Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
The Efficacy and Safety of Chinese Medicine Fufang Zhenzhu Tiaozhi Capsule (FTZ) in the Treatment of Diabetic Coronary Heart Disease: Study Protocol for Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Authors Wang L , Xiang L, Piao S, Gong X, Zhou W, Feng W, Li H, Li L, Wei A, Zhu Q, Rong X, Guo J
Received 3 March 2021
Accepted for publication 13 May 2021
Published 14 June 2021 Volume 2021:14 Pages 2651—2659
DOI https://doi.org/10.2147/DMSO.S309419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Lexun Wang,1 Lei Xiang,1,2 Shenghua Piao,1 Xiao Gong,3 Wanxing Zhou,4 Weixun Feng,5 Huilin Li,6 Leyu Li,7 Aisheng Wei,8 Qing Zhu,1 Xianglu Rong,1 Jiao Guo1,2
1Guangdong Metabolic Diseases Research Center of Integrated Chinese and Western Medicine; Key Laboratory of Glucolipid Metabolic Disorder, Ministry of Education of China; Institute of Chinese Medicine, Guangdong Pharmaceutical University; Guangdong TCM Key Laboratory for Metabolic Diseases, Guangzhou, People’s Republic of China; 2The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China; 3School of Public Health, Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China; 4Department of Internal Cardiology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China; 5Famous Doctor’s Studio, Qingyuan Hospital of Traditional Chinese Medicine, Qingyuan, People’s Republic of China; 6Department of Endocrinology, Shenzhen Traditional Chinese Medicine Hospital, The Fourth Clinical Medical College of Guangzhou University of Chinese Medicine, Shenzhen, People’s Republic of China; 7Department of Endocrinology, Zhongshan Hospital of Traditional Chinese Medicine, Zhongshan, People’s Republic of China; 8Department of Endocrinology, Foshan Hospital of Traditional Chinese Medicine, Foshan, People’s Republic of China
Correspondence: Jiao Guo
Guangdong Metabolic Diseases Research Center of Integrated Chinese and Western Medicine; Key Laboratory of Glucolipid Metabolic Disorder, Ministry of Education of China; Institute of Chinese Medicine, Guangdong Pharmaceutical University; Guangdong TCM Key Laboratory for Metabolic Diseases, Guangzhou, People’s Republic of China
Tel +86-02-39352818
Fax +86-02-39352607
Email [email protected]
Background: Diabetic coronary heart disease (DCHD), the main macrovascular complication of type 2 diabetes mellitus (T2DM), is greatly harmful to T2DM patients. Traditional Chinese medicine (TCM) is an alternative and effective therapy to delay the development of macrovascular diseases, but the existing evidence of its efficacy and safety is insufficient. The aim of this multicenter, randomized, double-blind, placebo-controlled trial is to evaluate the efficacy and safety of Chinese Medicine Fufang Zhenzhu Tiaozhi capsule (FTZ) in treating DCHD.
Patients and Methods: This study includes a 2-week run-in, 52-week treatment, and 52-week post-treatment follow-up. A total of 160 participants will be recruited and randomized into two groups. The treatment group will receive FTZ and basic treatment, while the control group will receive the placebo and basic treatment. The primary outcome is the combined outcome including the major adverse cardiovascular events, coronary restenosis, and unplanned revascularization. The combined secondary outcomes include all-cause mortality, acute coronary syndrome, ischemic stroke, heart failure, unplanned re-hospitalization mainly caused by acute complications of diabetes, other thromboembolic events, and TCM symptom indicators. The safety outcomes and adverse events will also be evaluated in this trial.
Discussion: This trial evaluates the clinical effectiveness and safety of FTZ in patients with DCHD. The results are important to further explore the effectiveness of the comprehensive strategy “Tiao Gan Qi Shu Hua Zhuo” (modulating Gan, trigging key metabolic system to resolve pathogenic factors such as phlegm retention and dampness) in the prevention and control of glucolipid metabolic disorders (GLMD) including DCHD and T2DM. On the other hand, this study is the first trial of FTZ to observe cardiovascular outcomes through long-term follow-up after treatment of DCHD, which is of great value.
Trial Registration: This trial was registered in the Chinese Clinical Trial Registry on April 07, 2019 (No. ChiCTR1900022345).
