Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
The efficacy of a “cocktail therapy” on Parkinson’s disease with dementia
Authors Zhang C , Zang Y, Song Q, Li H, Hu L, Zhao W, Feng S, Gu F, Zhao F, Zhang C
Received 7 July 2018
Accepted for publication 8 April 2019
Published 24 June 2019 Volume 2019:15 Pages 1639—1647
DOI https://doi.org/10.2147/NDT.S179453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Chenhao Zhang,1 Yanjing Zang,2 Qin Song,3 Hongxuan Li,1 Lei Hu,1 Weidong Zhao,3 Shanshan Feng,1 Fang Gu,4 FengLi Zhao,1 Chunliang Zhang1
1Department of Neurology, The Second Hospital of Baoding City, Baoding, Hebei 071051, People’s Republic of China; 2Department of Geriatric, The Second Hospital of Baoding City, Baoding, Hebei 071051, People’s Republic of China; 3Second Neurology Department, The Second Hospital of Baoding City, Baoding, Hebei, 071051, People’s Republic of China; 4Fifth Department of Internal Medicine, Baoding Children’s Hospital, Baoding, Hebei 071051, People’s Republic of China
Objective: To explore the efficacy and safety of “cocktail therapy” in Parkinson’s disease with dementia (PDD).
Patients and methods: Sixty patients with PDD were randomly assigned to the test group (n=30) and a control group (n=30). The control group received 10 mg of donepezil hydrochloride tablets orally, once a day. The test group was treated with a “cocktail therapy”, which consisted of 10 mg of donepezil hydrochloride tablets taken orally, once a day; 200 mg of dl-3n-butylphthalide soft capsules taken orally, 15 mins before each meal, three times daily; 800 mg of oxiracetam capsules taken orally, three times daily; and 80 mg of Ginkgo biloba extract tablets taken orally, three times daily. Treatment was administered for six months. The Montreal cognitive assessment scale (MoCA), Blessed-Roth dementia scale, and Clinical Dementia Rating Scale sum of boxes (CDR-SB) were used before treatment and on the third and sixth month after treatment. Adverse events were also monitored.
Results: There were no statistical differences in MoCA, CDR-SB, or Blessed-Roth dementia scale scores between the two groups before treatment. MoCA scores of the test group at six months were significantly higher than those before the treatment and at three months, while both the Blessed-Roth and CDR-SB scores were significantly lower than those before treatment and at three months (p<0.05). Compared with the control group, MoCA scores of the test group were significantly higher, while both the Blessed-Roth and CDR-SB scores were significantly lower (p<0.05), at six months after treatment. There was no statistical difference (p>0.05) between the test group (16.67%) and the control group (13.33%) in the rate of abnormal liver function after treatment.
Conclusion: Treatment with “cocktail therapy” was safe and improved the conditions of patients with PDD, as well as the quality of life of the patients.
Keywords: cocktail therapy, Parkinson’s disease with dementia, efficacy, donepezil hydrochloride, cocktail therapy
Introduction
Parkinson’s disease with dementia (PDD) refers to dementia during Parkinson’s disease (PD), and especially to dementia in the advanced stages of PD. Research shows that 46% of the patients with PD progress to PDD within 10 years of being diagnosed with PD.1,2 PDD seriously reduces patients’ quality of life and increases the burden on caregivers, and there are presently fewer methods to treat PDD and treatments are less effective.3–5 Studies6–9 have reported that butylphthalide significantly improves cognitive function in patients with vascular cognitive impairment with no dementia, with good safety. In addition, butylphthalide is reported to protect mitochondria, improve energy metabolism in the brain, improve microcirculation, and suppress nerve cell apoptosis. Butylphthalide also increased NR2B and synaptophysin expression in the rat hippocampus after chronic cerebral ischemia, and increased acetylcholine levels in the brain and decreased a reduction in learning and memory in a rat model of stroke. On the other hand, oxiracetam reportedly promotes synthesis of phosphorylcholine and phosphoethanolamine, promotes brain metabolism, improves memory, and reduces intellectual decline.10,11 Finally, research12–15 has shown that Ginkgo biloba extract (EGb761) can relieve symptoms in a rat model of PD, improve learning and memory ability in a rat model of dementia, and continuously improve cognitive function in patients with mild cognitive impairment in Parkinson’s disease (PD-MCI) over six months.
