Back to Journals » Cancer Management and Research » Volume 10
The efficacy and safety of olaparib in the treatment of cancers: a meta-analysis of randomized controlled trials
Authors Guo XX, Wu HL, Shi HY, Su L, Zhang X
Received 30 March 2018
Accepted for publication 12 June 2018
Published 10 August 2018 Volume 2018:10 Pages 2553—2562
DOI https://doi.org/10.2147/CMAR.S169558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Antonella D'Anneo
Xiao Xia Guo,1,* Hong Li Wu,2,* Hong Yun Shi,3 Lei Su,3 Xi Zhang3
1Department of Obstetrics and Gynecology, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, Chengdu, 2Department of Obstetrics and Gynecology, Hebei University Affiliated Hospital, 3Department of Radiation Oncology, Hebei University Affiliated Hospital, Baoding, China
*These authors contributed equally to this work
Purpose: PARP inhibition is an exciting new anticancer strategy. As the first PARP inhibitor approved for the treatment of advanced BRCA-mutated ovarian cancer, olaparib has proven to be effective in the treatment of several solid tumors. We performed a meta-analysis of published randomized controlled trials to evaluate the efficacy and safety of olaparib in cancer patients.
Methods: PubMed, Embase, and oncology-conference proceedings were searched for relevant studies. End points were overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and grade 3/4 adverse events. Pooled hazard ratio (HR)/risk ratio (RR) and 95% CI were calculated using random or fixed-effect models.
Results: Eight trials involving 1,957 patients were ultimately identified. The pooled analysis demonstrated that olaparib treatment significantly improved PFS (HR 0.62, 95% CI 0.47–0.82; P=0.001), OS (HR 0.82, 95% CI 0.73–0.93; P=0.001), and ORR (RR 1.38, 95% CI 1.16–1.65; P<0.001) when compared with therapy not containing olaparib. This association was further confirmed by sensitivity analysis. Additionally, olaparib treatment offered a significant survival benefit for patients with BRCA mutation. Moreover, treatment with olaparib was associated with a significant increase in risk of severe anemia.
Conclusion: Olaparib treatment has better treatment response compared with therapy not containing olaparib, whereas olaparib can increase the risk of severe anemia.
Keywords: olaparib, efficacy, safety, cancers, meta-analysis, RCTs
Introduction
As our knowledge of cancer biology grows, cancer therapy has evolved significantly. However, with approximately 14.1 million new cancer cases and 8.2 million deaths according to global cancer statistics in 2015, there is a clear need for continued improvement in cancer therapy. Agents targeting specific molecular defects that characterize certain cancer cells are a novel cancer therapy to increase treatment efficacy and reduce toxicities. The discovery of the PARP family of nuclear enzymes, which is recruited to repair DNA damage in cells, opened the possibility of developing a new class of antineoplastic drugs. PARPs are a family of enzymes composed of 17 members,1 and PARP1 is the protein that is best described. PARP1 plays a critical role in the base-excision-repair pathway, controlling the repair of single-strand breaks in DNA.2 With the ability to interfere with the DNA damage repair systems, PARP inhibitors (PARPis) can effectively eliminate a cell’s capacity to repair single-strand breaks, leading to double-strand breaks. Other DNA-repair mechanisms, specifically homologous recombination and the nonhomologous end-joining pathways, have been utilized.3,4 However, BRCA1- and BRCA2-mutation carriers are unable to utilize homologous recombination fully to repair double-strand breaks and low-fidelity repair by nonhomologous end joining is activated, and this absence of an accurate repair mechanism results in cell death.5,6 Until now, the most investigated PARPi has been olaparib (AZD2281, KU0059436), which is an orally available compound with activity against PARP1 and PARP2. As an important novel agent of anticancer drugs, olaparib has undergone comprehensive clinical evaluation as single and combination therapy in several cancer types, like ovarian cancer, breast cancer, and gastric cancer.7–9 It is important to summarize those results, offering evidence-based references for clinicians. We thus performed this meta-analysis of all published Phase II–III randomized controlled trials (RCTs) to evaluate the efficacy and safety of olaparib in the treatment of various advanced or metastatic cancers.