Keywords: type 2 diabetes mellitus, traditional Chinese medicine, clinical study
Introduction
Diabetic coronary heart disease (DCHD), the major macrovascular complication of type 2 diabetes mellitus (T2DM), is the leading cause of death among adults with T2DM.1–3 Recent clinical study has shown that 73.1% of T2DM patients have cardiovascular diseases.4 Moreover, some reports show that T2DM patients with DCHD are at a 2-fold to 4-fold higher risk of mortality compared to those without DCHD.4,5 Despite there are the significant progresses in basic research, medications, and intervention devices, the clinical prognosis of DCHD is still poor.6
In the treatment of T2DM, blood glucose control is the main goal. However, several clinical studies show that the effect of intensive glucose control in diabetic macrovascular disease is still uncertain.7–10 The cause of this situation might be that various factors such as inflammation, oxidative stress, and lipotoxicity lead to diabetic macrovascular injury. DCHD is not only directly related to abnormal glycometabolism (such as elevated fasting blood glucose, insulin resistance, and abnormality of glycosylated hemoglobin) but also closely associated with cardiovascular risk factors (including hypertension, obesity, atherosclerosis, dyslipidemia, etc.).11–14 In recent years, the concept of comprehensive prevention and control of the risk factors in the treatment of DCHD is gradually accepted.1,15,16
Based on holism and syndrome differentiation, traditional Chinese medicine (TCM) has certain advantages in the prevention and treatment of glucolipid metabolic disorders (GLMD) including diabetes and CHD.15 In the theory of TCM, the incidence of diabetes is fundamentally due to damage to the body. The decline of the body’s function can produce hidden pathogenic factors and continuously damage arteries.17 Moreover, the concept that “blood stasis” plays a key role in the occurrence and development of CHD has been widely accepted by TCM doctors and researchers.18,19 Currently, the number of clinical trials about the efficacy of Chinese herbal medicine (CHM) in treating CHD increases greatly.20–24
Different from CHD, however, DCHD is also related to various factors of glucolipid metabolism disorder. In the theory of TCM, the key pathogenesis of DCHD is “Gan-depression and Pi-mistransportion as fundamental, phlegm-damp-stasis stagnation as manifestation“.25 Based on this concept, our research group proposed “Tiao Gan Qi Shu Hua Zhuo” (modulating Gan, trigging key metabolic system of Pi to resolve pathogenic factors such as phlegm retention and dampness) in prevention and control of DCHD. “Tiao Gan” means to regulate Gan qi, as Gan qi up and down smoothly, the whole body qi will not be obstructed, then “Five Zang” get peace and cooperation. “Qi Shu” means to trigger the key to the regulation of the metabolism thereafter maintaining the normal functions of Pi and Wei (digestion and the absorption and transporting of nutrition). “Hua Zhuo” refers to the continuous elimination of pathogenic factors, such as phlegm turbidity, waste metabolites, and finally prevents and controls the development of disorders in the metabolism of glucose and lipid.15 And the CHM formula Fufang Zhenzhu Tiaozhi capsule (FTZ) was innovated under this rationale. Our previous studies have demonstrated that due to its multi-components, FTZ improved glucose and lipid metabolism by regulating various targets, such as HMG-CoA reductase, cytochrome P450 7A1 (CYP7A1), insulin receptor substrate 1 (IRS1), and phosphatidylinositol 3 kinase (PI3K).26–28 In addition, FZT dose-dependently suppressed the atherosclerotic plaques and reduced the cholesterol contents in the aorta of ApoE−/- mice by reducing the expressions of CD36 and scavenger receptor A (SR-A).27 Furthermore, FTZ has been used as a hospital preparation (Yue pharmaceutical word: Z20110029) and is effective and safe in the treatment of hyperlipidemia, T2DM, atherosclerosis, etc. in clinical practice. However, the effectiveness of FTZ in the treatment of DCHD is unclear.
This study protocol describes a planned clinical trial to assess the efficacy and safety of FTZ to treat DCHD.