Donepezil is one of the cholinesterase inhibitors. According to the Diagnosis and Treatment Guidelines for PDD formulated by PD and Movement Disorders Group, and neuropsychology and behavioral neurology group of the Chinese Medical Association-Neurology Branch in 2011, Donepezil is recommended to treat PDD in China.3
Thus, in the current study, a “cocktail therapy”, consisting of a combination of dl-3n-butylphthalide soft capsules, oxiracetam capsules, Ginkgo biloba extract tablets, and donepezil hydrochloride tablets, was used to treat patients with PDD, and the efficacy and safety of this treatment were observed.
Patients and methods
The study cohort consisted of 60 patients with PDD who were treated in the Second Hospital of Baoding from January 2011 to October 2016. Patient characteristics were as follows: male: 32 cases, female: 28 cases, age: 60–81 years, mean age: 68.6±9.9 years. The patients were randomly divided into test and control groups according to a random number table. Subjects and their families and caregivers, evaluators, and investigators were blinded to the group status of each patient and the experimental drugs. Characteristics of the test group and control group are shown in Table 1. There was no significant difference between the two groups in any of the recorded characteristics. (all p>0.05). This research was approved by the Ethics Committee of the Second Hospital of Baoding, and written informed consent was obtained from patients and their family members.
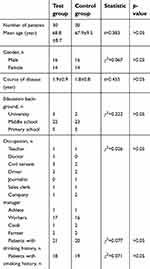 | Table 1 Comparison of baseline data between two groups |
Diagnostic and exclusion criteria for PDD
Diagnostic criteria for PDD3–5
Primary PD was confirmed according to the PD diagnostic criteria of the UK PD Society Brain Bank criteria16 and by the PD diagnostic criteria prepared by PD and dyskinesia groups of the Chinese Medical Association-Neurology Branch.17 Patients needed to have symptoms of hypokinesia and one of: static tremor, myotonia, or posture and gait disturbance. Patients also needed to show sensitivity to levodopa treatment. For a diagnosis of PDD, two further conditions needed to be met: that the cognitive disorder was sufficient to reduce the patient’s daily living ability, and that the cognitive disorder was observed at least one year after PD dyskinesia was evident. The presence of the following behavioral symptoms support the diagnosis of PDD: 1) Emotional or personality changes; 2) Visual hallucination; 3) Excessive daytime sleepiness; 4) Various different types of delusions and hallucinations. The diagnosis was made by the investigators for each subject with DSM-IVR criteria. The cognitive disorder was evaluated based on the DSM-IVR, which includes seven fields: Directive force, memory function, speech function, application function, attention, feeling (visual, hearing, and perception), and execution function.
Exclusion criteria of PDD3–5
There were five possible reasons to exclude patients, as follows: (1) Dementia was caused by other reasons, such as dementia with Lewy bodies and Alzheimer’s disease. Secondary parkinsonism and parkinsonism-plus syndromes were also excluded, as were patients with dementia caused by the following reasons: Systemic disease, drug poisoning, vitamin deficiency, and iatrogenic factors; (2) Disturbance of consciousness, mental diseases, and metabolic diseases; (3) Use of other drugs such as huperzine A, memantine, rivastigmine, piracetam, sedatives, anxiolytics, hypnotics, and cholinergic drugs; (4) Patients who were allergic to celery, oxiracetam, ginkgo leaf, donepezil, or piperidine derivatives; (5) PDD patients with other serious diseases who were expected to be dead within one year.
Randomization and allocation sequence
Participants were enrolled by dementia specialists. According to the diagnostic and exclusion criteria for PDD, 60 patients with PDD were enrolled and then randomly allocated into two groups. Third-party doctors who were not involved in participant enrollment, diagnosis and assessment were responsible for the random grouping and drug delivery. The third-party physicians generated random number sequence using SAS software for the participants with PDD. Then sealed envelopes with the serial number outside, group number and treatment allocations inside were produced, and kept in a locked drawer which was inaccessible to all the researchers. The envelopes were opened sequentially by the third-party physicians after baseline assessments, and participants were assigned to two groups (control group and test group) equally according to the group number printed inside the envelopes. The subjects and their families and caregivers, evaluation personnel, and investigators were blinded to each patient’s group status and the distribution of experimental drugs.