Methods
Data sources and search strategy
Study selection was conducted according to the Preferred Reporting Items for Systemic Reviews and Meta-Analyses statement.10 An independent review of citations from PubMed and Embase from January 2000 to January 2018 was conducted. We also searched abstracts and meeting presentations from the annual meetings of the American Society of Clinical Oncology and the European Society of Medical Oncology to identify relevant clinical trials. Additionally, we searched the clinical trial-registration website http://www.clinicaltrials.gov to obtain information on the registered RCTs. The search was conducted using the keywords “olaparib”, “AZD2281”, “KU-0059436”, “RCT”, “trial”, and “cancer”, and was restricted to RCTs published in English. When duplicate publications were identified, only the most complete, recent, and updated report of clinical trials was included in the meta-analysis.
Study selection
Clinical trials that met the following criteria were included in this meta-analysis: prospective randomized controlled Phase II and III clinical trails in cancer patients, participants assigned to treatment with olaparib containing therapy or control (placebo or chemotherapy), and the study had included sufficient data for extraction. Phase I and single-arm Phase II trials were excluded, due to lack of control groups.
Data extraction and quality of studies
Information extracted comprised the study, trial phase, underlying malignancy, dosage of olaparib, dosing schedules used in treatment and control arms, median age, median progression-free survival (PFS) and median overall survival (OS). Data extraction was performed independently by two reviewers, and any discrepancies were resolved by consensus. The quality of clinical trial reports was assessed according to the five-item Jadad scale, including randomization, double-blinding, and withdrawals.11 Severe (grade 3 or 4) adverse events (AEs) were defined according to the third or fourth version of the Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute.
Statistical analysis
The efficacy of olaparib in treating cancers was evaluated by calculating pooled OS, PFS and overall response rate (ORR), and 95% CIs based on data from all studies. ORR was defined as the sum of partial- and complete-response rates according to the response-evaluation criteria in solid tumors. To assess the stability of the pooled results, sensitivity analysis was performed by sequential omission of individual studies. Statistical analysis of overall HRs for OS and PFS and RRs for ORR and grade 3 or 4 AEs was calculated using version 2 of the Comprehensive Meta Analysis (Biostat, Englewood, NJ, USA) program. For the meta-analysis, both fixed-effect (weighted with inverse variance) and random-effect models were considered.12,13 The χ2-based Q statistic was applied to estimate between-study heterogeneity, and inconsistency was quantified with the I2 statistic, which represents the percentage of total variation across studies that is attributable to heterogeneity rather than chance.14 Heterogeneity was considered statistically significant when P<0.1. When substantial heterogeneity was not observed, the pooled estimate was calculated based on the fixed-effect model. If substantial heterogeneity existed, data were analyzed using the random-effect model. Publication bias was evaluated by Begg’s and Egger’s tests.15,16 A two-tailed P-value <0.05 was considered statistically significant.
Results
Search results
A total of 214 potentially relevant studies were retrieved electronically. Then, 203 studies were excluded according to the inclusion and exclusion criteria by screening the title, abstract and keywords of each record. Finally, eight RCTs were included after removal of duplicated reports and reports with both arms receiving olaparib. The detailed selection process and reasons for exclusion are presented in Figure 1.
