Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
The Efficacy of Combination Therapy Involving Excision Followed by Intralesional 5-Fluorouracil and Betamethasone, and Radiotherapy in the Treatment of Keloids: A Randomized Controlled Trial
Authors Li Y , Zhang D, Hang B, Wang H
Received 4 September 2022
Accepted for publication 15 November 2022
Published 23 December 2022 Volume 2022:15 Pages 2845—2854
DOI https://doi.org/10.2147/CCID.S388717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yazhuo Li, Dewu Zhang, Bing Hang, Hao Wang
Department of Dermatology, the Second Affiliated Hospital of Xi’an Jiaotong University (Xibei Hospital), Xi’an, People’s Republic of China
Correspondence: Hao Wang, Department of Dermatology, the Second Affiliated Hospital of Xi’an Jiaotong University (Xibei Hospital), No. 157, West 5th Road, Xi’an, Shaanxi Province, 710004, People’s Republic of China, Tel +86 182 9183 9156, Email [email protected]
Background: Combined therapy for keloids is currently recommended. Surgery is one of the main options, but the measures to prevent recurrence after excision are still being explored.
Objective: The randomized controlled study aimed at evaluating the efficacy of excision followed by intralesional low concentrations of 5-fluorouracil (5-FU)(12.5 mg/mL) and betamethasone.
Methods: Sixty patients were randomly assigned to three groups. Patients in group A had excision followed by 5-FU and betamethasone intralesional injections, group B had 5-FU and betamethasone intralesional injections, and group C had excision followed by radiotherapy. Efficacy parameters were assessed from 8 to 12 months, including improvement on the Vancouver Scar Scale (VSS) and the Patient and Observer Scar Scale (POSAS), as well as side effects and recurrence. Trial registration number: ChiCTR2100046025.
Results: After 4 months’ treatment, the improvement of the VSS and POSAS scores in group A was not different from that in group C (P > 0.05) but was superior to that in group B (P < 0.05); the pain and pruritus of the three groups were relieved more than 50%. After 8 to 12 months’ follow-up, there was no statistical difference in the incidence of side effects and recurrence among the groups (P > 0.05).
Conclusion: Excision followed by intralesional low concentrations of 5-FU (12.5mg/mL) with betamethasone is a safe and sustainable treatment for keloid, with no significant difference from excision followed by radiotherapy.
Keywords: keloid, excision, 5-fluorouracil, betamethasone, radiotherapy
Introduction
As the largest organ of the human body, the skin is the first defence against external damage. Any trauma including surgery, puncture, acne, folliculitis, tattoos, insect bites, burns, abrasions, and other processes that cause skin inflammation, the body automatically turns on the repair system. However, this process will have the possibility of excessive repair and proliferation, resulting in abnormal wound healing and subsequent development of hypertrophic scars or keloids.1 This not only makes aesthetically impaired, keloids growing in the joint affects motor function to varying degrees but also causes obstacles to the quality of life. The mechanism involves the excessive proliferation of fibroblasts in tissues2 and abnormal activation of the proinflammatory cascade,3 which leads to the deposition of a large amount of extracellular matrix such as collagen. There is a variety of treatments including surgery, radiation, intralesional injection of corticosteroids and anti-tumor drugs (5-fluorouracil, mitomycin C, bleomycin, etc), laser, topical silicone drugs, etc, but still lack of an effective monotherapy. It is believed that multimodal combination therapy has better results and fewer side effects.4 Excision followed by intralesional 5-fluorouracil (5-FU) and corticosteroid injection is a classical effective therapy, showing a better comprehensive treatment effect.5 Most studies have used 40–50 mg/mL 5-FU in the treatment of keloid.6,7 Considering the physical factors of Asians, we tried to use a low concentration of 5-FU (12.5mg/mL). In addition, we used Diprospan, which is a compound betamethasone preparation containing short-term and long-term effects. With these changes, we developed the new treatment regimens and have achieved effective results in previous clinical studies.8 In this study, we conducted a randomized controlled clinical trial to demonstrate that our new regimen has good efficacy compared with intralesional low concentrations of 5-FU and betamethasone injections, and excision followed by radiotherapy.
