Back to Journals » Drug Design, Development and Therapy » Volume 15
The Efficacy and Safety of Remimazolam Tosilate versus Etomidate-Propofol in Elderly Outpatients Undergoing Colonoscopy: A Prospective, Randomized, Single-Blind, Non-Inferiority Trial
Authors Liu X, Ding B, Shi F, Zhang Y, Liu L, Sha Y, Zhao T
Received 15 September 2021
Accepted for publication 7 November 2021
Published 16 November 2021 Volume 2021:15 Pages 4675—4685
DOI https://doi.org/10.2147/DDDT.S339535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Xianwen Liu,1 Baofeng Ding,2 Fu Shi,1 Yang Zhang,1 Lei Liu,1 Yongwei Sha,3 Tonghang Zhao1
1Department of Anaesthesiology, Liaocheng People’s Hospital, Liaocheng, Shandong, People’s Republic of China; 2Department of Anaesthesiology, Liaocheng Second People’s Hospital, Liaocheng, Shandong, People’s Republic of China; 3Department of Anaesthesiology, Guanxian Central Hospital, Liaocheng, Shandong, People’s Republic of China
Correspondence: Tonghang Zhao Email [email protected]
Objective: The optimal sedation regime during endoscopy remains controversial, especially for elderly outpatients. In this study, we compared the efficacy and safety between remimazolam tosilate (RT) and etomidate-propofol (EP) in elderly outpatients undergoing colonoscopy.
Methods: A total of 260 elderly outpatients undergoing sedative colonoscopy were randomized into two groups. Patients in the RT group received a 0.075-mg/kg maintenance dose of remimazolam following an initial dose of 0.15 mg/kg, whereas patients in the EP group (10 mL:20 mg etomidate plus 10 mL:100 mg propofol) received a 0.05-mL/kg maintenance dose following an initial dose of 0.1 mL/kg to maintain a Modified Observer’s Assessment of Alertness/Sedation score of ≤ 3 during the procedure. The primary endpoint was the success of the procedure. Secondary endpoints included time metrics, hemodynamics, consumption of fentanyl, etomidate, propofol, and remimazolam, intraoperative body movement, patient and endoscopist satisfaction scores, supplemental dose of sedative and fentanyl, and incidence and severity of adverse events.
Results: The procedure success rate was 96.52% in the RT group and 100% in the EP group. The difference in procedure success rate between the RT and EP groups was − 3.48% (95% confidence interval: − 6.81%, − 0.15%). Four patients in the RT group required rescue midazolam. Compared with patients in the RT group, the onset time of the EP group was significantly lower (p < 0.05), whereas time to fully alert (p = 0.001), ready for discharge (p = 0.001), and hospital discharge (p = 0.002) were all significantly higher in the EP group. However, there were no significant differences in procedure time (p = 0.846) or cecal intubation time (p = 0.320) between the two groups. Although the frequency of intraoperative body movement was higher in the RT group, the difference was not significant (p = 0.508). There were no significant differences in patients’ demographic and baseline characteristics, supplemental doses of sedative and fentanyl, or patient and endoscopist satisfaction scores (p > 0.05). Muscular tremor and pain on injection were recorded more frequently in the EP group (p < 0.05). However, there were no significant differences in hypoxia, respiratory depression, or incidence of postoperative nausea and vomiting. The severity of adverse events was all mild (grade 1) across both groups.
Conclusion: RT may have non-inferior efficacy and a higher safety profile than EP in elderly outpatients undergoing colonoscopy, which suggests that RT may be more suitable for elderly outpatients undergoing colonoscopy.