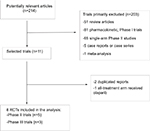  | Figure 1 Flowchart of selection process for trials included in meta-analysis. Abbreviation: RCTs, randomized controlled trials. |
Study characteristics
In total, 1,957 patients from eight RCTs7–9,17–21 were available for the meta-analysis, of whom 786 had ovarian cancer,7,18–20 302 breast cancer,9 649 gastric cancer,8,17 and 220 small-cell lung cancer (SCLC).21 Of the included studies, three were Phase III RCTs7–9 and five were Phase II RCTs.17–21 All studies reported sufficient data on PFS7–9,17–21 and OS,7–9,17–21and six reported sufficient data on ORR.8,9,17–20 Additionally, five trials reported sufficient data on efficacy of olaparib in patients with BRCA mutations,7,9,18–20 and two trials reported efficacy data in patients with ATM deficiency.8,17 Two schedules of olaparib oral tablets were investigated in the analysis conducted by Woll et al: 300 mg twice daily or 200 mg thrice daily.21 Jadad scores are listed for each trial in Table 1. The mean Jadad score was 4.4 (range 3–5), indicating that overall methodological quality of the included studies was generally good and fair. All trials reported grade 3 or 4 AEs according to CTCAE version three or four criteria. All selected trials included patients with an Eastern Cooperative Oncology Group performance status of ≤2 and with adequate organ, coagulation, and hematological functions.
Progression-free survival
All studies reported data on PFS. The combined HR for PFS was 0.62 (95% CI 0.47–0.82, P=0.001; Figure 2) indicating a significant improvement in PFS with olaparib treatment when compared with control therapy. There was significant heterogeneity among trials (I2=84.9%, P<0.001), and the pooled HR was calculated by using the random-effect model. Subgroup analysis based on tumor type was conducted to investigate the source of the heterogeneity (Table 2). We found olaparib treatment significantly improved PFS in ovarian cancer (HR 0.44, 95% CI 0.30–0.67; P<0.001), gastric cancer (HR 0.83, 95% CI 0.70–0.99; P=0.036), and breast cancer (HR 0.58, 95% CI 0.43–0.79; P=0.001), but not for SCLC (HR 0.88, 95% CI 0.69–1.13; P=0.321). In the BRCA-mutation group, the pooled HR showed that olaparib-containing therapy significantly improved PFS (HR 0.37, 95% CI 0.22–0.63; P<0.001).
  | Figure 2 Forest plots of pooled HRs for PFS by random-effect model. Abbreviations: HR, hazard ratio; PFS, progression-free survival. |
Overall survival
All trials reported data on OS. The pooled HR for OS favored olaparib therapy, yielding an HR of 0.82 (95% CI 0.73–0.93, P=0.001; Figure 3) using a fixed-effect model (I2=6%, P=0.385). Subgroup analysis by tumor type showed that olaparib significantly improved OS in gastric cancer (HR 0.75, 95% CI 0.62–0.90; P=0.002), but not for ovarian cancer (HR 0.83, 95% CI 0.68–1.02; P=0.075), breast cancer (HR 0.90, 95% CI 0.63–1.29; P=0.564), or SCLC (HR 0.99, 95% CI 0.71–1.39; P=0.980). Five trials evaluating BRCA-mutated patients demonstrated a significant improvement in OS, giving an HR of 0.78 (95% CI 0.62–0.98, P=0.030).
  | Figure 3 Forest plots of pooled HRs for OS by fixed-effect model. Abbreviations: HR, hazard ratio; OS, overall survival. |
Overall response rate
Six of the eight included trials reported data on ORR, and the pooled RR for ORR showed that there was a significant improvement for olaparib-containing therapy, with an RR of 1.38 (95% CI 1.16–1.65, P<0.001; Figure 4). There was no significant heterogeneity between the trials (I2=41.1%, P=0.132), and the pooled RR was performed using the fixed-effect model. Additionally, subgroup analysis according to tumor type showed that olaparib-containing therapy significantly improved ORR in gastric cancer (RR 1.51, 95% CI 1.04–2.21; P=0.032) and breast cancer (RR 2.08, 95% CI 1.40–3.10; P<0.001). Although there was a trend toward improving ORR in ovarian cancer (RR 1.16, 95% CI 0.92–1.47; P=0.205), the pooled RR did not achieve statistical significance. Moreover, olaparib-containing therapy showed a significant improvement in ORR (RR 2.01, 95% CI 1.42–2.85; P<0.001) in BRCA-mutated patients.
