Back to Journals » Cancer Management and Research » Volume 13
The Efficacy and Safety of the Shouzu Ning Decoction Treatment for Multi-Kinase Inhibitors-Associated Severe Hand–Foot Skin Reaction
Authors Shou L, Shao T, Zhao F, Chen S, Chen Q, Shu Q
Received 2 October 2020
Accepted for publication 16 December 2020
Published 7 January 2021 Volume 2021:13 Pages 45—53
DOI https://doi.org/10.2147/CMAR.S285002
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Liumei Shou,1,2,* Tianyu Shao,2,* Fangmin Zhao,2 Shuyi Chen,1 Qunwei Chen,1 Qijin Shu1
1Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2The First Clinical Medicine College, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qijin Shu
Department of Oncology, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310006, People’s Republic of China
Tel +86-13605706566
Fax +86-571-6707-2196
Email [email protected]
Background: Multi-kinase inhibitors (MKIs) treatment plays an important role in cancer therapy, but still suffers from a high incidence of hand–foot skin reaction (HFSR), leading to MKIs dose modification or termination. Thus, there is a high need for therapeutic strategy for HFSR.
Patients and Methods: This prospective analysis included twenty patients, who were continuously administered with MKIs treatment and presented with a grade 3 HFSR during January 2018 to December 2019. All the patients were treated with the Shouzu Ning Decoction (SND) twice a day, in addition to the MKIs treatment. Grading of HFSR was assessed by National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Pain intensity was evaluated using the numerical rating scale (NRS). Quality of life was assessed using the Hand–Foot Quality of Life Scale (HF-QoLS).
Results: The median time from MKIs initiation to onset of grade 3 HFSR was 26.2 days. Following the SND treatment, seventeen (17/20) patients displayed grade 2 HFSR with a median time of 5.1 days. Among whom, seven (7/17) finally transformed to grade 1 with a median time of 9.9 days. While all of the grade 1 patients (7/7) had local recurrence, and retreatment of the SND was effective. In addition, after the SND treatment, the score of NRS and HF-QoLS decreased to 1.60 ± 1.14 (P < 0.01) and 26.75 ± 11.76 (P < 0.01), respectively.
Conclusion: The SND treatment could alleviate symptoms, relieve pain and improve quality of life in HFSR patients. The SND treatment was proved to be an effective and well-tolerated treatment for MKIs-associated grade 3 HFSR patients for the first time. Indeed, further randomized controlled trails with large-scale, multi-center are require to fully determine the clinical application of the SND in MKIs-associated HFSR.
Keywords: hand-foot skin reaction, multi-kinase inhibitors, Shouzu Ning Decoction, efficacy, safety
Introduction
Multi-kinase inhibitors (MKIs) targeting specific tyrosine kinases have become one of the most promising classes of cancer-fighting agents.1 Currently, more than twenty MKIs have been approved by the US Food and Drug Administration for the treatment of advanced solid tumors.2 Some of them have been recommended as standard treatments for certain clinical indications3–5 [eg sorafenib in hepatocellular carcinoma, regorafenib in colorectal cancer, apatinib in gastric cancer, and anlotinib in non-small cell lung cancer].6–8 However, despite their clinical success, MKIs are prone to induce significant dermatologic adverse events.9,10
Hand–foot skin reaction (HFSR) is the most common dermatologic event developed in patients treated with MKIs.11,12 The incidence of HFSR ranges from 24.5% to 44.7% in various clinical trials.13–15 In all published Phase III trials, patients with grade ≥2 HFSR required dose modifications or treatment discontinuation.16,17 Although some drugs, such as topical urea-containing moisturizing cream, steroids, and celecoxib, have been suggested to reduce the incidence and severity of HFSR,18,19 their effectiveness were far from satisfactory. Dose modification or termination is a compromising strategy recommended in clinic treatment,2 but usually lead to disease progression. There remains an urgent need for effective therapeutic options to treat HFSR.
