Back to Journals » Cancer Management and Research » Volume 14
The Efficacy and Safety of PD-1 Inhibitors Combined with Nab-Paclitaxel Plus Gemcitabine versus Nab-Paclitaxel Plus Gemcitabine in the First-Line Treatment of Advanced Pancreatic Cancer: A Retrospective Monocentric Study
Authors Zhang F, Wang Y, Yang F, Zhang Y, Jiang M, Zhang X
Received 16 November 2021
Accepted for publication 22 January 2022
Published 9 February 2022 Volume 2022:14 Pages 535—546
DOI https://doi.org/10.2147/CMAR.S349442
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Feng Zhang, Yuyang Wang, Fangfang Yang, Yuming Zhang, Man Jiang,* Xiaochun Zhang*
Precision Medicine Center of Oncology, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, 266003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Man Jiang; Xiaochun Zhang, Precision Medicine Center of Oncology, The Affiliated Hospital of Qingdao University, Qingdao University, 59Haier Road, LaoShan District, Qingdao, 266003, People’s Republic of China, Tel/Fax +86053282913271, Email [email protected]; [email protected]
Purpose: The purpose of this study was to evaluate the efficacy and safety of PD-1 inhibitor combined with nab-paclitaxel plus gemcitabine (AG) chemotherapy versus AG chemotherapy in the first-line treatment of advanced pancreatic cancer.
Patients and Methods: This study included the application of AG treatment and PD-1 combined with AG treatment with advanced pancreatic ductal adenocarcinoma at the Affiliated Hospital of Qingdao University from September 2018 to July 2020. Clinical information and next-generation sequencing (NGS) reports of patients were collected to compare the effectiveness and adverse events of the two treatments and analyze the risk factors affecting the prognosis of patients.
Results: There was no difference in PFS between the AG group and the PD-1+AG group (4.9 months vs 5.0 months, P = 0.154), but the difference in OS was statistically significant (9.3 months vs 12.1 months, P < 0.001). Compared with the AG group, the PD-1+AG group reduced the risk of death about 20.0% (HR = 0.203, 95% CI, 0.090− 0.459, P < 0.001). In terms of safety, the incidence of hypothyroidism and reactive skin capillary hyperplasia in PD-1 + AG group was higher than that in AG group (P < 0.050) in grade 1– 2; grade 3– 4 adverse reactions were mainly hematologic AEs and abnormal liver function. The incidence of grade 3– 4 adverse reactions in the two groups was 38.7% (95% CI, 20.5– 56.9%) and 35.3% (95% CI, 10.0– 60.6%), respectively. In addition, PD-1+ AG regimen improved the OS of patients with KRAS and TP53 co-mutations (8.0months vs 10.2 months, P = 0.004).
Conclusion: PD-1 inhibitors combined with AG chemotherapy have shown good efficacy and safety in the first-line treatment of patients with advanced pancreatic ductal adenocarcinoma. This regimen similarly improved OS in patients with KRAS and TP53 co-mutations.