  | Figure 4 Forest plots of pooled RRs for ORR by fixed-effect model. Abbreviations: ORR, overall response rate; RR, risk ratio. |
Safety
To evaluate the safety of olaparib, pooled analysis of reported grade 3 and 4 AEs of interest was also performed. Neutropenia and anemia were the most common severe AEs, with an incidence of 23.1% (95% CI 10.3%–43.9%) for neutropenia and 12.5% (95% CI 9.2%–16.7%) for anemia. However, the severity of neutropenia may have been driven by combination chemotherapy. The pooled incidence of severe neutropenia was 7.2% (95% CI 4.0%–12.5%) in patients treated with olaparib monotherapy, which was significantly lower than the incidence of combined olaparib and chemotherapy (41.9%, 95% CI 27.4%–57.9%). Other less common olaparib related grade 3 and 4 AEs were fatigue (4.8%), nausea (2.3%), vomiting (2.2%), thrombocytopenia (2.1%), diarrhea (1.4%), increased AST (1.8%), increased ALT (1.5%), decreased appetite (1.0%), headache (0.8%), and urinary tract infection (0.5%). There was more grade 3 or 4 anemia (RR 2.21, 95% CI 1.53–3.49; P<0.001) and decreased appetite (RR 3.50, 95% CI 1.08–11.33; P=0.037) incidents in olaparib-containing therapy when compared with control therapy. With regard to the risk of grade 3 or 4 neutropenia, thrombocytopenia, fatigue, nausea, vomiting, diarrhea, increased AST, increased ALT, headache, and urinary tract infection, equivalent frequencies were found between the two groups (Table 3).
Sensitivity analysis
Sensitivity analysis was performed to examine the stability and reliability of pooled HRs and RRs by sequential omission of individual studies. Our results showed that significance estimates of PFS, OS, and ORR were not significantly influenced by omitting any single study (Figure 5).
Publication bias
We used Begg’s funnel plot and Egger’s test to assess the publication bias of literature. Begg’s funnel plots did not reveal any evidence of obvious asymmetry (P=0.92 for OS, P=0.75 for PFS, P=0.71 for ORR). Egger’s test further confirmed these results, and did not suggest any evidence of publication bias (P=0.74 for OS, P=0.65 for PFS, P=0.23 for ORR).
Discussion
As a first-in-class PARPi, olaparib has undergone comprehensive clinical evaluation as single and combination therapy in various malignancies. Olaparib has demonstrated stable response in diseases like ovarian cancer, breast cancer, and gastric cancer, and its efficacy seems more marked in patients with BRCA-mutation tumors.7–9 A previous meta-analysis demonstrated that the use of olaparib could improve PFS, but showed no significant efficacy in OS.22 However, only three Phase II RCTs evaluating the efficacy of olaparib in ovarian cancer were analyzed in that meta-analysis. Therefore, limited information about olaparib treatment was able to be gained. Recently, several new RCTs studying olaparib in ovarian cancer, breast cancer, gastric cancer, and SCLC have finished, and thus the efficacy and safety of olaparib should be reassessed. As a result, we systematized the information available to perform this meta-analysis to investigate the role of olaparib in cancer treatment. Our study, including 1,957 patients from eight RCTs, demonstrated that olaparib treatment provided substantial benefit in terms of PFS, OS, and ORR. Moreover, similar risks of severe hematologic and nonhematologic toxicities were found between olaparib-containing therapy and therapy not containing olaparib, except for anemia and decreased appetite.