Methods and Design
Study Design
This protocol is a prospective, multicenter, randomized, double-blind, placebo-controlled clinical trial for assessing the efficacy and safety of FTZ in the treatment of DCHD, which was developed following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist (Figure 1, Table 1).29 The initiating sponsor and coordinator of this clinical trial is the First Affiliated Hospital of Guangdong Pharmaceutical University. The other four participants are Foshan Hospital of TCM, Zhongshan Hospital of TCM, Qingyuan Hospital of TCM, and Shenzhen TCM Hospital. We will recruit 160 eligible patients and randomly assign them to the FTZ group and the placebo group at a ratio of 1:1. After 2-week of the baseline assessment, patients will accept a 52-week treatment and 52-week post-treatment follow-up.
 |
Table 1 Schedule of Enrolment, Interventions and Assessments |
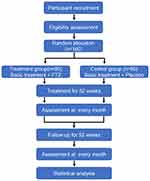 |
Figure 1 Study flowchart detailing randomization of patients with treatment and clinical follow-up. |
Participants
All in- or out-patients with DCHD from five hospitals will be referred for this study. Besides, open recruitment will be made by putting posters in clinics and advertising via WeChat. Patients retain the right to continue and discontinue the trial at any time during treatment and follow-up without prejudice.
Diagnostic Criteria
Diagnosis of DCHD must meet the T2DM diagnostic criteria and the CHD diagnostic criteria simultaneously.
The diagnostic criteria of T2DM will be defined on the basis of the China guidelines for type 2 diabetes (version 2013), as promulgated by the Chinese Diabetes Society.30 Diagnosis T2DM can be made if one of the following items is met:
- typical T2DM symptoms and random blood glucose ≥11.1 mmol/L;
- fasting blood glucose ≥7 mmol/L;
- 2 h blood glucose after the oral glucose tolerance test ≥11.1 mmol/L.
Referring to the Guideline for diagnosis and treatment of patients with chronic stable angina (version 2007),31 the diagnostic criteria of CHD are as follows: coronary angiography or CT angiography (CTA) indicating coronary stenosis of≥50% of at least one coronary artery.
Diagnostic criteria of TCM syndromes will be defined on the Standard of TCM diagnosis and treatment for diabetes mellitus complicated with heart disease (version 2011)32 and expert advice. The included patients need to meet all the main symptoms, the secondary symptoms [at least one of (1) and (2), at least one of (3)–(5), and at least one of (6) and (7)], as well as the tongue (8) and pulse (9) presentation, to be diagnosed with liver-stagnation, spleen-deficiency, and stasis and turbidity internal obstruction syndrome.
- Main symptoms: Sense of suppression in the chest; Chest pain;
- Secondary symptoms: (1) Emotional depression, irritability or sighing frequently; (2) Hypochondriac pain or distension in the hypochondriac region; (3) Torpid intake, sloppy stool, abdominal distension, or abdominal pain relief after catharsis; (4) Pale white complexion; (5) Lassitude of spirit and talking laziness, or fatigue and lack of strength; (6) Being overweight; (7) Copious phlegm; (8) Tongue signs: pale tongue, purple and dark tongue, enlarged tongue, or teeth-marked tongue; white fur, slimy fur or thick and slimy fur; (9) Pulse sings: string-like pulse, fine pulse, slippery pulse, fine and rough pulse, or bound and intermittent pulse.
Inclusion Criteria
The same criteria and rigidity for subject inclusion will be used in the five trial centers. Inclusion criteria are as follows:
- The patient simultaneously meets the diagnostic criteria of T2DM, CHD, and TCM diagnosis of liver-stagnation, spleen-deficiency, and stasis and turbidity internal obstruction syndrome;
- Aged between 18 and 85 years old, regardless of gender;
- Willingness to participate in the trial and to sign the informed consent, with a high degree of compliance and a cooperative attitude.
Exclusion Criteria
Patients will be excluded with any of the following:
- Type 1 diabetes mellitus or secondary diabetes;
- Unstable conditions, such as acute coronary syndrome, acute left heart failure, and acute complications of diabetes within three months;
- Plan that carry out cardiac intervention or operation within one year;
- Allergy to FTZ;
- Complicated with malignant tumors and life expectancy less than two years;
- Severe organic diseases (eGFR≤30%, abnormal expression of alanine aminotransferase (ALT) and creatine kinase (CK) of 2 times higher than the normal upper limit, acute and chronic respiratory failure, etc.);
- Women in pregnant or lactating;
- Psychiatric patients;
- Patients who are not considered by the researchers to meet the inclusion criteria.