Methods
Ethical Statement
This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University and registered in the Chinese Clinical Trial Registry (ChiCTR2100046025) before recruitment. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Patients
From May 2021 to August 2021, we included 60 patients diagnosed with keloid in the Second Affiliated Hospital of Xi’an Jiaotong University. Informed consent was obtained. Inclusion criteria were aged 18–70 years old; did not experience any keloid treatment within 3 months; the lesions without progression within 3 months and were assessed by the VSS with a score more than 4 and less than 13. Exclusion criteria included pregnancy and lactation, systemic disease or tumor, infection of lesions, allergic to corticosteroids or 5-FU. Patients were randomly divided into three groups. Group A: excision followed by 5-FU and betamethasone intralesional injections, group B: 5-FU and betamethasone intralesional injections, group C: excision followed by radiotherapy.
Treatment
Collected patients’ information before treatment. One doctor assessed the severity of the keloid, completed the scar scales and took photographs (camera: Leica C Typ112). Excision and injection were performed in the same surgeon’s visit, who only knew the results of randomization.
Group A: keloids were removed completely to minimize wound tension. Injection: 2mL 5-FU (250mg/10mL), 1mL betamethasone (7mg/mL) and 1mL lidocaine (2mg/mL) mixed well until there was no white precipitate. Using a 1-mL syringe, 0.5cm on parallel lines on both sides from the suture, with the interval of 1cm as an injection point, 0.1mL at each point injected slowly, the volume was limited to 4mL. Sterile dressing was applied after the end, and local drying was advised for 24 hours. The first injection was performed immediately after excision of keloids and every 4 weeks thereafter. The course of treatment was ended after 4 times.
Group B: The drugs preparation is the same as group A. Injection: Using a 1-mL syringe, the mixed solution was injected into keloid lesions and the volume was limited to 4mL. The injection was once every 4 weeks, and the course of treatment was ended after 4 times.
Group C: The surgical excision same as group A. We used high-energy electron beam superficial radiotherapy. Within 24 hours of surgery, the patients were treated for the first time. A Trilogy electron linear accelerator (Varian, USA, energy 6 MeV) was used, with wound and 1cm around it as the irradiation range, 3.5–4 Gy per fraction, and 3 times consecutive radiotherapy on the second, third and fourth days after surgery.
Efficacy Evaluation
The main criteria of assessment were the clinical evaluation of the Vancouver Scar Scale9 (VSS) and the Patient and Observer Scar Assessment Scale10 (POSAS). The latter includes two parts: the patient scale (PSAS) and the observer scale (OSAS). The work was always done by one doctor. Data were collected prior to treatment and after 4 months. Record all side effects that occurred during treatment and follow-up. Recurrence is defined as the presence of one or more of the following situations: the pruritus or pain increased, keloid appearing again and exceeding the original range. Follow-up was stopped when the patient experienced recurrence, and treatment was given immediately.
Statistical Analysis
All the data were analyzed by SPSS (version 20). Categorical variables were presented as numbers (%), and the Chi square or Fisher’s test were used. Continuous variables satisfying a normal distribution were presented as mean ±standard deviation (x±s), and the one-way ANOVA and paired t-test were used. Continuous variables that did not show normality were presented as median (Interquartile Range) [M (P25,P75)], and the Kruskal–Wallis test and Wilcoxon rank sum test were used. A P-value <0.05 was considered to be statistically significant.
Results
A total of 55 patients completed the treatment and follow-up. There was no statistical difference in sex, age, course of the disease, BMI, location of lesions, inducement, previous treatment history and family history among the three groups (P>0.05) (Table 1).
 |
Table 1 Patient Characteristics |
Improvement of Keloid Treatment
Before treatment, there was no statistical difference in VSS, OSAS, PSAS scores among the three groups (P>0.05). However, each group’s scores were significantly lower than themselves after 4 months’ treatment (P<0.01).