Keywords: remimazolam tosilate, etomidate, propofol, elderly outpatients, colonoscopy
Introduction
With the popularization of noninvasive digestive endoscopy, which is considered the gold standard for the diagnosis of digestive system diseases, there is an increasing demand for endoscopy in outpatients, particularly in countries with aging societies.1,2 To relieve patients’ anxiety, discomfort, pain, and potential vasovagal reactions, sedative endoscopy is widely used, especially following the development of new sedative and anesthetic drugs over the last decade.3,4 However, the optimal sedation regime during endoscopy remains controversial; the ideal agent should have a rapid onset, high controllability, and minimal side effects.5
Propofol, etomidate, and midazolam combined with opioids are the most commonly used drugs globally for sedation during endoscopic procedures.6 However, each drug has both advantages and disadvantages. The disadvantage of propofol is injection pain, aspiration pneumonia due to loss of protective reflexes, lack of an analgesic effect and specific antagonists, risk of developing propofol infusion syndrome and bacterial contamination, metabolic acidosis, a narrow therapeutic index (which increase the risk of hypoxia), and cardiovascular and respiratory depression, especially when combined with opioids.7 As a result, guidelines introduced by major anesthesia societies have recommended that propofol be used only by anesthesiologists, alongside constant monitoring and preparation for urgent endotracheal intubation.8 Etomidate may be an appropriate hypnotic agent for colonoscopy, especially for elderly patients, because of its relatively rapid onset and recovery and minimal adverse effects on the cardiovascular system owing to its lack of inhibitory effect on sympathetic tone and myocardial function.9 A previous study reported that histamine release and allergic reactions to etomidate are less common than to barbiturates or propofol.10 However, taking etomidate alone can cause muscle tremor and rigidity and increase postoperative nausea and vomiting (PONV), which may impact the procedure and reduce the satisfaction of both the patient and endoscopist.10 Midazolam has an elimination half-life of approximately 4.3 hours and provides excellent amnesia and a wide margin for safety, which makes it suitable for use by both anesthesiologists and non-anesthesiologists for procedural sedation. The obvious drawback includes a lack of analgesic effect, longer induction and recovery periods, even with relatively short procedures.11 Moreover, the effects of midazolam have considerable interindividual variability, which reduces its controllability. Furthermore, there are concerns regarding accumulation and the potential for repeat sedation because the active metabolite of midazolam has the potential to induce sedative effects.12 Given the characteristics of the aforementioned drugs, a previous study proposed that etomidate-midazolam should be administered to patients with a high American Society of Anesthesiology (ASA) score, whereas propofol-midazolam may be more suitable for patients with a low ASA score.13
Remimazolam tosilate (RT), which acts on gamma-aminobutyric acid (GABA) receptors, is a new ultrashort-acting benzodiazepine. It offers more rapid recovery and earlier restoration of cognitive function than does midazolam because of the high clearance, small volume of distribution, and susceptibility to ester hydrolysis by carboxylesterase-1 to an inactive carboxylic acid metabolite, which is in contrast to all the other benzodiazepines. Furthermore, the effects of RT can be fully reversed by flumazenil.14 Recent studies have reported that RT is suitable for short operations, such as gastrointestinal endoscopy, hysteroscopy, bronchoscopy, and closed reductions of long-bone fractures.15 In addition, it can also be used for the induction and maintenance of anesthesia, and its efficacy is non-inferior to propofol.16 However, the efficacy and safety of RT compared with etomidate-propofol (EP) in patients undergoing colonoscopy are unclear. Thus, we performed a prospective, randomized, single-blind, non-inferiority trial to compare the efficacy and safety of RT versus EP in elderly outpatients undergoing colonoscopy.
Methods
Patients
This single-center, prospective, randomized, single-blind, non-inferiority trial was performed from February 2021 to July 2021 at the Department of Gastroenterology and Digestive Endoscopy at Liaocheng People’s Hospital. The protocol was approved by the institutional review board of our center (NO.2020045). Written informed consent was obtained from all patients before the procedures were performed. The study was registered in the Chinese Clinical Trial Registry (ChiCTR2000041524).
Patients who underwent colonoscopy with sedation anesthesia who were aged between 65 and 75 years were recruited. Patients were excluded if they had contraindications to endoscopy (eg, acute upper respiratory tract infection, asthma attack, acute severe throat disease, and abnormal liver function); had an ASA ≥ III; required trachea cannula; had known sensitivity to benzodiazepines, flumazenil, opiates, propofol, egg products, or soybeans; had adrenocortical insufficiency, porphyria or received chronic corticoid therapy; had difficult airways and hemodynamic instability (systolic blood pressure [SBP] < 90 mmHg or > 180 mmHg, diastolic blood pressure [DBP] > 110 mmHg, peripheral oxygen saturation [SpO2] < 90% in room air); had a history of sleep apnea; had an operation time longer than 30 minutes; or abused or were addicted to alcohol, opioids, or sedative-hypnotic drugs. Patients who had participated in trials of other drugs during the 3 months before the study were also excluded.
Randomization and Blinding
Patients were randomized by an independent anesthetist into the RT or EP group using a computer-generated random number table prior to undergoing colonoscopy. Because of the different appearance of the two drugs, a single-blind design was adopted for this study. All endoscopists, nurses, and patients were blinded to group allocation.