Termination Criteria
The trial will be terminated in the participant if he/she has:
- Deteriorative conditions during treatment or presence of severe AE (ALT and/or CK ≥5 times of the normal value);
- Death from all causes;
- Acute complications of T2DM;
- Presence of life-threatening disease;
- The national administration agency requires the clinical trial to be halted.
The whole study plan will be terminated as follows:
- Presence of serious AE related to the research medication with supportive evidence;
- Completion of all follow-up assessments.
Randomization and Blinding
Random numbers will be generated by using SAS 9.4 software by an independent statistician. Grouping information kept in sealed opaque envelopes will be used for patient allocation. Researchers, participants and outcome assessors will be blinded during the trial period. All of the researchers will be trained before the trial begins, and strictly implement the principle of separation of the task. Statistician who generated randomization sequences and drug management staff is not directly involved in the trial process.
Interventions
The basic treatment of controlling hyperglycemia contains metformin and/or acarbose and/or repaglinide and/or insulin. Atorvastatin is selected to control hyperlipidemia, CCB (NORVASC) and/or ARB (APROVEL) and/or diuretic and/or COAPROVEL to control hypertension, and aspirin and/or clopidogrel to inhibit platelet. During intervention and follow-up, the basic treatment will be carried out by doctors according to the condition of participant. After enrollment, participants in both groups will take FTZ or the placebo drugs three times daily (4 capsules at a time) for 52 weeks. The constituents of FTZ are summarized in Table 2. The placebo is mainly made from starch. Placebo and FTZ are close to the same in color, smell, taste, appearance, packaging, and tag. The drugs will be distributed on every month’s visit. Both FTZ and placebo drugs will be manufactured by the Affiliated Pharmaceutical Factory of Guangdong Pharmaceutical University according to good manufacturing practice guidelines. To assess adherence to the intervention, the subjects will be told to bring the empty packet of the ingested test drug and the leftover. Subjects who have <80% drug compliance at the end of the study will be dropped out. The research team will periodically contact subjects by phone, text message, or WeChat to improve compliance and check for safety.
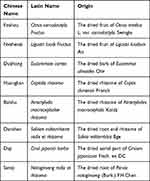 |
Table 2 Constituents of Chinese Medicine FTZ |
Follow-Up
The clinical follow-up will be performed every month for 12 months after drug intervention. The primary and secondary outcomes as well as safety will be evaluated at each follow-up.
Blood Glucose Detection
Blood glucose detection will be measured at the screening visit and every three months visit during treatment as well as follow-up. The blood glucose detection will include fasting blood glucose detection, 1h, 2h, and 3h of postprandial plasma glucose detection.
Electrocardiogram (ECG) and Blood Pressure Tests
ECG and blood pressure tests will be done at the screening visit and every three months visit. ECG, 24 h dynamic ECG, blood pressure, and 24 h ambulatory blood pressure will be assessed.
Ultrasonic Measurement
The cardiac structure and function, the carotid intima-media thickness, the liver structure, and the pulse wave velocity will be tested by color Doppler ultrasonography at the screening visit and every six months visit during treatment as well as follow-up.
Vessels Detection
At the screening visit and every six months visit, the vascular endothelial function will be evaluated through flow-mediated dilation (FMD) measurement of the brachial artery, the ankle-brachial index will be done, and the retinal blood vessel structure will be assessed by ophthalmoscope.
Blood Tests and Urine Routine Test
Urine routine test and blood tests will be done at the screening visit and every three months visit during drug intervention and follow-up. The blood tests will include blood routine, insulin, C-peptide, glycosylated hemoglobin, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, free fatty acid, apolipoprotein A, apolipoprotein B, lipoprotein-alpha, phospholipase A2, homocysteine, prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, hemorheology, alanine aminotransferase, aspartate aminotransferase, total protein, albumin, globulin, total bilirubin, direct bilirubin, indirect bilirubin, γ-glutamyltranspeptidase, leucyl aminopeptidase, creatine kinase, creatine kinase isoenzyme, lactate dehydrogenase, and lactate dehydrogenase isoenzyme.
TCM Parameters Assessment
The four-diagnostic information and the scores of TCM symptoms will be done at the screening visit and every month visit after intervention start.