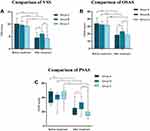
(1) After 4 months, there was statistical difference of VSS among the three groups (P<0.01), with similar improvement in group A and C (P=0.936), but superior to group B (P=0.028, P=0.001). The VSS scores in groups A, B, and C improved by 55.16%, 37.11%, and 54.11% after treatment, respectively (Table 2 and Figure 1).
 |
Table 2 Results: Comparison of VSS (Mean±std) |
(2) After 4 months, there was statistical difference of OSAS among the three groups (P<0.05), with similar improvement in group A and C (P=0.987), but superior to that in group B (P=0.008, P=0.01) The OSAS scores in group A, B, and C improved by 45.68%, 27.93%, and 41.77% after treatment, respectively (Table 3 and Figure 1).
 |
Table 3 Results: Comparison of OSAS (Mean±std) |
(3) After 4 months, there was statistical difference of PSAS among the three groups (P<0.01), with similar improvements in group A and C (P=0.09), but superior to that in group B (P=0.017, P=0.001). The PSAS scores in groups A, B, and C improved by 51.72%, 30.95%, and 63.63% after treatment, respectively (Table 4 and Figure 1).
 |
Table 4 Results: Comparison of PSAS [Median (Interquartile Range)] |
Recurrence
The median follow-up time of groups A, B, and C was 9 months, 9 months and 10 months, respectively, and the minimum follow-up time was 8 months and the maximum was 12 months in all the three groups. Comparison of recurrence among the three groups: 2 cases in group A (2/18), 4 cases in group B (4/20), 1 case in group C (1/17). The recurrence rate in group A (11.1%) and group B (20%) was higher than that in group C (5.9%), but there was no statistical difference among the three groups (P=0.535). (Table 5).
 |
Table 5 Results: Comparison of Recurrence and Adverse Side Effects |
Adverse Side Effects
The side effects included hyperpigmentation, scab, telangiectasia, and hypopigmentation. The incidence of side effects in group A, group B and group C was 44.44% (8/18), 25.00% (5/20) and 58.82% (10/17), respectively, but there was no statistical difference among the groups (P=0.423). There was none of the malignant transformation or systemic side effects (Table 5 and Figure 2).
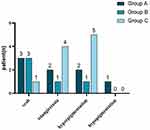 |
Figure 2 Adverse side effects resulting from treatments. The most common side effect in group A, B and C was scab, scab and hyperpigmentation, respectively. |
Pain and Pruritus
Among the 55 patients in our study, 44 (84.62%) patients reported pain and 52 (94.55%) patients reported pruritus at the site of keloid. We counted the pain and pruritus scores separately in the PSAS. Whether before or after 4 months’ treatment, there was no statistical difference in scores among the three groups (P>0.05). However, each group’s scores were significantly lower after 4 months of treatment (P<0.05). The pain scores in group A, B and C were improved by 75.00%, 66.67% and 80.00%, respectively, and the pruritus scores were improved by 66.67%, 50.00% and 60.00%, respectively (Tables 6 and 7).