Keywords: pancreatic cancer, immunotherapy, chemotherapy, KRAS, TP53
Introduction
Pancreatic cancer is hidden in location and difficult to diagnose at an early stage.1 About 50% of pancreatic cancer is accompanied by distant metastasis at diagnosis, and the 5-year survival rate is only 9%.2 According to National Cancer Comprehensive Network (NCCN) guidelines, chemotherapy is recommended as a first-line treatment for metastatic pancreatic cancer.3 FOLFIRINOX or gemcitabine-based chemotherapy is the mainstream first-line chemotherapy for advanced pancreatic cancer.4,5 However, pancreatic cancer is not sensitive to chemotherapy, targeted therapies have not made significant progress in the treatment of advanced pancreatic cancer.6
In recent years, PD-1/PD-L1 immune checkpoint inhibitors (ICIs) have made remarkable advances in the treatment of solid tumors, but previous immune-monotherapy for pancreatic cancer has mostly ended in failure.7,8 At present, only Pembrolizumab has been recommended by NCCN guidelines for second-line treatment of patients with microsatellite instability-high (MSI-H) and/or defect mismatch repair genes (dMMR),9 s, while MSI-H/dMMR incidence in pancreatic cancer is less than 1%.10 The presence of a large number of dense stromal components in the tumor microenvironment (TME) of pancreatic cancer and the dense fibrous tissue make it difficult for immune effector cells such as CD8 +T cells and NK cells to infiltrate in the tumor tissue and form a unique immunosuppressive microenvironment.11 The lack of infiltrating T cells makes it difficult for single-agent immunotherapy to reverse the immunosuppressive microenvironment.12
Fortunately, the research data of PD-1 inhibitors combined chemotherapy in clinical research are exciting. In a 2017 phase Ib/II study of Pembrolizumab, gemcitabine, and nab-paclitaxel for patients with metastatic pancreatic cancer, the disease control rate (DCR) reached 100%.13 A retrospective study result in 2020 ASCO reported that the median PFS of Toripalimab in combination with chemotherapy in the first-line treatment of advanced pancreatic cancer was 7.0 months.14
In China, independently developed PD-1 inhibitors are thriving. Sintilimab is the first innovative PD-1 inhibitor in China and has been approved for indications in Hodgkin’s lymphoma, non-small cell lung cancer (NSCLC), and hepatocellular carcinoma (HCC).15 Toripalimab is the first PD-1 immunotherapy approved worldwide for the treatment of nasopharyngeal carcinoma, and its indications also include melanoma and urothelial carcinoma.16 Tislelizumab, which remodels the Fc segment to abrogate antibody dependent cell-mediated phagocytosis, is the first PD-1 mAb approved for urothelial cancer indications in China, with other indications including Hodgkin’s lymphoma, NSCLC as well as HCC.17 Camrelizumab is one of the most approved indications for a PD-1 inhibitor in China, including Hodgkin’s lymphoma, HCC, NSCLC (squamous and non-squamous), esophageal squamous carcinoma, as well as nasopharyngeal carcinoma.18 These 4 PD-1 inhibitors all performed well in terms of safety. Findings from studies evaluating the safety of the 4 PD-1 inhibitors revealed that the majority of treatment-related adverse events (AEs) were grade 1 or 2, such as rash, hypertension, fatigue, diarrhea, paresthesia, anaemia and elevated aspartate aminotransferase.19,20
Due to the good safety and effectiveness of previous clinical studies of immune combined chemotherapy, we also observed benefits in patients with advanced pancreatic cancer using PD-1 combined chemotherapy in our center. Compared with foreign drugs, domestic PD-1 has price advantages and strong accessibility, and is widely used in Chinese patients. Therefore, this study retrospectively investigated the clinical efficacy of 4 Chinese domestic PD-1 inhibitors combined with chemotherapy (gemcitabine + nab-paclitaxel, AG) versus chemotherapy (AG) alone in advanced pancreatic cancer and further explored the influencing factors associated with prognosis.
Methods
Study Subjects
This study retrospectively collected all clinical information of patients with advanced metastatic (stage IV) pancreatic ductal adenocarcinoma who visited the cancer precision medicine center of the Affiliated Hospital of Qingdao University from September 2018 to July 2020.
Inclusion criteria: 1. Diagnosed pathologically (surgical pathology slides or needle biopsies) as pancreatic ductal adenocarcinoma; 2. Stage IV (according to the 8th edition of the TNM staging criteria for pancreatic cancer published by the American Joint Committee on cancer); 3. Clinical stage I, II, or III, local recurrence or distant metastasis after radical surgery; 4. Presence of evaluable lesions on imaging; 5. ECOG score of ≤2 points; 6. No previous chemoradiotherapy, or treatment discontinuation for at least 6 months; 7. Agree to provide a next-generation sequencing (NGS) test report.
Exclusion criteria: 1. patients who had received previous immunotherapy with CTLA-4 inhibitors, PD-1/PD-L1 inhibitors; 2. ECOG > 2 and expected survival in less than 3 months; 3. recent presence of systemic active infection or acute on chronic disease phase with inability to tolerate chemotherapy; 4. Previous autoimmune disease, hematologic disease; 5. Patients with malignancies other than pancreatic cancer or severe, uncontrolled disease.