Procedures
All patients underwent bowel preparation in accordance with the standard protocol.17 Supplemental oxygen at a rate of 4 L/min by nasal cannula was administered to all patients immediately after they entered the examination room until they were fully alert, according to sedation guidelines.18 Patients with ASA standard monitoring (blood pressure, heart rate [HR], pulse oxygen saturation, and end-tidal carbon dioxide) received 0.5 μg/kg fentanyl for analgesia after the introduction of 500 mL of a 0.9% sodium chloride solution. After 5 minutes, 0.15 mg/kg RT or 0.1 mL/kg EP (10 mL:20 mg etomidate plus 10 mL:100 mg propofol) was administered to the RT or EP group, respectively, over 1 minute to induce sedation. When the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score was ≤3, colonoscopy was performed by the same endoscopist who had over 10 years of experience, in accordance with standard clinical practice to reduce deviations between endoscopists.19 Sedation level was maintained at an MOAA/S score of ≤3 during the procedure. Supplemental 0.5 μg/kg fentanyl was given for analgesia during the procedure if pain was not controlled, up to a maximum cumulative dose of 150 µg. If patients were not sufficiently sedated, a supplemental bolus of 0.075 mg/kg RT or 0.05 mL/kg EP was administered, up to a maximum of five doses every 15 min. If the initial and supplemental boluses were insufficient to maintain adequate sedation within a 15-minute window, midazolam (dosed per physician discretion) was administered as rescue sedative medication.
If patients experienced hypoxia or respiratory depression for more than 10 seconds, the flow of inhaled oxygen was increased, and the patient’s mandibular angle was raised. If oxygen saturation did not improve, the colonoscopy was suspended, and the patient’s thorax was squeezed slightly. If the situation still showed no improvement, the mandibular back mask was held up to pressurize to assist the patient to breathe, or endotracheal intubation was performed. Vasoactive drugs, such as phenylephrine, ephedrine, atropine, urapidil, and esmolol, were used to maintain hemodynamic stability at target values (fluctuation range did not exceed 20% of the baseline).
Clinical Outcomes
The primary endpoint was the success of the procedure based on a previous study, which included: (1) completion of the procedure, (2) no requirement for an alternative and/or rescue sedative, and (3) administered up to a maximum of five supplemental doses within 15 minutes of the initial dose.14 Secondary endpoints were as follows: time metrics, which included onset time (from the injection of the sedative drug to the start of the colonoscopy), procedure time (from the start to the end of the colonoscopy), cecal intubation time (from insertion of the colonoscope to reaching the cecum), time to fully alert (from completion of the colonoscopy to patients reaching an MOAA/S score of 5), time to ready for discharge using the Modified Aldrete Scoring System, and time to hospital discharge using the discharge score; hemodynamics, which were recorded at the following time points: arrival at the examination room (T1), after administration of intravenous fentanyl (T2), 5 minutes after administration of intravenous fentanyl (T3), immediately after insertion of the colonoscope (T4), reaching the cecum (T5), end of the colonoscopy (T6), 5 minutes after the colonoscopy (T7), 10 minutes after the colonoscopy (T8), and at hospital discharge (T9); consumption of fentanyl, etomidate, propofol, and RT; intraoperative body movement; patient and endoscopist satisfaction scores (on a 10-point scale: 1, most dissatisfied to 10, most satisfied); supplemental dose of sedative and fentanyl; and adverse events, including hypotension (SBP < 90 mmHg, DBP < 50 mmHg, or a mean blood pressure (MBP) decrease of 20% or more below baseline), bradycardia (< 50 beats per minute or a decrease in HR of 20% or more from baseline), hypertension (SBP > 180 mmHg, DBP > 110 mmHg, or an MBP increase of 20% or more from baseline), hypoxia (SpO2 < 90% on 4 L/min oxygen), respiratory depression (respiratory rate < 8 breaths per minute), prolonged sedation (MOAA/S score ≤ 4 for longer than 30 minutes after the end of the colonoscopy), muscular tremor (on a 4-point scale: 0, muscle contraction not observed; 1, weak contractions of face and extremities; 2, facial, body, and limb muscles have mild contractions; 3, strong contractions of facial, body, and limb muscles), and pain on injection and PONV (on a 4-point scale: 0, none; 1, mild; 2, moderate; 3, severe). The severity of adverse events was classified using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (on a 4-point scale: 1, mild; 2, moderate; 3, severe; 4, life-threatening or disabling).20
Statistical Analysis
The sample size calculation was based on a previous study.14 We assumed that the success rates of EP and RT sedation would both be 95%. The predefined non-inferiority margin was an absolute difference of 10% between groups for the primary endpoint. With a non-inferiority margin of 10% on the relative scale, a power of 90%, and a one-sided alpha of 2.5%, the total sample size needed was 200. Assuming a dropout rate of 15%, a minimum of 115 patients were recruited for each group.