 |
Table 6 Results: Comparison of Pain [Median (Interquartile Range)] |
 |
Table 7 Results: Comparison of Pruritus [Median (Interquartile Range)] |
Discussion
As a benign fibroproliferative disease, the final process of keloid development remains to be elucidated. The main purpose of current treatments is to inhibit fibroblast proliferation and collagen deposition, as well as reduce inflammation. All of these therapies are prone to relapse and cannot be completely cured. Many years of research have found that monotherapy of keloids is difficult to achieve satisfactory efficacy, so experts recommend the combination of multiple measures for treatment currently.4
Corticosteroids can inhibit inflammation,11 collagen and glycosaminoglycan synthesis, fibroblast proliferation, and also can prevent the denaturation of collagen and fibroblasts.12 Corticosteroids are the traditional treatment of keloids, which can effectively improve the flexibility of the tissue, reduce its thickness, relieve pruritus and pain in patients.13 The most commonly used corticosteroid is triamcinolone acetonide, with an efficiency of 50–100% in different studies.6 However, after long-term observation, it has been found that the recurrence rate of corticosteroids is up to 50%, and 63% of the patients have local side effects, including skin atrophy, hypopigmentation and telangiectasia.14 Whereas, the side effects appear to be reduced when combining corticosteroids with 5-FU.15 The cells and tissues of keloids are in an excessive metabolic state,16 so antimetabolites are logical. 5-FU is a pyrimidine analogue with antimetabolite activity that inhibits cell division at all stages of the cell cycle, leading to growth arrest.17 It has been shown that 5-FU inhibits fibroblast proliferation in vitro.18 Therefore, international guidelines formally incorporated 5-FU into new advances in keloid treatment in 2014.15,19 More and more studies have observed that the efficacy of 5-FU combined with corticosteroids in the treatment of keloid and hypertrophic scar is superior to single-drug injections.20,21 The combination of these two drugs is also recommended for the prevention of postoperative scar hyperplasia.1
After years of treatment for keloid patients, we found that triamcinolone acetonide has poor solubility and is easy to block the syringe, the liquid always splashing to the skin and eyes. White spots with a hard texture and difficulty to be absorbed are often observed. Betamethasone has better solubility and less bolus pressure. It also has similar efficacy and its side effects do not increase either. Currently, there are still limited studies about betamethasone in the treatment of keloids.8,22,23 Due to the abnormal appearance, cannot resolve spontaneously, pain and pruritus and other characteristics of keloid, many patients often require excision to achieve rapid efficacy. However, the recurrence rate of surgery alone can be 45–100%,24 scholars found that keloid surgery combined with other treatments can reduce the risk of recurrence from 50% to 8%.25 Even keloids with postoperative hyperplasia will be more sensitive to treatment than old lesions. Excision followed by 5-FU and corticosteroids can significantly reduce the risk of recurrence and achieve better efficacy.25,26 Immediately or the early period after excision, injection of antimetabolites and corticosteroids near the incision has no adverse effect on healing. What’s more, it has been proposed that early injection can inhibit the secretion of inflammatory factors and growth factors that promote scar formation.27
This study aims to demonstrate the efficacy of excision followed by intralesional low concentrations of 5-FU (12.5mg/mL) and betamethasone in the treatment of keloids, compared with 5-FU (12.5mg/mL) and betamethasone intralesional injection, as well as excision followed by radiotherapy. After excision of keloids, a low concentration of 5-FU (12.5mg/mL) and betamethasone (1.75mg/mL) mixture was injected around the edge of the incision. The maximum dose as 50mg 5-FU and 7mg betamethasone each time. The dosage of 5-FU used in our study is much less than the chemotherapeutic dose of 200–600mg/m2.28 In the present study, the appearance, clinical symptoms and patient satisfaction of keloids were assessed with VSS, OSAS and PSAS. We found no significant difference in the scores of the two treatments in group A and group C, but they were superior to group B. The results showed comparable efficacy in group A and group C, providing convincing evidence for our new therapy. We observed a positive response in all the three groups, and the improvement of scores between the baseline and 4 months was statistically significant in three groups. The reduction of PSAS, pain, and pruritus scores indicated that the three treatments could relieve uncomfortable symptoms and improve the quality of life for patients. In this study, some patients experienced local side effects, including scab, telangiectasia, hyperpigmentation and hypopigmentation. These complications had also been reported in previous studies.29 The most common side effect in group A and group B was scab on the surface of the injection, but all of them could heal spontaneously without serious infection. The scab did not affect the subsequent treatment for patients. In group C, perilesional hyperpigmentation was most common, which occurs frequently in radiotherapy.30 The 8 to 12 months’ follow-up showed that side effects and recurrence rates in three groups showed no statistical difference, which may be affected by a small sample size.