Treatment Program
The specific medication of chemotherapy group: gemcitabine 1000 mg/m2 intravenous drip, nab-paclitaxel 125 mg/m2 intravenous drip, day 1–8, every 3 weeks as a treatment cycle. PD-1 + chemotherapy group: PD-1 inhibitors are one of the following four: Toripalimab 240 mg (Junshi Biosciences Co., Ltd. Shanghai, China); Camrelizumab 200 mg (Hengrui Pharma Co., Ltd. Jiangsu, China); Tislelizumab 200 mg (BeiGene Shenzhou Biotechnology Co., Ltd. Beijing, China); Sintilimab 200 mg (Cinda Biopharmaceutical Co., LTD. Suzhou, China); All PD-1 inhibitors were administered intravenously at day 1, every 3 weeks; nab-paclitaxel and gemcitabine at the same dose as the other arm. Immunotherapy was applied before chemotherapy, and chemotherapy was given after an interval of at least 30 minutes, the adverse reactions were observed and treated in time.
Efficacy Evaluation Criteria
The efficacy was evaluated every 6 weeks, and the maximum diameter of the target lesion was measured by CT or MRI. According to the guideline of response evaluation solid tumors (RECIST) 1.1, the evaluation standard is: complete response (CR): all target lesions disappear completely and the maintenance time is not less than 4 weeks; Partial response (PR): the reduction of the sum of the maximum diameters of all target lesions after treatment, which is greater than or equal to 30%; Stable disease (SD): the sum of the maximum diameters of the target lesions decreased by less than 30% or increased by less than 20% compared with baseline; Progressive disease (PD): the sum of the maximum diameters of the target lesions increased by more than 20% compared with the baseline or new lesions appeared. Progression-free survival (PFS) was defined as the time from the beginning of treatment to disease progression; overall survival (OS) is defined as the time from the beginning of treatment to death. Objective response rate (ORR) refers to the proportion of the total number of CR + PR patients in the total number of evaluable cases after treatment. The disease control rate (DCR) is the ratio of the sum of all CR, PR, and SD patients to the total number of all patients. Adverse events were evaluated using the common terminology criteria adverse events (CTCAE) version 5.0. The primary end points of this study were PFS and OS; The secondary end points were adverse reactions, ORR and DCR.
Clinical Information Collection and Follow Up
Factors that may be associated with patient outcomes were collected, including: gender, age, ECOG score, time of first diagnosis, tumor stage at the time of medication, primary tumor site, distant metastasis site, whether surgery was performed, treatment regimen, efficacy and adverse effects (AEs). Telephone follow-up was conducted for patients who did not come to the outpatient clinic for review or admission at 3 months intervals.
The NGS of the 48 patients collected in this study were all reported from Guangzhou Burning Rock Medical Institute Co., Ltd. A total of 520 genes closely related to cancer mechanism and targeted therapy were examined, and probe hybridization and high-throughput sequencing were used to examine the whole exonic regions of 310 genes and the hotspot mutation regions (exons, introns, or promoter regions) of 210 genes. The test report includes somatic gene variants and abundance, tumor mutation burden (TMB), and MSI.
Statistic Analysis
Patient information was collected by Microsoft Excel, and the collected data were processed with SPSS 23.0 and GraphPad Prism 8.0. The baseline characteristics of patients were assessed by χ2 test. PFS and OS survival curves were obtained by Kaplan–Meier method, and the differences between groups were compared by Log rank nonparametric test. Univariate and multivariate analyses were performed using Cox proportional hazards models. In all analyses, two-sided P < 0.05 were considered statistically significant.