Treatment method
Both two groups were given general treatment for six months, including oral administration of levodopa and benserazide hydrochloride tablets, and pramipexole dihydrochloride, and regulation of blood pressure, blood sugar, and blood fat. Anti-infection and symptomatic treatment were also given to both groups. The control group received 5 mg of donepezil hydrochloride in tablet form (Eisai China Inc., SFDA Approval No. H20050978) orally, once a day for four weeks, and then the dose was increased to 10 mg once a day, for six months. The test group was treated with the “cocktail therapy”, which consisted of 5 mg donepezil hydrochloride in tablet form (Eisai China Inc., SFDA Approval No. H20050978) orally, once a day for four weeks, and then the dose was increased to 10 mg once a day; 0.2 g of dl-3n-butylphthalide in soft capsules (CSPC NBP Pharmaceutical Co., Ltd, approval No.: H20050299) orally, 15 mins before each meal, three times a day; 0.8 g of oxiracetam in capsules (Hunan Jianlang Medicine, batch No.: H20030037) orally, three times a day; 80 mg of Ginkgo biloba extract in tablets (Beaufour Ipsen Industrie, H20030122) orally, three times a day. The course of treatment was six months.
Observation indicators
Patients’ cognitive function was evaluated with The Montreal cognitive assessment scale (MoCA),18 while the severity of dementia was evaluated with Clinical Dementia Rating Scale sum of boxes (CDR-SB) and the patients’ basic living habits, daily living ability, and changes in personality were evaluated using the Blessed-Roth dementia scale. These evaluations were carried out before the treatment and at three and six months after the treatment.
Observation of adverse events
Adverse events over the course of the treatments were monitored. Patients’ 4 mL of empty-stomach venous blood was taken before the treatment and at six months after the treatment. Blood urea nitrogen, creatinine, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were inspected using the oxidation resistance method, and any abnormalities were treated according to their symptoms.
Statistical analysis
Measurement data were described as mean ± standard deviation. The ANOVA with repeated measurement design data was used for assessing changes in multiple follow-up indicators at different time points, and two-sample t-test was used for comparison between the groups in the study. Count data were expressed as rates and absolute numbers, and comparisons between groups were calculated using a χ2 test. Differences were considered statistically significant if p<0.05.
Results
Comparisons of MoCA scores between test and control groups before and after treatment
There was no difference in MoCA scores between the test and control groups before treatment (t=0.292, p>0.05). However, the MoCA scores of the test group at six months after treatment were significantly increased compared with the control group (t=2.539, p=0.015); these scores were also higher than those before the treatment and at three months in the test group (F=5.047, p=0.016). The MoCA scores of the control group at six months were increased compared with before the treatment and at three months, but there was no statistical significance in the difference (F =2.943, p=0.069) (Table 2).
 | Table 2 Comparison of MoCA scores in the test and control groups before and after treatment (mean ± SD) |
At the different time after treatment, the scores for the different components of MoCA in the test group were significantly increased compared with the control group (F =4.311, P=0.032), the scores for the different components of MoCA after treatment were significantly increased than before treatment in the test group (F=5.719, P=0.004). The improvement in visual-spatial ability and executive function (F=4.093, p=0.037), naming (F=4.325, p=0.031), attention (F=3.597, p=0.044), language (F=5.528, p=0.011),abstract ability (F=3.487, p=0.046),delayed memory (F=5.241, p=0.013) and orientation (F=3.4498, p=0.045) were more obvious in the test group (Table 3).
 | Table 3 Comparison of different components of MoCA in the test and control groups before and after treatment (mean ± SD) |
Comparisons of blessed-roth scores between test and control groups before and after treatment
There was no difference in Blessed-Roth scores between the test and control groups before treatment (t=0.128, p>0.05). The Blessed-Roth scores of the test group at six months were significantly decreased compared with the control group (t=2.601, p=0.013); they were also lower than those before the treatment and at three months in the test group (F =3.713, p=0.042). The Blessed-Roth scores of the control group at six months were decreased compared with before the treatment and at three months, but there was no statistical significance in the difference (F=2.876, p=0.074) (Table 4).