A meta-analysis that included four studies with 254 patients found that intralesional triamcinolone acetonide injection after excision of keloids was invalid in reducing the recurrence rate, while after analysis of two studies with 107 patients, it was shown that treated with 5-FU after surgery was effective in the prevention of recurrence.31 This suggests that 5-FU can prevent recurrence of keloid excision, but the combination with corticosteroids can optimize therapeutic effect and reduce side effects. Most studies have treated keloids with (40–50mg/mL) 5-FU, and a total injection dose of 50–150mg.32 Research has proposed a recurrence rate of 19% when excision followed by intralesional 5-FU.33 In a randomized controlled study of ear keloids, excision followed by intralesional of 9:1 5-FU versus triamcinolone injection, the recurrence rate was 26.67% at a follow-up time of 20 months.34 We kept the observations for 8 to 12 months, showed that the recurrence rate was 11.1% in group A and 20% in group B. The results of the low concentrations of 5-FU combined betamethasone injections in our study are comparable with these previous studies.
Reinholz et al treated keloids with 50mg/mL 5-FU and triamcinolone acetonide, the overall incidence of side effects was 80%, including hyperpigmentation (36%), telangiectasia (24%), and ulcers (20%). In addition, pruritus and pain were reduced by 57% and 55%, respectively, the PSAS improved by 39%, and the OSAS improved by 52% after treatment.28 In our study, the incidence of complications in group A and group B was 44.44% and 25.00%, respectively. Moreover, the pain and pruritus scores in group A and group B were also improved more than 50.00%. Compared with the data from these studies using higher concentrations and doses of 5-FU, our therapy with 12.5mg/mL 5-FU still achieved comparable comprehensive results. Many studies suggest that excision followed by radiotherapy is an effective and safe treatment. Mankowski et al conducted a review of 72 studies involving radiation therapy with 9048 keloids, the data showed that excision followed by radiotherapy prevented recurrence better than radiotherapy alone (recurrence rates of 22% and 37%, respectively, P=0.005).35 However, as a benign disease, the choice of radiation therapy for keloid must be very cautious, especially in young women and special sites such as the thyroid gland, gonads and breast. Whether as the postoperative prevention for recurrence or monotherapy, low concentrations of 5-FU (12.5mg/mL) combined and betamethasone improved lesions significantly and achieved a lower incidence of side effects and recurrence. It can treat keloids where radiotherapy is not recommended, or patients who cannot accept radiotherapy due to various contraindications. In conclusion, we think excision followed by low concentrations of 5-FU and betamethasone intralesional injections has a wider application, higher security and more rapid efficacy (especially for patients with severe symptoms). Furthermore, injection is economical, easy to operate, does not require special equipment and convenient to carry out the treatment, especially suitable for primary hospitals. However, this study has some limitations. Patients were followed up for 8 to 12 months, and the recovery of lesions still required longer observation. We could not carry out blinding because of the limitation of treatment design. In addition, total number of patients included in the study is small and a large-scale study may be necessary.
Conclusion
In summary, the results of the present study showed that excision followed by intralesional low concentrations of 5-FU with betamethasone could be a safe and effective method to treat keloid and prevent postoperative hyperplasia, with no significant difference from excision followed by radiotherapy.
Representative Cases
Representative cases of three groups are presented in Figures 3–6.
 |
Figure 3 Example of keloid in epigastric region treated with excision followed by intralesional 5-FU and betamethasone. (A) Before treatment, (B) at 6 months of follow-up. |
 |
Figure 4 Example of keloid in pubic region treated with excision followed by intralesional 5-FU and betamethasone. (A) Before treatment, (B) at 6 months of follow-up. |
 |
Figure 5 Example of keloid treated with intralesional 5-FU and betamethasone. (A) Before treatment, (B) at 6 months of follow-up. |
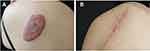 |
Figure 6 Example of keloid treated with excision followed by radiotherapy. (A) Before treatment, (B) at 6 months of follow-up. |
Data Sharing Statement
The authors intend to share individual deidentified participant data. The data that support the findings of this study are available from the corresponding author (Hao Wang, Email: [email protected]), which is available during the publication of the article.