The main tumor types included in this analysis were ovarian cancer, breast cancer, gastric cancer, and SCLC. According to subgroup analysis, olaparib significantly improved PFS in ovarian cancer, breast cancer, and gastric cancer, but not in SCLC. PARPis have demonstrated greater efficacy in cancers with deleterious mutations in BRCA1 or BRCA2 or when used in clinical situations where homologous recombination deficiency is operative. Compared with wild-type cells, BRCA1 and BRCA2-deficient cells were up to 1,000-fold more sensitive to PARP inhibition.6 Similarly, our analysis found that patients with BRCA mutations showed significant improvement in OS, but the increased OS did not translate to trials that did not require patients to be BRCA-mutated (HR 0.91, 95% CI 0.57–1.43; P=0.671), which may indicate that mutations in BRCA1/BRCA2 are a marker for olaparib response. In addition, it has been reported that the estimated prevalence of a BRCA1/BRCA2 mutation in patients with newly diagnosed high-grade serous ovarian cancer is 20%–25%, which might be higher in patients with platinum-sensitive, relapsed ovarian cancer.23,24 Approximately 5% of unselected patients with breast cancer carry a germ-line BRCA mutation, which is more likely to be present in patients with a strong family history of breast cancer, younger patients, and triple-negative breast cancer patients.25 Therefore, the reason may be that most patients in the ovarian and breast cancer groups had BRCA mutations, while those in SCLC group did not. In addition to BRCA mutation, deficiency in ATM, a key activator of the DNA-damage response to double-strand breaks, has been associated with increased sensitivity of olaparib treatment in gastric cancer, lung adenocarcinoma, pancreatic ductal adenocarcinoma, and chronic lymphocytic leukemia.26–30 Our subgroup analysis demonstrated that olaparib-containing therapy provided substantial benefit for gastric cancer in terms of OS, but this benefit was not found in patients with ATM deficiency. However, Bang et al found the median OS in ATM-negative patients was longer than that in the overall population.8,17 One possible explanation may be that the ATM-negative population might have been too small to determine a difference between treatment groups. To further explore the role of olaparib in the treatment of ATM-negative patients, future studies with a larger proportion of ATM-negative patients are recommended.
PARPis work based on the concept of synthetic lethality, and the synergy effect of a combined PARPi and chemotherapy has been well established. For example, PARPis are highly synergistic when used in combination with topoisomerase I inhibitors and alkylating agents, such as temozolomide.31,32 However, combinations of PARPis and chemotherapy agents may lead to overlapping toxicities, such as myelosuppression. In this analysis, severe neutropenia, which occurred in 23.1% of patients treated with olaparib, was the most common AE. Similarly, our previous analysis found that olaparib was associated with an increased risk of severe neutropenia.33 However, those results should be explained carefully, because the severity of neutropenia may be driven by combination chemotherapy. With new RCTs evaluating olaparib monotherapy published, we found the calculated summary incidence of severe neutropenia was only 7.2%, which is significantly lower than olaparib combined with chemotherapy. In addition, the use of olaparib treatment was associated with an increased risk of severe anemia, with an RR of 2.21 (95% CI 1.53–3.49, P<0.001). Sensitivity analysis also confirmed these results. Moreover, subgroup analysis showed the overall incidence of severe anemia was not significantly influenced by combination therapy, with an incidence rate of 13.1% for olaparib combined with chemotherapy and an incidence rate of 12.3% for olaparib monotherapy. PARPis are well known for interfering with successful DNA repair, especially for rapidly dividing cell populations, such as in gut lining and bone marrow. As one of the most common severe hematologic AEs, anemia can cause dose discontinuation, interruption, and modification of olaparib. Early detection and effective management of hematologic toxicities that can occur with olaparib treatment is crucial for safer use of this drug. To reduce the risk of olaparib-related hematologic toxicities, drug interactions should be considered, especially when drugs are coadministered with other myelosuppressive anticancer agents. Moreover, CYP3A inhibitors and inducers should not be used concomitantly with olaparib. As olaparib is primarily metabolized by CYP3A enzymes, coadministration with CYP3A inhibitors or inducers may affect metabolic clearance and alter plasma concentration of this drug.34