Results
Patient Characteristics
The enrollment period of this study was from September 2018 to July 2020, during which time a total of 126 advanced pancreatic cancer patients were hospitalized in our center, and a total of 48 patients met the enrollment criteria after strict screening. Thirty-one patients were included in AG chemotherapy group, and 17 patients were included in PD-1+AG group. In the PD-1+AG group, 7 patients used Camrelizumab, 4 patients used Toripalimab, 3 patients used Tislelizumab, and 3 patients used Sintilimab. The baseline characteristics were no statistically significant differences, as shown in Table 1.
 |
Table 1 Baseline Characteristics |
NGS Test Results
A total of 21 types of mutation were detected in the 48 patients in this study, with the highest frequency of KRAS mutations (36/48, 75.0%), followed by TP53 mutations (32/48, 66.7%), 20 patients had both KRAS and TP53 mutations. Other relatively rare mutations include: CDKN2A, ARID1A, FANCA, APC, KDM6A, FGFR1, SMAD4, and so on. The mutation types and abundances of all patients are presented in Figure 1. TMB was less than or equal to 10 mutations/MB in all patients, and MSI was microsatellite stable (MSS).
 |
Figure 1 Next generation sequencing heat map of 48 patients. |
There are 4 KRAS mutation subtypes: G21D mutation, G12V mutation, G12C mutation and K117N mutation. The types of TP53 gene mutations varied, including p.H214R missense mutation in exon 6, p.Y327fs frameshift mutation in exon 9, p.R249M missense mutation in exon 7, p.C141R missense mutation in exon 5, p.R715H missense mutation in exon 5, nonsense mutation in exon 6, and so on.20 patients had both KRAS and TP53 mutations, the specific mutation types and mutation abundance of patients as shown in Table 2.
 |
Table 2 KRAS/TP53 Co-Mutation Patients (N = 20) |
Clinical Efficacy
The follow-up was ended in October 2021. Among the 31 patients in AG group, 8 patients achieved PR, 15 patients were maintained SD, and 8 patients were PD, as shown in Figure 2A. The ORR was 25.8% (95% CI, 9.5–42.1%), DCR was 74.2% (95% CI, 57.9–90.5%). Among the 17 patients in PD-1 combined with AG group, PR was observed in 6 patients, SD in 8 patients, and PD in 3 patients, as shown in Figure 2B. ORR in this group was 35.0% (95% CI,10.0–60.6%), and DCR was 82.4% (95% CI, 62.1–100.0%). There was no significant difference in ORR and DCR between PD-1 combined with AG group and AG group by χ2 test (P > 0.050).
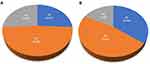 |
Figure 2 Comparison of efficacy between the two groups. (A) Efficacy evaluation results of AG group; (B) Efficacy evaluation results of PD-1+AG group. |
Survival Analysis
Kaplan–Meier curves were used to compare the survival of the two groups. The study found that the median PFS was 4.9 months (95% CI, 4.1–5.7 months) in the AG group and 5.0 months (95% CI, 3.3–6.7 months) in the combination therapy group. There was no significant difference in PFS between the two groups (P = 0.154), as shown in Figure 3A. But the median OS was 2.8 months longer in the combination therapy group than in the AG group. The median OS was 9.3 months (95% CI, 8.8–9.8 months) in AG group, whereas the median OS reached 12.1 months (95% CI, 8.1–16.1 months) in the combination group, and the difference between the two groups was statistically significant (P < 0.001), see Figure 3B.
 |
Figure 3 (A) Kaplan–Meier PFS curves of patients treated with PD-1 +AG versus AG (P=0.154); (B) Kaplan–Meier OS curves of patients treated with PD-1+AG versus AG (P<0.001). |
Univariate and Multivariate Analysis of Patients’ Prognosis
Next, Cox univariate analysis model was used to explore the factors that may affect patients’ OS. Age, gender, ECOG score, surgery, metastasis site, treatment and KRAS types were analyzed, respectively. The results showed that ECOG score (P < 0.001), treatment regimen (P < 0.001) and KRAS types (P < 0.001) were closely related to patients’ OS, and other variables had no significant effect on OS (P > 0.200), as shown in Figure 4A.