Si-Miao-Yong-An decoction (SMYAD), a traditional Chinese medicine formula, was used for centuries to treat gangrene patients via eliminating heat and promoting blood circulation, according to the traditional Chinese medicine theory.20,21 Recently, the SMYAD were reported to be effective in relieving pressure ulcers, diabetic ulcers and venous stasis ulcers.22–24 These symptoms are some kind of similar to those of HFSR, which presenting with peeling, blister, bleeding, edema, or hyperkeratosis in the palms and soles.2 We previously observed that the SMYAD could partially suppress the development of HFSR, but the results were still far from satisfactory.
Shouzu Ning Decoction (SND) was empirically modified from the SMYAD, and was used to treat HFSR for the first time in clinical applications by our team. Our clinical observation showed that the remission rate for HFSR symptoms was significantly higher in the SND group compared to the SMYAD group (unpublished data). Meanwhile, no studies focused on the treatment of severe HFSR have been reported till now. In this study, 20 severe HFSR patients who previously received various treatments (steroids, celecoxib, and urea cream) were treated on a novel schedule by using the SND treatment. Our results showed that the SND treatment could alleviate symptoms, relieve pain and improve quality of life in the grade 3 HFSR patients.
Methods
Study Design
We prospectively collected data from all patients who received MKIs therapy at the First Affiliated Hospital of Zhejiang Chinese Medical University and developed grade 3 HFSR during January 2018 to December 2019. Patients must meet all of the following criteria: i) at least 18 years old, ii) were continuously administered with MKIs treatment and presented with a grade 3 HFSR; iii) experienced failure of treatments such as topical urea-containing moisturizing cream, steroids, or NSAIDs. Patients were excluded if they had preexisting skin lesions, including peripheral neuropathy caused by diabetes or chemotherapy, hand and foot syndrome, hand and foot fungal infection, skin trauma. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang Chinese Medical University and conducted in accordance with the principles of Helsinki Declaration. Written informed consent for both study participation and publication of identifying information/images was obtained from all patients.
Patient Characteristics
For included patients, charts were further reviewed for demographic characteristics, tumor histology and stage, prior history of HFSR or HFS, and types of MKIs in use. Demographic characteristics included age and sex. The histology consisted of hepatocellular carcinoma, lung cancer, gastrointestinal cancer, esophageal cancer, and renal cancers. The tumor stage was determined according to the American Joint Committee on Cancer’s Cancer Staging Manual, 8th edition. Use of other chemotherapeutic agents that were reported to cause HFS was recorded (ie, capecitabine, epirubicin, or docetaxel).
HFSR
The degree of HFSR was graded from 1 to 3 in accordance with the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0 (shown in Table 1). Relevant clinical data of HFSR, including skin changes, dermatitis, pain, and activities of daily living (ADL) were collected. Photos of palms and soles were recorded for clinical purposes. Pain intensity was evaluated using the numerical rating scale (NRS) from 0 (no pain) to 10 (most severe pain). Patients’ HFS-related restrictions in daily activities were assessed using the Hand-Foot Quality of Life Scale (HF-QoLS).16,25 HF-QoLS consists of four subscales: physical (8 items), self-care (3 items), social (3 items), and psychological (4 items). Each item is scored on a scale of 0–4, with a higher score indicating greater difficulty in daily activities affecting HFSR-related quality of life. The timing of HFSR relative to MKIs treatment initiation was assessed. All patients had experienced treatment failure of topical urea-containing moisturizing cream, steroids, or NSAIDs.