According to this analysis, severe fatigue and gastrointestinal (GI) toxicities were less common AEs in patients treated with olaparib. The pooled incidence of severe fatigue was 4.8%. Although the incidence was relatively low, severe fatigue can be particularly problematic in cancer patients. As many patients may have baseline fatigue symptoms from disease burden, all patients taking olaparib should be screened for fatigue, and its management should be a routine part of care. Patients who suffer from severe fatigue require timely intervention. Nonpharmacologic approaches include massage, exercise, maintenance of physical fitness, sleep hygiene, distractions, and other psychosocial methods. Pharmacological interventions, such as psychostimulants, sleep-aid medication, and treatment of underlying pain or depression, may help alleviate fatigue.35,36 Additionally, the experience of severe GI toxicity is one of the challenges for olaparib treatment.37 Fortunately, the incidence of severe GI events is relatively low. In our analysis, severe nausea was one of the most common GI AEs, but <2.5% patients experienced it. Counseling patients proactively about the risk of nausea and vomiting and providing upfront prescriptions for prochlorperazine and lorazepam as needed is key to helping patients maintain adherence to the prescribed dose of olaparib. Thorough evaluation and aggressive management are required in patients with severe diarrhea, dose interruption, or dose modification of olaparib, and is an acceptable way to manage significant treatment-related diarrhea.38
Another concern with combinations of PARPis and chemotherapy agents is the increased severity of mutations, and it has been postulated that DNA-damaging chemotherapy in combination with impaired DNA-repair pathways may prime patients for the development of myelodysplastic syndrome/acute myeloid leukemia. Monitoring of complete blood counts is warranted for patients receiving PARPis, and further investigations should be conducted for prolonged hematological toxicities. If myelodysplastic syndrome/acute myeloid leukemia is confirmed, discontinue olaparib.39
Despite PARPi therapy’s clinical promise, resistance to PARPis remains a challenge for the implementation of olaparib. Multiple mechanisms of resistance to PARPi therapy have been identified. The development of secondary mutations that restore BRCA functionality is one of the established mechanisms of resistance to PARPi.40,41 The second mechanism involves upregulation of P-glycoproteins with consequent increasing PARPi efflux from tumor cells.42 The third mechanism of PARPi resistance is based on loss of 53BP1, which is able to increase the activity of BRCA1 or BRCA2 variants encoded by hypomorphic alleles and rescue of DNA end resection in BRCA1-deficient tumors, which ultimately leads to reduced sensitivity to PARPi.43 The existence of resistance mechanisms is not unexpected in the context of increased clinical use of PARPis, and strategies to overcome acquired resistance will be pertinent.
There are several limitations that need to be considered in our meta-analysis. Firstly, this analysis was not based on individual patient data; therefore, confounding variables at the patient level, such as age, race, and disease status, could not be assessed properly or incorporated into the analysis. In addition, there were potential differences among the studies included, including different types of malignancies, dosage, and concomitant therapies. All of these would increase the clinical heterogeneity among included trials, which also makes the interpretation of a meta-analysis more problematic. Secondly, OS was not the primary end point for most trials included in this analysis, except for Bang et al,8 which may lead to a question of immature data for OS. As such, the efficacy of olaparib in cancer patients still needs to be investigated during the long-term follow-up of these trials. Thirdly, not all articles had available data on OS, PFS, ORR, and some adverse effects.
Conclusion
In conclusion, our meta-analysis demonstrates that olaparib treatment has better treatment response compared with therapy not containing olaparib. The profile of BRCA mutation may allow expansion of the population able to derive clinical benefit from PARP inhibition, and should be further investigated in future trials. Treatment with olaparib is associated with an increased risk of developing severe anemia. Clinicians should be aware of the risk and perform regular hematological monitoring.
Disclosure
The authors report no conflicts of interest in this work.