Shapiro–Wilk and Levene tests were used to assess the data distribution and homogeneity of variance, respectively. Quantitative data are presented as means ± standard deviations (SDs) or medians and interquartile ranges, as appropriate. Qualitative data are presented as numbers and frequencies. Between-groups comparisons of quantitative variables were analyzed using Student’s t-test or Kolmogorov–Smirnov Z-test. Between-groups comparisons of qualitative variables were analyzed using χ2 of Fisher’s exact tests. A p < 0.05 was considered to be statistically significant. Statistical analyses were performed on SPSS for Windows, version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient Demographics and Baseline Characteristics
Figure 1 shows the patient enrollment process for this trial. We recruited 345 elderly outpatients who underwent colonoscopy between February 2021 and July 2021. Of these, 85 elderly outpatients were excluded because of the following reasons: 25 patients with contraindications to endoscopy; 7 patients with an ASA ≥ III; 3 patients required trachea cannula; 8 patients had a known sensitivity to egg or soybeans; 1 patient had adrenocortical insufficiency; 2 patients received chronic corticoid therapy; 11 patients had difficult airways; 2 patients had hemodynamic instability; 13 patients had a history of sleep apnea; 9 patients had an addiction to alcohol or hypnotic drugs; and 4 patients participated in other drug trials during the previous 3 months. As a result, 260 patients were divided into two groups, among which 28 patients were excluded because the procedure took longer than 30 minutes (n = 14, eight patients in the RT group and six patients in the EP group) or patients were lost to follow-up (n = 14, six patients in the RT group and eight patients in the EP group). The final sample comprised 115 patients in the RT group and 117 patients in the EP group. Patients’ demographics and baseline characteristics were comparable across the two groups (p > 0.05; Table 1).
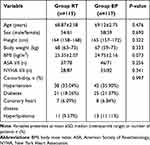 |
Table 1 Demographic and Baseline Characteristics of Patients |
 |
Figure 1 Patient flowchart with CONSORT guidelines. |
Primary Outcome
The procedure success rate was 96.52% (111/115, 95% confidence interval [CI]: 93.19–99.85%) in the RT group and 100% (117/117, 95% CI: 100.00–100.00%) in the EP group. Four patients in the RT group required rescue midazolam. The difference in the procedure success rate between the RT and EP groups was −3.48% (95% CI: −6.81%, −0.15%). RT was considered non-inferior to EP in elderly outpatients undergoing colonoscopy because the lower limit of the 95% CI for the difference in procedure success rate was not greater than the non-inferiority limit of −10% (Table 2).
 |
Table 2 Difference of Primary Outcome Between the Two Groups |
Secondary Outcomes
Compared with patients in the RT group, the EP group’s onset time was significantly higher in (41 [32–52] s vs 32 [26–41] s, p < 0.05; Table 3). However, there were no significant differences in the procedure time (p = 0.846) or cecal intubation time (p = 0.320) between the two groups (Table 3). Time to be fully alert (p = 0.001), time to be ready for discharge (p = 0.001), and time to hospital discharge (p = 0.002) were all significantly lower in the RT group (Table 3).
 |
Table 3 Difference of Second Outcomes Between the Two Groups |
There were no significant differences in hemodynamics during the trial, except for SBP at T6 and RR at T5 (Figure 2). Consumption of fentanyl, etomidate, propofol, and RT is shown in Table 3. Although the frequency of intraoperative body movement was higher in the RT group, this difference was not statistically significant (p = 0.508; Table 3). There were no significant differences in either the patients’ or endoscopist’s satisfaction scores (p > 0.05; Table 3). Supplemental doses of sedatives and fentanyl were similar across the two groups (p = 0.783; Table 3).
 |
Figure 2 Hemodynamics. There were no significant differences in hemodynamics during the trial, except for SBP at T6 and RR at T5. *P< 0.05 vs RT group. |
Safety Assessment Outcomes
Although the number of patients who required vasopressors was higher in the EP group, this difference was not statistically significant (p = 0.311; Table 4). The incidence of PONV was similar across the two groups (p = 0.449; Table 4). However, muscular tremors and pain during injection were more frequently recorded in the EP group than in the RT group (p < 0.05; Table 4). There were no significant differences in either hypoxia or respiratory depression, which were resolved by increasing the flow of inhaled oxygen and raising the patient’s mandibular angle (p > 0.05; Table 4). Only one patient in the EP group had to have their colonoscopy suspended and thorax squeezed slightly. None of the patients required manual or mechanical ventilation. The proportions of patients who had hypotension, hypertension, and bradycardia were similar across the two groups (p > 0.05; Table 4). Of the 27 patients with hypotension, 19 received a supplemental dose of fentanyl. Three patients with moderate hypotension received a supplemental dose of fentanyl more than once. The severity of all adverse events was mild (grade 1) in both groups (p = 0.797; Table 4).