 |
Table 1 NCI-CTCAE Version 5.0 Grading of HFSR |
Intervention
SND herbs (150 g) were consisted of 30 g Lonicerae japonicae Flos (Jin Yin Hua), 30 g Angelicae sinensis Radix (Dang Gui), 30 g Lonicerae japonicae Caulis (Ren Dong Teng), 30 g Spatholobi Caulis (Ji Xue Teng), and 30 g Glycyrrhizae Radix et Rhizoma (Gan Cao). All of them were purchased from the Zhejiang Chinese Medical University (Zhejiang, China), and were further authenticated by pharmacologist, Professor Jingxia Wang. A total of 150 g SND herbs were decocted twice with eightfold of water for 1 h. The extracts were combined, filtered, and then concentrated to a volume of 200 mL at 100 °C. The extracts were named as SND. SND used in this study were analyzed by a validated reversed-phase HPLC system (Thermo Dionex Ultimate 3000 HPLC system, Thermo Fisher Scientific, Waltham, MA, USA) using an Agilent Zorbax SB C18 column (4.6×150 mm, 5µm). A representative HPLC chromatogram of the SND was shown in Figure 1.
 |
Figure 1 HPLC analysis of the Shouzu Ning Decoction (SND) |
Administration method: The patients were treated with the SND twice a day. For each treatments, 200 mL SND was diluted with water to a final volume of 1000 mL. Temperature was kept at 35 °C to 40 °C in a thermostatic bath. Then the hands and feet of the patients were soaked in the bath for 20 minutes.
Statistical Analysis
Standard descriptive and analytical methods were used to describe the baseline characteristics, symptom characteristics, and clinical outcomes of the patients. Data analyses were performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). A p < 0.05 (two-tailed) was considered as statistically significant.
Results
Patient Characteristics
Twenty-two patients who developed grade 3 HFSR during MKIs therapy were enrolled in this study. Two patients who had discontinued MKIs treatment after the disease progression dropped out. The remaining twenty patients were included in the analysis. The patient characteristics were shown in Table 2. Patient age ranged from 18 to 76 years, with a median age of 58 years. The male to female ratio was 15:5. Among these twenty patients, six patients were on stage III and fourteen patients were on stage Ⅳ. The most common types of cancers were hepatocellular carcinoma (50%), followed by lung cancer (20%) and gastric cancer (10%). The types of MKIs included sorafenib (n = 8 patients), anlotinib (n = 4 patients), apatinib (n = 3 patients), lenvatinib (n = 3 patients), and regorafenib (n = 2 patients). Two patients and one patient had a history of grade ≥2 HFS/HFSR caused by capecitabine and sorafenib, respectively.
 |
Table 2 Patient Characteristics(n=20) |
Diagnosis, Management, and Evaluation of MKIs-associatedInd HFSR
The median time from MKIs initiation to onset of grade 3 HFSR was 26.2 days (range 8–60 days) (Table 3). The patients were initially diagnosed with HFSR and treated in accordance with the international recommendations, including topical urea-containing moisturizing cream, steroids, or NSAIDs (Table 3), but had no clinical benefits which prompted the use of SND.
 |
Table 3 MKIs-associated HFSR Characteristics and the SND Treatment |
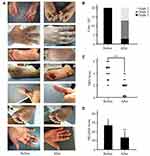
Prior to the SND treatment, all the patients presented with grade 3 HFSR according to NCI-CTCAE, version 5.0. The presenting symptoms of HFSR in patients: hyperkeratosis (17 of 20, 85%), erythema (13 of 20, 65%), edema (6 of 20, 30%), blisters (6 of 20, 30%), peeling (3 of 20, 15%), and bleeding (1of 20, 5%), hand and foot pain (20 of 20, 100%), limiting instrumental ADL (20 of 20, 100%) (Table 3).
SND Treatment
The patients were continuously treated with the SND at a dose of 200 mL twice daily in addition to the treatment of MKIs until the clinical regression of grade 1 was observed. If the symptoms of HFSR were not relieved after two weeks of treatment, we also terminated the administration of both MKIs and SND. Meanwhile, MKIs dosage was not reduced during the combination treatment. Symptom remission was observed as early as 2 days after the treatment in patient NO. 18. Most of the patients (17/20) displayed grade 2. The median time from the starting of the SND treatment to HFSR symptom remission was 5.1 days (ranging from 3 days to 14 days). Among whom, seven patients (7/17) finally transformed to grade 1 with a median time of 9.9 days . As already known, patients who reach grade 1 HFSR could continue MKIs treatment at the same level as the initial dose, and those who reach grade 2 HFSR may need a dose reduction. While all of the grade 1 patients (7/7) had local recurrence. Retreatment of the SND was effective, which indicated that interruption of the SND treatment did not result in drug resistance.