 | Table 4 Comparison of Blessed-Roth scores in the test and control groups before and after treatment (mean ± SD) |
Comparisons of CDR-SB scores between test and control groups before and after treatment
There was no difference in CDR-SB scores between the test and control groups before treatment (t=0.092, p>0.05). The CDR-SB scores of the test group at six months were significantly decreased compared with the control group (t=2.421, p=0.017); they were also lower than those before the treatment and at three months in the test group (F=3.591, p=0.045). The CDR-SB scores of the control group at six months were decreased compared with before the treatment and at three months, but there was no statistical significance in the difference (F=2.517, p=0.083) (Table 5).
 | Table 5 Comparison of CDR-SB scores in the test and control groups before and after treatment (mean ± SD) |
Safety analysis
Before treatment, there was one patient with abnormal liver function in each of the two groups. Over the course of the treatment, patients in the two groups showed stable vital signs, with no adverse events in the digestive system, nervous system, renal function, or with allergies. In the test group, there were four patients with elevated ALT levels and one patient with elevated AST levels; all of them had levels of less than 80 U/L. In the control group, there were two patients with elevated ALT and two patients with elevated AST; both of them had levels of less than 80 U/L. The liver protection therapy (by the administration of vitamins and glucuronolactone) was given and these levels returned to normal. There were no significant differences (p>0.05) between the results of AST and ALT in the test group (16.67%) and those of the control group (13.33%) (Table 6).
 | Table 6 Comparison of cases with abnormal liver function in the two groups before treatment |
Discussion
PD is a common degenerative disease in the central nervous system, and patients with PD may present with dementia to different degrees. PDD in the later stages can seriously affect a patient’s cognitive function and self-care ability, which in turn significantly reduces the patient’s quality of life and increases the burden placed on society and the patient’s family. This study showed that, before treatment, the MoCA scores of PDD patients were significantly decreased while the Blessed-Roth dementia scale, and CDR-SB scores were significantly increased. These findings were consistent with the results from previous investigations.1,2 In the current study, a “cocktail therapy”, consisting of dl-3n-butylphthalide, oxiracetam, Ginkgo biloba extract, and donepezil hydrochloride, was used to treat patients with PDD. The efficacy and safety of this “cocktail therapy” treatment was also observed, and it was found to be safe, which is consistent with previous research.6
Previous studies19–22 have shown that the impairment in cognition in PD is related to many factors, including a reduction in brain tissue perfusion, destruction of the cholinergic system, reduction in the brain’s metabolic rate, structural impairment of brain tissue, toxicity of excitatory amino acids, and oxidative stress. Winklhofer et al,21,22 found that mitochondrial dysfunction plays a core role in the pathogenesis of PD, and that functional lesions in mitochondria could cause cellular damage, aging, and neurodegenerative disease.
In this study, at six months after treatment with the “cocktail therapy”, MoCA scores were obviously improved compared with both pretreatment and control scores. These results show that cognitive function in PPD patients can be improved, and that the degree of dementia can be relieved over time. They also show that our “cocktail therapy” gives better results than treatment with just donepezil hydrochloride. We also showed that “cocktail therapy” treatment resulted in a decrease in both Blessed-Roth and CDR-SB scores compared with pretreatment scores, and that at six months after treatment, scores were also obviously decreased in these patients compared with those in the control group. These results show that the quality of life of the patients with PDD was improved over the course of the therapy, and that our “cocktail therapy” was more effective than treatment with just donepezil hydrochloride. The reason for this improvement with a combination of therapies may be related to the fact that: multiple studies6–9 have demonstrated that by triggering various pathways, dl-3n-butylphthalide seems to interrupt the development of physiopathological processes that lead to cerebral damage, increase the number of blood capillaries and reconstruct microcirculation in the ischemic region, and improve brain perfusion. Following ischemia, dl-3n-butylphthalide has also been shown to protect mitochondria and improve energy metabolism, suppress the release of glutamic acid and the generation of free radicals, reduce apoptosis, reduce the degree of damage to neurological function, increase the synthesis of acetylcholinesterase, and improve acetylcholine levels in the brain, so that learning and memory functions can improve.