 |
Table 4 Difference of Adverse Events Between the Two Groups |
Discussion
We found that in combination with fentanyl, the procedure success rate was 96.52% in the RT group and 100% in the EP group. Compared with patients in the RT group, the onset time of the EP group was significantly lower, whereas time to be fully alert, ready for discharge, and hospital discharge were all significantly higher in the EP group. At the same time, muscular tremor and pain on injection were recorded more frequently in the EP group.
An increasing number of patients who undergo colonoscopy are elderly, with cardiac or respiratory diseases or electrolyte disturbances due to colonic preparation.21 Currently, two or more sedatives or anesthetic drugs are often used during gastrointestinal endoscopy. However, drug interactions may change the pharmacological effects of individual drugs and increase the occurrence of adverse reactions.22 Remimazolam, a recently developed ultrashort-acting benzodiazepine, shows a high affinity to GABA receptors and increases the frequency of chloride channel opening in the cerebral cortex, limbic system, midbrain, brainstem, and spinal cord.23,24 The pharmacokinetics and pharmacodynamics of RT are similar to those of remimazolam.25 In addition, remimazolam did not differ between elderly and younger patients or between patients with normal renal function and end-stage renal failure.16 However, its use requires caution in patients with severe hepatic impairment,26 which is why we excluded patients with abnormal liver function. We also excluded patients with opioid or alcohol abuse because mortality has been shown to be higher for certain categories of patients who use opioids for pain reduction or abuse drugs, such as opioids or alcohol.27 In contrast to previous studies, we administered fentanyl rather than remifentanil 5 minutes before sedation. This was performed for two reasons: the incidence of hyperalgesia and muscle rigidity of remifentanil, and the synergy for propofol/remifentanil is only 61%, which is considerably lower than that for remimazolam/remifentanil (94% and 98%, respectively).27,28
The success of the procedure using only a single dose of remimazolam is 32%, 56%, and 64% in the 0.10, 0.15, and 0.20 mg/kg groups, respectively.29 A previous study reported that 0.15 mg/kg of remimazolam is sufficient to produce adequate sedation. Moreover, a higher initial dose of remimazolam may result in deeper sedation, which may not be required for routine colonoscopies, although remimazolam has a wide therapeutic window for sedation.30 Furthermore, remimazolam induces loss of consciousness at mean (5–95%) cumulative doses of 0.16 (0.11–0.24) mg/kg in ASA Class III patients.31 Thus, we administered an initial dose of 0.15 mg/kg in the RT group in this trial. Previous study has reported that most adverse reactions to propofol, particularly serious complications, are related to deep sedation levels, dosage, and injection speed of propofol.32 Thus, we administered 0.1 mL/kg EP over a 1-minute period to induce sedation, in accordance with a previous study.33 Similar to the results of a previous study, our study showed that the procedure success rate was 96.52% (95% CI: 93.19–99.85%) in the RT group vs 100% (95% CI: 100.00–100.00%) in the EP group. The difference in the procedure success rate between the RT and EP groups was −3.48%, which indicated that RT was non-inferior to EP in elderly outpatients undergoing colonoscopy.14
Compared with the RT group, the time to achieving an MOAA/S score of 3 was shorter in the EP group. However, increasing the initial dose of remimazolam may eliminate this difference. A previous study reported that cumulative doses of remimazolam were largely similar among the three groups (12.28, 11.70, and 10.93 mg).29 However, the consumption of RT was significantly increased in our study, which was partly due to the larger initial dose, more additional doses, and lower consumption of opioids. Consistent with the results of a previous study, the mean supplemental doses of sedatives and fentanyl were similar across the two groups (3 [2–4] vs 3 [1–4]).34 There were no significant differences in hemodynamics during the trial between the two groups. However, both SBP at T6 and RR at T5 were significantly increased in the RT group, which was puzzled and needed further research to explore this confusion.