Intriguingly, the results showed that the effect of the SND differed by symptoms of HFSR. Erythema and edema were completely remitted in all patients, whereas peeling, blister, and bleeding were partially reduced. Unfortunately, the symptoms of patient No. 3, 12, 14 characterized by severe hyper-keratinization in hands and feet were not alleviated after continuously treated with the SND for 2 weeks and the desired effect of the SND was not achieved. In addition, no side effects related to the SND treatment were observed (Table 3) except that the skin color changed to light brown. Skin discoloration disappeared within one week and did not need any treatment.
Numerical rating scale (NRS) of pain and HF-QoLS of daily activity subscale were taken in all patients before and after the SND treatment. Median NRS score and HF-QoLS score were 4.30 ± 1.26 and 53.80 ± 4.16 at the onset of grade 3 HFSR, respectively. After the SND treatment, the score of NRS and HF-QoLS decreased to 1.60 ± 1.14 (P < 0.01) and 26.75 ± 11.76 (P < 0.01), respectively (Figure 2). Our results indicated that the SND treatment could decrease pain and improve quality of life in patients with HFSR. We also compared baseline characteristics between the 15% of patients who did not respond to the SND to the 85% of patients who were treated effectively. No significant difference were observed between groups (Table 4).
 |
Table 4 Comparison of Patient Characteristics in Effective and Non-effective Samples |
Discussion
MKIs are a promising cancer therapeutic strategy, but usually lead to a high incidence of HFSR, which limits their clinical application.2 To date, only prevention measures have been reported to reduce the severity of HFSR.26 Once severe HFSR occurs, dose modification or termination are taken for a comprising therapy, which may lead to tumor progression. At present, evidence from clinical trials and real-world experience determines the interventions to manage HFSR. In a randomized, open-label trial, advanced hepatocellular carcinoma (HCC) patients with initial sorafenib treatment were co-treated with 10% urea-based cream (UBC) plus best supportive care (BSC) or BSC alone. The results showed that the prophylactic use of UBC could reduce the incidence of HFSR and prolong the time of first onset.27 In addition, there was another similar trial using combined oral nutritional supplement and sorafenib in patients with HCC, the results suggested that prophylactic HMB, L-arginine and L-glutamine supplementation effectively prevented sorafenib-associated HFSR in patients with advanced HCC.28 However, these studies only provided some prophylaxis strategies but not the treatment strategy of HFSR.
Previous studies showed that modified Taohongsiwu decoction and Compound Danxiong Granules (CDG) could effectively attenuate MKIs-associated HFSR.29,30 However, previous topical use of Chinese herbal was applicable to all-grade HFSR especially grade 1 and 2.31 In this study, we reported the effectiveness of our strategy for grade 3 HFSR. Topical urea-containing moisturizing cream, steroids, and NSAIDs were applied in accordance with the international recommendations, which were not feasible in these patients. Whereas MKIs therapy was a long-term treatment to control the disease, and these patients thus represented a group of difficult-to-treat cases. We found that administration of two weeks of SND was sufficient to achieve the remission. The median duration of response was less than 5 days from initiation of the SND treatment. It is less likely that the severe HFSR could be eliminated without additional novel therapeutic strategies. Alternatively, SND could be chosen to suppress MKIs-associated HFSR and extend the therapeutic window of SND.