 |
Figure 4 (A) Forest plot for univariate analysis. (B) Forest plot of multivariate analysis. |
The three variables were then included to construct a multifactor Cox proportional risk model. The results showed that the risk of death in patients with an ECOG score of 2 was nearly 4 times higher than that in patients with an ECOG score of 0–1 (HR = 4.015, 95% CI 1.784–9.032, P = 0.001). Patients with KRAS wild types had a significantly lower risk of death (HR 0.3.938; 95% CI,1.356–11.434; P = 0.012). In addition, immunotherapy combined with chemotherapy reduces the risk of death in advanced pancreatic cancer by about 20.0% compared with chemotherapy alone (HR = 0.203, 95% CI, 0.090–0.459, P < 0.001). See Figure 4B.
AEs
Reactive cutaneous capillary hyperplasia is an adverse effect specific to the immunotherapy group. The majority of AEs reported in this study were grade 1–2, with no statistically significant differences in the incidence of leukopenia, neutropenia, anemia, thrombocytopenia, transaminase elevations, rash, diarrhea, nausea and vomiting, fatigue, and fever between the two groups (P > 0.05). The incidences of hypothyroidism and reactive cutaneous capillary hyperplasia were higher in the immunochemotherapy group than in the chemotherapy alone group (P < 0.05) and are shown in Table 3. The incidence of grade 3–4 AEs in the two groups was 38.7% (95% CI, 20.5–56.9%) and 35.3% (95% CI, 10.0–60.6%), respectively. Grade 3–4 adverse reactions were mainly hematologic AEs and abnormal liver function, which are shown in Table 4. There were no fatal adverse events in either group, which were well tolerated and safe by patients.
 |
Table 3 Comparison of Grade 1–2 Adverse Events |
 |
Table 4 Comparison of Grade 3–4 Adverse Events |
Subgroup Analysis of KRAS and TP53 Co-Mutated Patients
Among the 20 patients with KRAS and TP53 co-mutation, there were 13 patients treated with AG chemotherapy and 7 patients treated with PD-1 + AG chemotherapy. PFS and OS were compared between the two groups, see Figure 5A and B. The results showed that the addition of immunotherapy did prolong the OS of patients with KRAS and TP53 co-mutation (8.00 months vs 10.20 months, P = 0.004). There was no difference in PFS between the two groups (3.70 months vs 4.50 months, P = 0.373).
 |
Figure 5 (A) Kaplan–Meier PFS curves of patients with KRAS and TP53 co-mutations (P=0.323); (B) Kaplan–Meier OS curves of patients with KRAS and TP53 co-mutations (P=0.004). |
Discussion
Pancreatic cancer remains one of the most aggressive and intractable human malignancies. Over the past few decades, chemotherapy has been the main treatment for metastatic pancreatic adenocarcinoma. Compared with gemcitabine chemotherapy, AG regimen significantly improved the overall survival rate, progression-free survival rate and remission rate of patients with advanced pancreatic cancer.21 Both FOFIRINOX and AG regimens are internationally recognized first-line chemotherapy regimens for advanced pancreatic cancer.22 In consideration of the large side effects of chemotherapy regimen with FOLFIRINOX, which are often difficult to be tolerated by patients, we chose the AG regimen in this study. Previous studies have shown that the combination of immunotherapy and chemotherapy has achieved better results than chemotherapy alone in the treatment of various solid tumors.23–25 Clinical trials are also being carried out in combination with PD-1 inhibitors for advanced pancreatic cancer. This study mainly retrospectively collected the patients treated with AG chemotherapy alone and PD-1 + AG for follow-up observation, and compared the curative effect and the incidence of adverse events. The results showed that compared with the AG chemotherapy regimen, the addition of PD-1 inhibitor effectively prolonged the OS of patients with advanced pancreatic cancer and had good safety and reduced the risk of death by about 20.0% (HR = 0.203, 95% CI, 0.090−0.459, P < 0.001).