Etomidate is non-inferior to propofol in terms of occurrence of respiratory events and has better sedation efficacy even during advanced endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography.35 Although EP may increase the recovery time of patients undergoing gastroscopy, etomidate-remifentanil and EP were more suitable than propofol-remifentanil for the elderly for providing more stable hemodynamic responses and fewer adverse events than etomidate, remifentanil, or propofol alone.13 However, because of the high medical expenses that may be incurred by patients, we used etomidate-propofol for the control group. A previous study reported that a combination of 10 mL 1.0% propofol and 5 mL 0.2% etomidate (ie, a 1:2 EP mixture) for painless gastroscopy reduces the occurrence of adverse reactions without affecting patients’ respiratory function.36 In our study, we adopted a 1:1 EP mixture to allow longer examination time and stimulation needed for colonoscopy compared with gastroscopy.6 A previous study reported that 70% of the usual dose could be administered to complete endoscopy in elderly patients, although the number of remediations was more frequent.37 Consistent with the results of previous studies, time to be fully alert, ready for discharge, and hospital discharge were all significantly higher in the EP group which may be due to the rapid breakdown of RT into an inactive metabolite.25,29,36
Bispectral index (BIS) has been used previously to assess the level of patient sedation.38 However, we used the MOAA/S because a previous study found that the sedation depth as determined by these two methods is similar. In addition, the BIS index was originally developed for propofol, and is less accurate for midazolam.39 A recent study highlighted the clinical benefits of using electroencephalogram measures, which significantly correlate with sedation scale scores and do not require patient stimulation.39,40 We did not apply routine flumazenil reversal at the end of the colonoscopies because of the possibility of resedation and economic reasons.41 Dehydration after bowel preparation combined with the vasodilatory effects of fentanyl is considered the main causes of perioperative hypotension.41 Though the incidence of intraoperative hypotension was relatively low in our study, of the 27 patients with hypotension, 19 required a supplemental dose of fentanyl. Furthermore, three patients with moderate hypotension required a supplemental dose of fentanyl more than once, indicating that fentanyl combined with opioids contributed to the hypotension. In addition, injection pain was commonly reported in the EP group. The underlying mechanism may be related to the direct stimulation of blood vessels or the indirect stimulation of the production of bradykinin and prostaglandins by propofol.9 The incidence of muscular tremor with etomidate has been reported to be approximately 20%–80%.42 However, only 11% of our patients in the EP group experienced muscular tremor, which may be because both opioid pretreatment and propofol inhibit muscular tremor and the lower dose of etomidate. A previous study reported that muscular tremor increases intragastric pressure and the risk of PONV and aspiration, which are related to etomidate dose.43 However, the incidence of PONV was similar across the two groups. This may be due to the different mixing ratios of EP in different studies as well as the preinjection of opioids in our study. All muscular tremors and injection pain were mild, short-lasting, recovered spontaneously, and did not influence the performance of the colonoscopy. None of the elderly outpatients experienced a serious treatment-emergent adverse event that required hospitalization.
This study has the following limitations. First, we did not assess the adrenal function of patients in the EP group, such as plasma cortisol and adrenocorticotropic hormone. However, the effect of etomidate on the function of the adrenal cortex is usually transient and reversible and does not increase the mortality rate if the infusion is not prolonged.44 In addition, we initially used 0.1 mg/kg of etomidate, which is less than the recommended dose (0.15–0.20 mg/kg). Second, muscular tremor can increase postoperative serum potassium and hyperuricemia, and elevated bilirubin has been reported previously in patients receiving RT.30 However, electrolyte changes before and after the operation were not recorded in this study because all were outpatients. Third, we only used one dose of RT; however, establishing optimized dosages is warranted because previous reports have suggested that even higher doses of remimazolam do not cause severe hemodynamic fluctuations and may have a slight advantage during the induction period.31 Fourth, we did not record the recall of the procedure using the Brice questionnaire or cognition and memory function using the Hopkins Verbal Learning Test-Revised™. Finally, multicenter randomized trials are required to confirm the results of this single-center study.
In conclusion, we found that in combination with fentanyl, RT has non-inferior efficacy and a higher safety profile than EP for elderly outpatients undergoing colonoscopy, which suggests that RT is more suitable for elderly outpatients undergoing colonoscopy.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the privacy policy but are available from the corresponding authors on reasonable requests.