Acknowledgments
This work was supported by the Key Research and Development Program of Shaanxi, China. Grant number: 2021SF-280. The authors wish to express their gratitude to the patients and their families.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Molr Med. 2011;17(1–2):113–125. doi:10.2119/molmed.2009.00153
2. Zhang Z, Cheng L, Wang R, Cen Y, Li Z. Effects and safety of triamcinolone acetonide-controlled common therapy in keloid treatment: a Bayesian network meta-analysis. Ther Clin Risk Manag. 2018;14:973–980. doi:10.2147/TCRM.S162315
3. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi:10.1172/JCI24282
4. Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. 2018;19(3). doi:10.3390/ijms19030711
5. LaRanger R, Karimpour-Fard A, Costa C, Mathes D, Wright WE, Chong T. Analysis of keloid response to 5-fluorouracil treatment and long-term prevention of keloid recurrence. Plast Reconstr Surg. 2019;143(2):490–494. doi:10.1097/PRS.0000000000005257
6. Nanda S, Reddy BSN. Intralesional 5-fluorouracil as a treatment modality of keloids. Dermatol Surg. 2004;30(1):1.
7. Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: an open clinical and histopathologic study. J Am Acad Dermatol. 2005;52(3 Pt 1):474–479. doi:10.1016/j.jaad.2004.09.018
8. Wang H, Zhao W, Xu M, An L. Treatment of keloids by surgical excision combined with 5-fluorouracil and betamethasone injection. Dermatol Surg. 2021;47(5):740–741. doi:10.1097/DSS.0000000000002438
9. Baryza MJ, Baryza GA. The Vancouver Scar Scale: an administration tool and its interrater reliability. J Burn Care Rehabil. 1995;16(5):535–538. doi:10.1097/00004630-199509000-00013
10. Draaijers LJ, Tempelman FRH, Botman YAM, Tuinebreijer WE, Middelkoop E, Kreis RW, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113(7)
11. Wang Z-C, Zhao W-Y, Cao Y, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187. doi:10.3389/fimmu.2020.603187
12. Berman B, Maderal A, Raphael B. Keloids and Hypertrophic Scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(1):S3–S18. doi:10.1097/DSS.0000000000000819
13. Hawash AA, Ingrasci G, Nouri K, Yosipovitch G. Pruritus in keloid scars: mechanisms and treatments. Acta Derm Venereol. 2021;101(10):adv00582. doi:10.2340/00015555-3923
14. Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40(7):1255–1266. doi:10.1016/j.burns.2014.02.011
15. Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatol Surg. 2014;40(8):825–831. doi:10.1111/dsu.0000000000000050
16. Speranza G, Sultanem K, Muanza T. Descriptive study of patients receiving excision and radiotherapy for keloids. Int J Radiat Oncol Biol Phys. 2008;71(5):1465–1469. doi:10.1016/j.ijrobp.2007.12.015
17. Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153(2):295–300.