The results of this study showed that PD-1 plus chemotherapy significantly prolonged OS without prolonging PFS in patients with advanced pancreatic cancer. The reasons may be as follows: First of all, both PFS and OS are two key observations in the clinical application of antineoplastic drugs, PFS focuses on observing the effect of drugs on reducing patients’ symptoms, improving patients’ quality of life, and reflecting the short-term efficacy of drugs. And OS focuses on observing the effect of medication on the overall survival time of patients.26 In addition, chemotherapy works quickly and is prone to drug resistance. Immunotherapy responds slowly but has a special trailing effect that continues to affect subsequent survival and has a greater impact on OS.27 Furthermore, there was pseudoprogression with immunotherapy in terms of efficacy assessment, leading to a possible misjudgment of PFS.28 Therefore, the efficacy of immunotherapy cannot be assessed solely by conventional imaging and hematologic indices. More attention should be paid to patients’ systemic symptoms and physical performance status when assessing efficacy. In this study, Immunotherapy makes a significant contribution in prolonging the OS, and patients who originally survived for a shorter period of time were given the opportunity to live longer.
It is particularly noteworthy that grade 3 AEs, abnormal liver function and bone marrow suppression will seriously affect the quality of life of patients. Prompt attention should be paid to while enhancing supportive and symptomatic treatment. Cutaneous AEs are one of the most common immune related AEs with ICIs. Reactive cutaneous capillary hyperplasia is a typical AEs of Camrelizumab, which is closely related to efficacy. Patients who develop reactive cutaneous capillary hyperplasia often have better clinical efficacy.29
Chemotherapy has a positive effect on the tumor immune microenvironment. Dendritic cells (DCs) play a key role in the cross presentation of tumor antigens and are essential for the induction of tumor-specific cytotoxic T lymphocyte (CTL) effects.30 However, tumor infiltrating DCs in the immune micro-environment exhibit defective cross presentation of tumor antigens. Tumor infiltrating T cells (TILs) cannot be activated by DCs, deregulating tumor growth.31 Studies have shown that gemcitabine can reverse this deficit in tumor infiltrating DCs.32 Gemcitabine can initiate the host immune system, and its induced destruction by tumor cell apoptosis may expose the immune system to a large number of tumor antigens, reduce immunosuppression, and aid immunotherapy. Gemcitabine enables tumor infiltrating CD8 + T cells to exert full effector effects, a process that could be further exploited for cancer immunotherapy. The PD-1 inhibitors used by the patients in this study are self-developed products in China. The mechanism is the same as that of PD-1 imported from abroad, but the antibody structure design is different. They can restore the killing effect of T lymphocytes on tumor cells by inhibiting the binding of PD-1 and its ligand PD-L1.33 Therefore, it is a promising option to synergize both chemotherapy and immunotherapy to treat advanced pancreatic cancer.
It has been previously reported in the literature that approximately 90% of pancreatic cancer patients are KRAS mutant,34,35 but the KRAS mutation rate among the 48 patients in this study is only 75%. The first reason may be geographical and ethnic differences. KRAS G12C mutation landscape analysis of 11,951 Chinese tumor samples showed that the mutation frequency of KRAS in pancreatic cancer was 81.5%.36 Secondly, it may be because the sample size of the people we included was small and screened. A portion of PDAC patients with KRAS mutation in our center received gemcitabine plus TS-1 or TS-1 monotherapy or FOLFIRINOX or other treatments, and their KRAS mutation rate was excluded, which may result in a mutation rate less than 90%. Thirdly, the low mutation frequency may be due to low tumor cell numbers.
KRAS is the most common genetic mutation in pancreatic cancer,37 which is usually associated with poor prognosis.38,39 KRAS mutation is a necessary condition for tumor initiation. KRAS and TP53 co mutations are potential immunotherapy predictors in patients with EGFR/ALK wild-type non-squamous NSCLC.40 In pancreatic cancer, the co-mutation of KRAS and TP53 was the most common, and the high co-mutation rate suggested that it might be related to the carcinogenic mechanism of pancreatic cancer. Aman Chandra Kaushik et al analyzed the mutation interaction in pancreatic cancer, and the results showed that KRAS gene mutations were associated with increased TGF-β pathway activity, TP53 gene mutation was closely related to the activation of PI3K pathway. It is known that KRAS mediates PI3K pathway. Therefore, KRAS and TP53 pathways may interfere with each other.41 According to a recent study, the mutation of KRAS and TP53 promotes the escape of the immune system by promoting ARF6 pathway, high-level expression of ARF6 and its downstream effector AMAP1 and promoting the expression of PD-L1 on the cell surface.42 The subgroup analysis of this study showed that chemotherapy combined with immunotherapy successfully improved the prognosis of patients with KRAS and TP53 co-mutations. In addition, this study needs to explore the relationship between the immunochemotherapy with the ARF6 pathway.