Ethical Approval
All procedures performed on the patients were in accordance with the 1964 Helsinki declaration and its later amendments. The study was approved by the institutional review boards of both Liaocheng People’s Hospitals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Special funds for comfortable medical anesthesia optimization of Shandong Provincial Medical Association (NO.YXH2021ZX004).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nagata N, Ishii N, Manabe N, et al. Guidelines for colonic diverticular bleeding and colonic diverticulitis: Japan Gastroenterological Association. Digestion. 2019;99(Suppl 1):1–26. doi:10.1159/000495282
2. Watanabe J, Ikegami Y, Tsuda A, et al. Lidocaine spray versus viscous lidocaine solution for pharyngeal local anesthesia in upper gastrointestinal endoscopy: systematic review and meta-analysis. Dig Endosc. 2021;33(4):538–548. doi:10.1111/den.13775
3. Chung JW, Kim N, Wee JH, et al. Clinical features of snoring patients during sedative endoscopy. Korean J Intern Med. 2019;34(2):305–314. doi:10.3904/kjim.2017.110
4. Kim DK. Nonoperating room anaesthesia for elderly patients. Curr Opin Anaesthesiol. 2020;33(4):589–593. doi:10.1097/ACO.0000000000000883
5. Laoveeravat P, Thavaraputta S, Suchartlikitwong S, et al. Optimal sequences of same-visit bidirectional endoscopy: systematic review and meta-analysis. Dig Endosc. 2020;32(5):706–714. doi:10.1111/den.13503
6. Dossa F, Megetto O, Yakubu M, et al. Sedation practices for routine gastrointestinal endoscopy: a systematic review of recommendations. BMC Gastroenterol. 2021;21(1):22. doi:10.1186/s12876-020-01561-z
7. Lee JG, Yoo KS, Byun YJ. Continuous infusion versus intermittent bolus injection of propofol during endoscopic retrograde cholangiopancreatography. Korean J Intern Med. 2020;35(6):1338–1345. doi:10.3904/kjim.2018.233
8. Smith I, Durkin D, Lau KW, et al. Establishing an anaesthetist-delivered propofol sedation service for advanced endoscopic procedures: implementing the RCA/BSG guidelines. Frontline Gastroenterol. 2018;9(3):185–191. doi:10.1136/flgastro-2017-100839
9. Kim MG, Park SW, Kim JH, et al. Etomidate versus propofol sedation for complex upper endoscopic procedures: a prospective double-blinded randomized controlled trial. Gastrointest Endosc. 2017;86(3):452–461. doi:10.1016/j.gie.2017.02.033
10. Doenicke A, Lorenz W, Hoernecke R, et al. Histamine release after injection of benzodiazepines and of etomidate. A problem associated with the solvent propylene glycol. Ann Fr Anesth Reanim. 1993;12(2):166–168. doi:10.1016/S0750-7658(05)81025-1
11. Kim J, Choi SM, Park YS, et al. Dexmedetomidine versus midazolam for sedation during endobronchial ultrasound-guided transbronchial needle aspiration: a randomised controlled trial. Eur J Anaesthesiol. 2021;38(5):534–540. doi:10.1097/EJA.0000000000001370
12. Li WX, Luo RY, Chen C, et al. Effects of propofol, dexmedetomidine, and midazolam on postoperative cognitive dysfunction in elderly patients: a randomized controlled preliminary trial. Chin Med J. 2019;132(4):437–445. doi:10.1097/CM9.0000000000000098
13. Lee JM, Min G, Lee JM, et al. Efficacy and safety of etomidate-midazolam for screening colonoscopy in the elderly: a prospective double-blinded randomized controlled study. Medicine. 2018;97(20):e10635. doi:10.1097/MD.0000000000010635
14. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, Phase III trial. J Gastroenterol Hepatol. 2021;36(2):474–481. doi:10.1111/jgh.15188
15. Sneyd JR, Gambus PL, Rigby-Jones AE, et al. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127(1):41–55. doi:10.1016/j.bja.2021.03.028
16. Keam SJ. Remimazolam: first Approval. Drugs. 2020;80(6):625–633. doi:10.1007/s40265-020-01299-8
17. Hernandez PV, Horsley-Silva JL, Snyder DL, et al. Effect of bowel preparation volume in inpatient colonoscopy. Results of a prospective, randomized, comparative pilot study. BMC Gastroenterol. 2020;20(1):227. doi:10.1186/s12876-020-01373-1
18. Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133(2):675–701. doi:10.1053/j.gastro.2007.06.002
19. Chandran S, Parker F, Vaughan R, et al. The current practice standard for colonoscopy in Australia. Gastrointest Endosc. 2014;79(3):473–479. doi:10.1016/j.gie.2013.10.050