18. Huang L, Wong YP, Cai YJ, Lung I, Leung CS, Burd A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br J Dermatol. 2010;163(6):1181–1185. doi:10.1111/j.1365-2133.2010.09939.x
19. Gold MH, Berman B, Clementoni MT, Gauglitz GG, Nahai F, Murcia C. Updated international clinical recommendations on scar management: part 1--evaluating the evidence. Dermatol Surg. 2014;40(8):817–824. doi:10.1111/dsu.0000000000000049
20. Ren Y, Zhou X, Wei Z, Lin W, Fan B, Feng S. Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: a systematic review and meta-analysis. Int Wound J. 2017;14(3):480–487. doi:10.1111/iwj.12629
21. Yang S, Luo YJ, Luo C. Network meta-analysis of different clinical commonly used drugs for the treatment of hypertrophic scar and keloid. Front Med. 2021;8:691628. doi:10.3389/fmed.2021.691628
22. Hao Y-H, Xing X-J, Zhao Z-G, et al. A multimodal therapeutic approach improves the clinical outcome of auricular keloid patients. Int J Dermatol. 2019;58(6):745–749. doi:10.1111/ijd.14413
23. Zouboulis CC, Zouridaki E. Cryosurgery as a single agent and in combination with intralesional corticosteroids is effective on young, small keloids and induces characteristic histological and immunohistological changes: a prospective randomized trial. Dermatology. 2021;237(3):396–406. doi:10.1159/000511624
24. Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110(2):560–571. doi:10.1097/00006534-200208000-00031
25. Uppal RS, Khan U, Kakar S, Talas G, Chapman P, McGrouther AD. The effects of a single dose of 5-fluorouracil on keloid scars: a clinical trial of timed wound irrigation after extralesional excision. Plast Reconstr Surg. 2001;108(5):1218–1224. doi:10.1097/00006534-200110000-00018
26. Bijlard E, Steltenpool S, Niessen FB. Intralesional 5-fluorouracil in keloid treatment: a systematic review. Acta Derm Venereol. 2015;95(7):778–782. doi:10.2340/00015555-2106
27. Del Toro D, Dedhia R, Tollefson TT. Advances in scar management: prevention and management of hypertrophic scars and keloids. Curr Opin Otolaryngol Head Neck Surg. 2016;24(4):322–329. doi:10.1097/MOO.0000000000000268
28. Reinholz M, Guertler A, Schwaiger H, Poetschke J, Gauglitz GG. Treatment of keloids using 5-fluorouracil in combination with crystalline triamcinolone acetonide suspension: evaluating therapeutic effects by using non-invasive objective measures. JEADV. 2020;34(10):2436–2444. doi:10.1111/jdv.16354
29. Hietanen KE, Järvinen TA, Huhtala H, Tolonen TT, Kuokkanen HO, Kaartinen IS. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections - a randomized controlled trial. JPRAS. 2019;72(1):4–11. doi:10.1016/j.bjps.2018.05.052
30. Ogawa R, Tosa M, Dohi T, Akaishi S, Kuribayashi S. Surgical excision and postoperative radiotherapy for keloids. Scars Burn Heal. 2019;5:2059513119891113. doi:10.1177/2059513119891113
31. Shin JY, Lee J-W, Roh S-G, Lee N-H, Yang K-M. A comparison of the effectiveness of triamcinolone and radiation therapy for ear keloids after surgical excision: a systematic review and meta-analysis. Plast Reconstr Surg. 2016;137(6):1718–1725. doi:10.1097/PRS.0000000000002165
32. Shin JY, Kim JS. Could 5-fluorouracil or triamcinolone be an effective treatment option for keloid after surgical excision? A meta-analysis. J Oral Maxillofac Surg. 2016;74(5):1055–1060. doi:10.1016/j.joms.2015.10.002
33. Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec. 2015;7(1–3):1–7. doi:10.1016/j.jccw.2016.10.001
34. Khalid FA, Farooq UK, Saleem M, et al. The efficacy of excision followed by intralesional 5-fluorouracil and triamcinolone acetonide versus excision followed by radiotherapy in the treatment of ear keloids: a randomized control trial. Burns. 2018;44(6):1489–1495. doi:10.1016/j.burns.2018.02.017
35. Mankowski P, Kanevsky J, Tomlinson J, Dyachenko A, Luc M. Optimizing radiotherapy for keloids: a meta-analysis systematic review comparing recurrence rates between different radiation modalities. Ann Plast Surg. 2017;78(4):403–411. doi:10.1097/SAP.0000000000000989
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.