Oxiracetam is a nootropic drug; it can increase the release of acetylcholine by promoting synthesis of phosphorylcholine and phosphorylethanolamine, stimulate specific central nervous pathways through the blood–brain barrier, promote synthesis of triphosadenine, increase the synthesis of proteins and nucleic acids in the brain, and improve the memory and learning of patients with memory and intelligence disturbances.10,11
The main components of the Gingko biloba extract that was used in this study, EGb761, are total flavonoid glycoside and bilobalide. These components may treat PD-related cognitive disorders through strengthening the brain’s oxygen supply, protecting ATP enzymatic activity of the nerve cell, scavenging free radicals and lipid peroxidase, preventing the accumulation of peroxide, suppressing the release of excitatory neurotransmitters, and protecting dopaminergic neurons, among other functions. Moreover, EGb761 can promote the secretion of neurotrophic factors, accelerate repair and proliferation of nerve cells, prevent neuron damage, improve learning and memory in a rat model of dementia, and improve the cognitive function of patients with PD-MCI.12–15
Table 2 shows that the MoCA scores of the control group at six months were increased compared with before the treatment and at three months, and Tables 4 and 5 show that the Blessed-Roth and CDR-SB scores of the control group at six months were decreased compared with before the treatment and at three months, but all of them were no statistical significance in the difference (P>0.05). However, in the test group, the MoCA scores of PDD patients were increased while the Blessed-Roth dementia scale and CDR-SB scores were decreased at three months, and they were particularly obvious at six months (P<0.05). Table 3 shows that the scores for the different components of MoCA in the test group were significantly increased compared with the control group after treatment. Visual-spatial ability, executive function, naming, attention, delayed memory, and orientation had improved more obvious than language and abstraction. This shows that the effect of the “cocktail therapy” was more effective than treatment with just donepezil hydrochloride. The Blessed-Roth dementia scale allows the assessment of social activity ability and self-care ability in daily life, so the improvement in the change of the Blessed-Roth scores in the test group at six months indicates that cocktail therapy seemed to reduce the motor symptoms of PD.
In addition to cognitive impairment, patients with PDD also have a variety of psychobehavioral symptoms, including hallucinations, illusions, delusions, mood changes (such as depression), apathy, REM sleep disorders, among which visual hallucinations and illusions are more common. Mood changes, in particular depression, are also common with dementia. Depression can affect cognitive performance. Patients with PDD who have depression should be given antidepressant treatment before evaluating their cognitive function.23,24 However, the association between mood states and cognitive function in patients with PDD after the treatment need to be further investigated.
Table 6 shows that there was no significant difference between the results of AST and ALT in the test group (16.67%) and the control group (13.33%), There were no adverse events in the digestive system, nervous system, renal function, or with allergies. The results indicate that the “cocktail therapy” was safety, which was consistent with the findings of the previous study.6 We considered that this may be related to the following reasons: 1) Some of the side effects in patients with dementia are often poorly recognized, which may look similar to the symptoms of PDD. 2) Virtually all patients with PD need medications, such as levodopa and benserazide hydrochloride to relieve their symptoms,25 and some of the side effects may be similar to those of levodopa and benserazide hydrochloride. 3) There may be a potential mechanism that can counteract any potential adverse effects of the drugs, which need further investigation.
The reason for this improvement with a combination of therapies may be related to the fact that butylphthalide, oxiracetam, and Ginkgo biloba extract can improve microcirculation in the brain, improve the use of glucose and oxygen in brain tissue, promote synthesis of triphosadenine and energy metabolism in brain cells, resist oxidative stress, protect nerve cells, and strengthen learning and memory, among other functions. These effects may be behind the improvement in cognitive function and self-care ability that we observed in patients with PDD following “cocktail therapy”.
This research shows that a “cocktail therapy” consisting of dl-3n-butylphthalide, oxiracetam, Ginkgo biloba extract, and donepezil hydrochloride, can be successfully used to treat patients with PDD. This treatment helped to improve patients’ symptoms and quality of life, with good safety. However, the sample size in this study is small and the observation time is relatively short; therefore, to verify the accuracy of these results, a trial of this “cocktail therapy” needs to be carried out using a larger sample size, longer time period, and multiple centers.
Abbreviation list
PDD, Parkinson’s disease with dementia; PD, Parkinson’s disease; MoCA, Montreal cognitive assessment scale; CDR-SB, Clinical Dementia Rating Scale sum of boxes; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PD-MCI, mild cognitive impairment in Parkinson’s disease.