The main limitation of our study is the relatively small sample size. In this study, a total of 4 PD-1 inhibitors were used, but the total number of cases was only 17. The cohort of patients for each PD-1 inhibitor was small, so the conclusions may not be reliable for a certain PD-1 inhibitor alone. In other words, immune checkpoint inhibitors combined with chemotherapy can lead to better overall survival. We should underline the fact that the study is only hypothesis generating, due to the limitation in design, population and the fact that the population is only asiatic. This study is necessary to increase the number of cases in further studies.
Conclusion
In conclusion, combination therapy is the trend in the era of precision therapy. Combination regimens based on immune or targeted therapy have shown better clinical outcomes in a variety of cancer treatments, and the combination of immune and chemotherapy also brings a new opportunity to improve the prognosis of advanced pancreatic patients.
Ethical Statement
This study is consistent with the Declaration of Helsinki. The Affiliated Hospital of Qingdao University Institutional Review Board approved this study, and all study subjects provided informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Acknowledgment
The authors thank all members of the cancer precision medical center for assistance in various aspects of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pereira SP, Oldfield L, Ney A, et al. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5(7):698–710. doi:10.1016/S2468-1253(19)30416-9
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. doi:10.6004/jnccn.2021.0017
4. Lund K, Olsen CE, Wong JJW, et al. 5-FU resistant EMT-like pancreatic cancer cells are hypersensitive to photochemical internalization of the novel endoglin-targeting immunotoxin CD105-saporin. J Exp Clin Cancer Res. 2017;36(1):187. doi:10.1186/s13046-017-0662-6
5. Manji GA, Olive KP, Saenger YM, Oberstein P. Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res. 2017;23(7):1670–1678. doi:10.1158/1078-0432.CCR-16-2319
6. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi:10.1016/S0140-6736(20)30974-0
7. Wainberg ZA, Hochster HS, Kim EJ, et al. Open-label, phase I study of nivolumab combined with nab-paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4814–4822. doi:10.1158/1078-0432.CCR-20-0099
8. Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi:10.1016/j.canlet.2017.08.006
9. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/JCO.19.02105
10. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733
11. Krishnamoorthy M, Lenehan JG, Burton JP, Vareki SM. Immunomodulation in pancreatic cancer. Cancers. 2020;12(11):3340. doi:10.3390/cancers12113340
12. Huber M, Brehm CU, Gress TM, et al. The immune microenvironment in pancreatic cancer. Int J Mol Sci. 2020;21(19):7307. doi:10.3390/ijms21197307
13. Weiss GJ, Waypa J, Blaydorn L, et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer. 2017;117(1):33–40. doi:10.1038/bjc.2017.145
14. Cheng K, Lv WR, Li X, et al. Toripalimab with nab-paclitaxel/gemcitabine as first-line treatment for advanced pancreatic adenocarcinoma: updated results of a single-arm, open-label, phase Ib/II clinical study. J Clin Oncol. 2021;39(suppl 15):abstr e16213. doi:10.1200/JCO.2021.39.15_suppl.e16213
15. Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: a promising anti-tumor PD-1 antibody. Front Oncol. 2020;10:594558. doi:10.3389/fonc.2020.594558
16. Keam SJ. Toripalimab: first global approval. Drugs. 2019;79(5):573–578. doi:10.1007/s40265-019-01076-2
17. Ye D, Liu J, Zhou A, et al. Tislelizumab in Asian patients with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Sci. 2021;112(1):305–313. doi:10.1111/cas.14681