20. Basch E, Becker C, Rogak LJ, et al. Composite grading algorithm for the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Clin Trials. 2021;18(1):104–114. doi:10.1177/1740774520975120
21. Causada-Calo N, Bishay K, Albashir S, et al. Association between age and complications after outpatient colonoscopy. JAMA Netw Open. 2020;3(6):e208958. doi:10.1001/jamanetworkopen.2020.8958
22. Seleem WM, El Hossieny KM, Abd-Elsalam S. Evaluation of different sedatives for colonoscopy. Curr Drug Saf. 2020;15(1):20–24. doi:10.2174/1574886314666190726154238
23. Chen W, Chen S, Huang Y. Induction and maintenance of procedural sedation in adults: focus on remimazolam injection. Expert Rev Clin Pharmacol. 2021;14(4):411–426. doi:10.1080/17512433.2021.1901575
24. Cornett EM, Novitch MB, Brunk AJ, et al. New benzodiazepines for sedation. Best Pract Res Clin Anaesthesiol. 2018;32(2):149–164. doi:10.1016/j.bpa.2018.06.007
25. Jia Z, Ren LX, Fan YT, et al. Observation of effective dosage of remimazolam tosilate used for moderate-to-deep sedation in fiberoptic bronchoscopy. Zhonghua Yi Xue Za Zhi. 2021;101(11):813–816. doi:10.3760/cma.j.cn112137-20200901-02524
26. Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi:10.1016/j.bja.2021.05.027
27. Kops MS, Pesic M, Petersen KU, et al. Impact of concurrent remifentanil on the sedative effects of remimazolam, midazolam and propofol in cynomolgus monkeys. Eur J Pharmacol. 2021;890:173639. doi:10.1016/j.ejphar.2020.173639
28. Ahmadi A, Amri P, Shokri J, et al. Comparison of the analgesic effect of intravenous paracetamol/midazolam and fentanyl in preparation of patients for colonoscopy: a double blind randomized clinical trial. Caspian J Intern Med. 2015;6(2):87–92.
29. Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–780. doi:10.1213/ANE.0000000000000548
30. Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–992. doi:10.1016/j.gie.2015.08.062
31. Doi M, Hirata N, Suzuki T, et al. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501. doi:10.1007/s00540-020-02776-w
32. Shimizu H, Homma Y, Norii T, et al. Incidence of adverse events among elderly vs non-elderly patients during procedural sedation and analgesia with propofol. Am J Emerg Med. 2021;44:411–414. doi:10.1016/j.ajem.2020.04.094
33. Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e6. doi:10.1016/j.gie.2018.04.2351
34. Han SJ, Lee TH, Yang JK, et al. Etomidate sedation for advanced endoscopic procedures. Dig Dis Sci. 2019;64(1):144–151. doi:10.1007/s10620-018-5220-3
35. Hao L, Hu X, Zhu B, et al. Clinical observation of the combined use of propofol and etomidate in painless gastroscopy. Medicine. 2020;99(45):e23061. doi:10.1097/MD.0000000000023061
36. Qureshi WA, Zuckerman MJ, Adler DG, et al. ASGE guideline: modifications in endoscopic practice for the elderly. Gastrointest Endosc. 2006;63(4):566–569. doi:10.1016/j.gie.2006.02.001
37. Bower AL, Ripepi A, Dilger J, et al. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc. 2000;52(2):192–196. doi:10.1067/mge.2000.107284
38. Toklu S, Iyilikci L, Gonen C, et al. Comparison of etomidate–remifentanil and propofol– remifentanil sedation in patients scheduled for colonoscopy. Eur J Anaesthesiol. 2009;26(5):370–376. doi:10.1097/EJA.0b013e328318c666
39. Schüttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I pharmacokinetics and clinical pharmacodynamics . Anesthesiology. 2020;132(4):636–651.
40. Yamamoto T, Kurabe M, Kamiya Y. A mechanism of re-sedation caused by remimazolam. J Anesth. 2021;35(3):467–468. doi:10.1007/s00540-021-02930-y
41. Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117(5):1093–1100. doi:10.1213/ANE.0b013e3182a705ae
42. Du X, Zhou C, Pan L, et al. Effect of dexmedetomidine in preventing etomidate-induced myoclonus: a meta-analysis. Drug Des Devel Ther. 2017;11:365–370. doi:10.2147/DDDT.S121979
43. St Pierre M, Dunkel M, Rutherford A, et al. Does etomidate increase postoperative nausea? A double-blind controlled comparison of etomidate in lipid emulsion with propofol for balanced anaesthesia. Eur J Anaesthesiol. 2000;17(10):634–641. doi:10.1097/00003643-200010000-00007
44. Zhou X, Li BX, Chen LM, et al. Etomidate plus propofol versus propofol alone for sedation during gastroscopy: a randomized prospective clinical trial. Surg Endosc. 2016;30(11):5108–5116. doi:10.1007/s00464-016-4861-6
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