Acknowledgments
The authors thank the patients and their families for their participation. The authors also thank the third-party doctors: physician Lan Yu and physician Liu Liu.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson‘s patients in the UK. The CamPaIGN study. Brain. 2004;127:550–560. doi:10.1093/brain/awh067
2. Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol. 2012;25(4):208–214. doi:10.1177/0891988712464823
3. PD and dyskinesia groups of Chinese medical association-neurology branch, neuropsychology and behavioural neurology groups of Chinese medical association-neurology branch. Guidance for diagnosis and treatment of PDD. Zhonghuashenjingkezazi. 2011;44(9):635–637.
4. Emre M, Tsolaki M, Bonuccelli U, et al. 11018 study investigators. Memantine for patients with Parkinson‘s disease dementia or dementia with lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(10):969–977. doi:10.1016/S1474-4422(10)70194-0
5. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson‘s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314–2324. doi:10.1002/mds.21844
6. Jia J, Wei C, Liang J, et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement. 2016;12(2):89–99. doi:10.1016/j.jalz.2015.04.010
7. Guo T, Shen RL, Wu YZ, Teng JF. The effect of dl-butylphthalide on NR2B and synaptophysin in hippocampus of aged rats after chronic cerebral hypoperfusion. Zhongguo Shiyong Shenjingjibing Zazhi. 2007;10:60–62.
8. Peng Y, Sun J, Hon S, et al. L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer‘s disease. J Neurosci. 2010;30(24):8180–8189. doi:10.1523/JNEUROSCI.0340-10.2010
9. Hu D, Zhang LY, Feng YP. Effect of dl-3-n-butylphthalide on memory disturbance induced by focal cerebral ischemia in rats. Zhongguo Yaoxue yu Dulixue Zazhi. 1997;11:14–16.
10. Guo YX. The efficacy observation for combination of oxiracetam and nimodipine in treating vascular dementia. Dangdai Yixue. 2013;19(2):136–137.
11. Xu YS. The clinical research for combination of nimodipine and oxiracetam in treating vascular dementia. Zhongguo Yixue Gongcheng. 2014;22(2):160–162.
12. Rojas P, Montes P, Rojas C, Serrano-García N, Rojas-Castañeda JC. Effect of a phytopharmaceutical medicine, Ginko biloba extract 761, in an animal model of Parkinson‘s disease: therapeutic perspectives. Nutrition. 2012;28(11–12):1081–1088. doi:10.1016/j.nut.2012.03.007
13. Zhang C, Feng J, Li HJ, Wang JQ, Ye JQ, Wang XJ. The efficacy observation for ginkgo biloba extract in treating mild cognitive impairment of PD. Zhongguo Xiandai Yisheng. 2013;51(31):109–111.
14. Zeng CM, Zhao W, Ding N, Zeng CQ. The research progress for action mechanism of ginkgo biloba extract in treating neurodegenerative disease. Zhongguo Heli Yongyao Tansuo. 2013;10(8):21–23.
15. Feng JJ, Li YL, Hou C, Li LB, Zhang QJ. The protection function of ginkgo biloba extract to dopaminergic neuron of the model rat with PD. Xi‘An Jiaotong Daxue Xuebao Yixueban. 2011;32(6):783–786.
16. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson‘s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184.
17. Dyskinesia and PD groups of Chinese medical association-neurology branch. Diagnosis of PD. Zhonghuashenjingkezazi. 2006;39(6):408–409.
18. Chen L, Liu WG, Zhao YY, Hua P, Wang Y, Zhang N. Application value of MoCA in treating the patients with PD cognitive disorde. Zhonghuashenjingkezazi. 2011;44(3):200–202.
19. Zhao MM, Chen WX. Epidemiology and hazard factors of PDD. Jiangsu Yiyao. 2013;39(6):710–712.
20. Liu JA. Research progress for mild cognitive impairment of PD. Jiefangjun Yixueyuan Xuebao. 2014;35(2):199.
21. Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson‘s disease. Biochim Biophys Acta. 2010;1802(1):29–44. doi:10.1016/j.bbadis.2009.08.013
22. Yang ZM, Li XZ, Lu F, Liu SM. The research progress for mitochondria pathogenesis of PD. Zhongguo Laonianxue Zazhi. 2014;34(21):6233–6235.
23. Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson‘s disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492–496.
24. Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with lewy bodies and Parkinson‘s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16(5):528–536.
25. Poewe W, Gauthier S, Aarsland D, et al. Diagnosis and management of Parkinson‘s disease dementia. Int J Clin Pract. 2008;62(10):1581–1587. doi:10.1111/j.1742-1241.2008.01869.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