18. Xu B, Sun H-C. Camrelizumab: an investigational agent for hepatocellular carcinoma. Expert Opin Investig Drugs. 2021;1–10. doi:10.1080/13543784.2022.2022121
19. Chen J, Hu X, Li Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients. Ann Transl Med. 2020;8(18):1187. doi:10.21037/atm-20-6063
20. Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer. 2020;8:1. doi:10.1136/jitc-2019-000437
21. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi:10.1056/NEJMoa1304369
22. Li X, Huang D-B, Zhang Q, et al. The efficacy and toxicity of chemotherapy in the elderly with advanced pancreatic cancer. Pancreatology. 2020;20(1):95–100. doi:10.1016/j.pan.2019.11.012
23. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi:10.1056/NEJMoa1809615
24. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2
25. Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi:10.1016/S1470-2045(21)00152-2
26. Pilz LR, Manegold C, Schmid-Bindert G. Statistical considerations and endpoints for clinical lung cancer studies: can progression free survival (PFS) substitute overall survival (OS) as a valid endpoint in clinical trials for advanced non-small-cell lung cancer? Transl Lung Cancer Res. 2012;1(1):26–35. doi:10.3978/j.issn.2218-6751.2011.12.08
27. Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother. 2020;124:109821. doi:10.1016/j.biopha.2020.109821
28. Frelaut M, Du Rusquec P, de Moura A, Le Tourneau C, Borcoman E. Pseudoprogression and hyperprogression as new forms of response to immunotherapy. BioDrugs. 2020;34(4):463–476. doi:10.1007/s40259-020-00425-y
29. Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47. doi:10.1186/s13045-020-00886-2
30. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi:10.1038/s41577-019-0210-z
31. Ataera H, Hyde E, Price KM, Stoitzner P, Ronchese F. Murine melanoma-infiltrating dendritic cells are defective in antigen presenting function regardless of the presence of CD4CD25 regulatory T cells. PLoS One. 2011;6(3):e17515. doi:10.1371/journal.pone.0017515
32. McDonnell AM, Lesterhuis WJ, Khong A, et al. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol. 2015;45(1):49–59. doi:10.1002/eji.201444722
33. Lipson EJ, Forde PM, Hammers H-J, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42(4):587–600. doi:10.1053/j.seminoncol.2015.05.013
34. Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi:10.1038/nature16965
35. Guo S, Shi X, Gao S, et al. The landscape of genetic alterations stratified prognosis in oriental pancreatic cancer patients. Front Oncol. 2021;11:2900.
36. Loong HH, Du N, Cheng C, et al. KRAS G12C mutations in Asia: a landscape analysis of 11,951 Chinese tumor samples. Transl Lung Cancer Res. 2020;9(5):1759–1769. doi:10.21037/tlcr-20-455
37. Mann KM, Ying H, Juan J, Jenkins NA, Copeland NG. KRAS-related proteins in pancreatic cancer. Pharmacol Ther. 2016;168:29–42. doi:10.1016/j.pharmthera.2016.09.003
38. Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17(3):153–168. doi:10.1038/s41575-019-0245-4
39. Bournet B, Buscail C, Muscari F, Cordelier P, Buscail L. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: hopes and realities. Eur J Cancer. 2016;54:75–83. doi:10.1016/j.ejca.2015.11.012
40. Dong Z-Y, Zhong W-Z, Zhang X-C, et al. Potential predictive value of and mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–3024. doi:10.1158/1078-0432.CCR-16-2554
41. Kaushik AC, Wang YJ, Wang X, Wei DQ. Irinotecan and vandetanib create synergies for treatment of pancreatic cancer patients with concomitant TP53 and KRAS mutations. Brief Bioinform. 2021;22(3). doi:10.1093/bib/bbaa149
42. Hashimoto S, Furukawa S, Hashimoto A, et al. ARF6 and AMAP1 are major targets of and mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc Natl Acad Sci U S A. 2019;116(35):17450–17459. doi:10.1073/pnas.1901765116
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