In this study, erythema and edema in hands and feet almost disappeared in the patients, hyper-keratinization were partially relieved. As illustrated by patient No. 3, 12, 14, with severe hyper-keratinization, the SND treatment was failed to release the symptoms, indicating the SND treatment might not suitable for patients with hyper-keratinization. It has been reported that HFSR-related hand and foot symptom significantly restricted patients’ daily activities, including barriers to social participation, lack of willingness to work and continue treatment.16,25 In addition to alleviating the symptoms, the SND treatment led to a significant lower NRS score and improved quality of life of patients. We believed that the SND treatment was an appropriate choice for most of the patients with MKIs-associated HFSR. Another situation where prophylactic treatment with the SND could be suggested.
Despite the strengths, there were several limitations of this study. First, the lack of a control group limited the ability to separate the effect of SND. Second, the sample size in this study was small. Therefore, the concept of the SND treatment in patients with HFSR should be explored in further larger scale studies. Last, the lack of biomarkers of HFSR makes the mechanistic inside remains to be explored.
Conclusions
This is a study to suggest that the SND treatment was an effective and well-tolerated treatment for MKIs-associated grade 3 HFSR patients for the first time. The results of this study showed that the SND treatment could alleviate symptoms, relieve pain and improve quality of life in HFSR patients. In addition, randomized controlled trails with large-scale, multi-center would fully determine the clinical application of the SND in MKIs-associated HFSR.
Acknowledgment
The study was supported by the Key Pilot Program of Integrative Chinese and Western Medicine for Refractoriness Disease (Gastric Cancer) and Project of Administration of Traditional Chinese Medicine of Zhejiang Province of China (No. 2020ZB076).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi:10.1634/theoncologist.2008-0131
2. Chanprapaph K, Rutnin S, Vachiramon V. Multikinase inhibitor-induced hand-foot skin reaction: a review of clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2016;17:387–402. doi:10.1007/s40257-016-0197-1
3. Hulin A, Stocco J, Bouattour M. Clinical pharmacokinetics and pharmacodynamics of transarterial chemoembolization and targeted therapies in hepatocellular carcinoma. Clin Pharmacokinet. 2019;58:983–1014. doi:10.1007/s40262-019-00740-w
4. Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, Phase 3 trial. Lancet. 2013;381:303–312. doi:10.1016/S0140-6736(12)61900-X
5. Abrahao ABK, Ko YJ, Berry S, Chan KKWA. Comparison of regorafenib and TAS-102 for metastatic colorectal cancer: a systematic review and network meta-analysis. Clin Colorectal Cancer. 2018;17:113–120. doi:10.1016/j.clcc.2017.10.016
6. Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78:747–758. doi:10.1007/s40265-018-0903-9
7. Chen R, Chen QT, Dong YH. Clinical efficacy of apatinib in treating metastatic gastric cancer and its effect on IL-17. Oncol Lett. 2019;17:5447–5452. doi:10.3892/ol.2019.10270
8. Si X, Zhang L, Wang H, et al. Quality of life results from a randomized, double-blinded, placebo-controlled, multi-center phase III trial of anlotinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2018;122:32–37. doi:10.1016/j.lungcan.2018.05.013
9. Robert C, Soria J-C, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi:10.1016/S1470-2045(05)70243-6
10. Kuzuya T, Ishigami M, Ito T, et al. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol Res. 2019;49(9):1054–1065. doi:10.1111/hepr.13358
11. Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology. 2009;77:257–271. doi:10.1159/000258880
12. Granito A, Marinelli S, Negrini G, et al. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Therap Adv Gastroenterol. 2016;9(2):240–249. doi:10.1177/1756283X15618129
13. Nonomiya Y, Yokokawa T, Kawakami K, et al. Regorafenib-induced hand-foot skin reaction is more severe on the feet than on the hands. Oncol Res. 2019;27:551–556. doi:10.3727/096504018X15291727589740
14. Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs. 2015;33(3):740–750. doi:10.1007/s10637-014-0154-x
15. Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi:10.1080/02841860701765675
16. Anderson RT, Keating KN, Doll HA, Camacho F. The hand-foot skin reaction and quality of life questionnaire: an assessment tool for oncology. Oncologist. 2015;20:831–838. doi:10.1634/theoncologist.2014-0219
17. Wang E, Xia D, Bai W, et al. Hand-foot-skin reaction of grade >/= 2 within sixty days as the optimal clinical marker best help predict survival in sorafenib therapy for HCC. Invest New Drugs. 2019;37:401–414. doi:10.1007/s10637-018-0640-7
18. Moreno RA. [Curettage and gingivectomy in periodontal therapy. Histological clinical tests]. Rev Fed Odontol Colomb. 1974;22:83–88.
