015;45(11):1411–1416. | ||
Mishra A, Nar AS, Bawa A, Kaur G, Bawa S, Mishra S. Pregabalin in chronic post-thoracotomy pain. J Clin Diagn Res. 2013;7(8):1659–1661. | ||
Brulotte V, Ruel MM, Lafontaine E, Chouinard P, Girard F. Impact of pregabalin on the occurrence of postthoracotomy pain syndrome: a randomized trial. Reg Anesth Pain Med. 2015;40(3):262–269. | ||
Kim JC, Byun S, Kim S, et al. Effect of preoperative pregabalin as an adjunct to a multimodal analgesic regimen in video-assisted thoracoscopic surgery: a randomized controlled trial. Medicine. 2017;96(49):e8644. | ||
Matsutani N, Dejima H, Nakayama T, et al. Impact of pregabalin on early phase post-thoracotomy pain compared with epidural analgesia. J Thorac Dis. 2017;9(10):3766–3773. | ||
Metin SK, Meydan B, Evman S, Dogruyol T, Baysungur V. The effect of pregabalin and methylcobalamin combination on the chronic postthoracotomy pain syndrome. Ann Thorac Surg. 2017;103(4):1109–1113. | ||
Lam DM, Choi SW, Wong SS, Irwin MG, Cheung CW. Efficacy of pregabalin in acute postoperative pain under different surgical categories: a meta-analysis. Medicine. 2015;94(46):e1944. | ||
Martinez V, Pichard X, Fletcher D. Perioperative pregabalin administration does not prevent chronic postoperative pain: systematic review with a meta-analysis of randomized trials. Pain. 2017;158(5):775–783. | ||
Fabritius ML, Strøm C, Koyuncu S, et al. Benefit and harm of pregabalin in acute pain treatment: a systematic review with meta-analyses and trial sequential analyses. Br J Anaesth. 2017;119(4):775–791. | ||
Nalamachu S, Gudin J, Datto C, et al. Efficacy and safety of naloxegol for opioid-induced constipation assessed by specific opioid medication, opioid dose, and duration of opioid use. J Opioid Manag. 2018;14(3):211–221. | ||
Griffin E, Brown JN. Pregabalin for the treatment of restless legs syndrome. Ann Pharmacother. 2016;50(7):586–591. | ||
Li F, Ma J, Kuang M, et al. The efficacy of pregabalin for the management of postoperative pain in primary total knee and hip arthroplasty: a meta-analysis. J Orthop Surg Res. 2017;12(1):49. | ||
Sawan H, Chen AF, Viscusi ER, Parvizi J, Hozack WJ. Pregabalin reduces opioid consumption and improves outcome in chronic pain patients undergoing total knee arthroplasty. Phys Sportsmed. 2014;42(2):10–18. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.