References
Amé JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26(8):882–893. | ||
Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2(12):1134–1149. | ||
Walsh CS. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecol Oncol. 2015;137(2):343–350. | ||
Brown JS, Kaye SB, Yap TA. PARP inhibitors: the race is on. Br J Cancer. 2016;114(7):713–715. | ||
Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–3790. | ||
Farmer H, Mccabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. | ||
Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. | ||
Bang YJ, Xu RH, Chin K, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy (GOLD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1637–1651. | ||
Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. | ||
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. | ||
Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Bang YJ, Im SA, Lee KW, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol. 2015;33(33):3858–3865. | ||
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. | ||
Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and PEGylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–379. | ||
Oza AM, Cibula D, Benzaquen AO, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97. | ||
Woll P, Gaunt P, Steele N, et al. STOMP: a UK National Cancer Research Network randomised, double blind, multicentre phase II trial of olaparib as maintenance therapy in SCLC. J Thorac Oncol. 2017;12(1 Suppl):S704–S705. | ||
Bao Z, Cao C, Geng X, et al. Effectiveness and safety of poly(ADP-ribose) polymerase inhibitors in cancer therapy: a systematic review and meta-analysis. Oncotarget. 2016;7(7):7629–7639. | ||
Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. | ||
Zhang S, Royer R, Li S, et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121(2):353–357. | ||
Malone KE, Daling JR, Doody DR, et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66(16):8297–8308. | ||
Hodgson D, Mason H, Oplustilova L, et al. Activity of the PARP inhibitor olaparib in ATM-deficient gastric cancer: from preclinical models to the clinic. Cancer Res. 2014;74(19 Suppl):2398. | ||
Kubota E, Williamson CT, Ye R, et al. Low ATM protein expression and depletion of p53 correlates with olaparib sensitivity in gastric cancer cell lines. Cell Cycle. 2014;13(13):2129–2137. | ||
Schmitt A, Knittel G, Welcker D, et al. ATM deficiency is associated with sensitivity to PARP1- and ATR inhibitors in lung adenocarcinoma. Cancer Res. 2017;77(11):3040–3056. | ||
Perkhofer L, Schmitt A, Romero Carrasco MC, et al. ATM deficiency generating genomic instability sensitizes pancreatic ductal adenocarcinoma cells to therapy-induced DNA damage. Cancer Res. 2017;77(20):5576–5590. | ||
Knittel G, Rehkämper T, Korovkina D, et al. Two mouse models reveal an actionable PARP1 dependence in aggressive chronic lymphocytic leukemia. Nat Commun. 2017;8(1):153. | ||
Patel AG, Flatten KS, Schneider PA, et al. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J Biol Chem. 2012;287(6):4198–4210. | ||
Murai J, Zhang Y, Morris J, et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther. 2014;349(3):408–416. | ||
Zhou JX, Feng LJ, Zhang X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2017;11:3009–3017. | ||
Dirix L, Swaisland H, Verheul HM, et al. Effect of itraconazole and rifampin on the pharmacokinetics of olaparib in patients with advanced solid tumors: results of two phase I open-label studies. Clin Ther. 2016;38(10):2286–2299. | ||
National Comprehensive Cancer Institute. NCCN guidelines for supportive care: cancer-related fatigue. 2015. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#fatigue. Accessed June 28, 2018. | ||
Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. | ||
Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. | ||
Moore KN, Monk BJ. Patient counseling and management of symptoms during olaparib therapy for recurrent ovarian cancer. Oncologist. 2016;21(8):954–963. | ||
Lynparza [homepage]. Available from: https://www.lynparza.com. Accessed June 27, 2018. | ||
Sakai W, Swisher EM, Jacquemont C, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69(16):6381–6386. | ||
Swisher EM, Sakai W, Karlan BY, et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68(8):2581–2586. | ||
Wurzer G, Herceg Z, Wesierska-Gadek J. Increased resistance to anticancer therapy of mouse cells lacking the poly(ADP-ribose) polymerase attributable to up-regulation of the multidrug resistance gene product P-glycoprotein. Cancer Res. 2000;60(15):4238–4244. | ||
Bunting SF, Callén E, Wong N, et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell. 2010;141(2):243–254. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