19. Fukuoka S, Shitara K, Noguchi M, et al. Prophylactic use of oral dexamethasone to alleviate fatigue during regorafenib treatment for patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2017;16:e39–e44. doi:10.1016/j.clcc.2016.07.012
20. Su C, Wang Q, Zhang H, et al. Si-Miao-Yong-An Decoction protects against cardiac hypertrophy and dysfunction by inhibiting platelet aggregation and activation. Front Pharmacol. 2019;10:990. doi:10.3389/fphar.2019.00990
21. Ren Y, Chen X, Li P, et al. Si-Miao-Yong-An decoction ameliorates cardiac function through restoring the equilibrium of SOD and NOX2 in heart failure mice. Pharmacol Res. 2019;146:104318. doi:10.1016/j.phrs.2019.104318
22. Wang L, Wang N, Tan HY, Zhang Y, Feng Y. Protective effect of a Chinese Medicine formula He-Ying-Qing-Re Formula on diabetic retinopathy. J Ethnopharmacol. 2015;169:295–304. doi:10.1016/j.jep.2015.04.031
23. Zhang C, Xu Y, Tan H-Y, et al. Neuroprotective effect of He-Ying-Qing-Re formula on retinal ganglion cell in diabetic retinopathy. J Ethnopharmacol. 2018;214:179–189. doi:10.1016/j.jep.2017.12.018
24. Shi S, Liu Z, Xue Z, Chen X, Chu Y. A plasma metabonomics study on the therapeutic effects of the Si-miao-yong-an decoction in hyperlipidemic rats. J Ethnopharmacol. 2020;256:112780. doi:10.1016/j.jep.2020.112780
25. Hsu YH, Shen WC, Wang CH, Lin YF, Chen SC. Hand-foot syndrome and its impact on daily activities in breast cancer patients receiving docetaxel-based chemotherapy. Eur J Oncol Nurs. 2019;43:101670. doi:10.1016/j.ejon.2019.09.011
26. Ai L, Xu Z, Yang B, He Q, Luo P. Sorafenib-associated hand-foot skin reaction: practical advice on diagnosis, mechanism, prevention, and management. Expert Rev Clin Pharmacol. 2019;12:1121–1127. doi:10.1080/17512433.2019.1689122
27. Ren Z, Zhu K, Kang H, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894–900. doi:10.1200/JCO.2013.52.9651
28. Naganuma A, Hoshino T, Ohno N, et al. β-hydroxy-β-methyl butyrate/L-arginine/L-glutamine supplementation for preventing hand–foot skin reaction in sorafenib for advanced hepatocellular carcinoma. In Vivo (Brooklyn). 2019;33:155–161. doi:10.21873/invivo.11452
29. Chen J, Yu B, Wu X, et al. Effect of modified Taohongsiwu decoction on patients with chemotherapy-induced hand-foot syndrome. J Tradit Chin Med. 2014;34:10–14. doi:10.1016/s0254-6272(14)60047-9
30. Tian A, Zhou A, Bi X, et al. Efficacy of topical compound danxiong granules for treatment of dermatologic toxicities induced by targeted anticancer therapy: a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2017;2017:3970601. doi:10.1155/2017/3970601
31. Wang G, Jia L, Pei Y, et al. Clinical study for external Chinese herbal medicine LC09 treating hand-foot skin reaction associated with the antitumor targeted drugs: protocol for a prospective, randomized, controlled, double-blind, and monocentric clinical trial. Medicine (Baltimore. 2020;99(4):e18849. doi:10.1097/MD.0000000000018849
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.