Questionnaire Assessment
The patient-reported outcomes questionnaire of GLMD was developed by Guangdong Pharmaceutical University to comprehensively and objectively assess the clinical effect of the treatment of GLMD.33 It and SF-36 health survey questionnaire (Supplementary Material) will be done at annual visits.
Medication Evaluation
The types and dosage of the medication for antiplatelet, lipid-lowering, anti-hypertensive, or antidiabetic therapy will be monitored at every visit by doctors.
Safety Assessment
Before the start of trial, the data and safety monitoring board included the principal investigator, researchers, physicians, etc. was formed to manage the safety assessment of research process and the data collection and management. The researchers record the occurrence and nature of each participant’s pre-existing conditions, including clinically significant signs and symptoms of the disease during the study. The frequency of adverse events (AEs) will be presented for the safety assessment. All AEs will be reported in written case report forms (CRF) to the Ethical Review Committee (ERC) of the First Affiliated Hospital of Guangdong Pharmaceutical University and each participating center. If the AEs occur, it will be reported to the ERC and the principal investigator as soon as possible. The study physicians will follow up with all participants reporting AEs and will perform medical evaluations to determine the cause of the AE and its possible relationship to the interventions.
Primary Outcome
The primary outcome is the combined outcomes including the major adverse cardiovascular events (MACE, defined as cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction), coronary restenosis (≥30% from baseline), and unplanned revascularization.
Secondary Outcomes
The combined secondary outcomes include all-cause mortality, acute coronary syndrome, ischemic stroke, heart failure, unplanned re-hospitalization mainly caused by acute complications of diabetes, other thromboembolic events, and the effective rate on the improvement of TCM syndromes during treatment and follow-up.
Blood glucose, blood lipid, hemorheology indexes, blood routine, urine routine, insulin, C-peptide, advanced glycation end products, electrocardiogram, cardiac structure and function, carotid intima-media thickness, liver structure and function, kidney function, pulse wave velocity, vascular endothelial function, ankle-brachial index, retinal blood vessel structure, and SF-36 health survey questionnaire will also be monitored periodically.
Data Collection and Management
All source documents from the five centers, including informed consent forms, questionnaires, and worksheets, will be collected in compliance with standard operating procedures. The researchers will fill in the CRF for each enrolled participant and collect demographic characteristics, baseline symptoms, data outcome at each visit, and AEs, etc. All data will be securely stored and accessible only by the study investigators. The collected data will not be linked to any individual or personal identifiers. Confidentiality will be maintained at all levels of data management. The principal investigator will coordinate the dissemination of data. If required, data transfer between centers will be encrypted, and any information capable of identifying individuals will be removed. Patient files and other source data must be kept for at least 10 years after the study is finished.
Sample Size Determination and Statistical Analysis
This is a phase-II study for evaluating the efficacy and safety of FTZ in treating DCHD. The sample size was calculated based on the expected reduction of primary outcome from pre- to post-treatment at two years. The primary outcome incidence in T2DM patients with CHD is about 19% within two years according to previous studies and expert advice.34–36 Assuming a primary outcome incidence reduction to 5% following treatment with FTZ added, and given a type I error rate of alpha = 0.05, a power of 70% (type II error rate of beta = 0.3), the sample size for one group needed to be 64, a total of 128 participants. Considering a dropout rate of 20%, a total of 160 patients needed to be allocated to reach the required number of patients for the efficacy analysis.
Baseline characteristics of study patients will be summarized in terms of frequencies and percentages for categorical variables and by means ± SD and median with quartile for continuous variables. The independent t-test or Wilcoxon rank-sum test and Pearson chi-test or Fisher’s exact test will be applied to analyze continuous and categorical variables, respectively. This study is a multi-center clinical trial. CMH Chi-square will be used for numeration data; analysis of variance (ANOVA) and an analysis of covariance (ANCOVA) will be used for measurement data; and the Log rank test will be used for outcomes. After deducting the influence of polycentric effect, comparing efficacy survival outcomes, endpoint outcome index and safety outcomes between two groups. If required, statistical analysis between subgroups will be performed. All statistical data analyses will be conducted with significance levels of 0.05 for both sides. SAS 9.4 will be used for data analyses.
Discussion
TCM has accumulated extensive experience in the diagnosis and treatment of diseases from long-term clinical practice, but it is a subjective evaluation that is considered as the low-quality evidence-based medicine (EBM) evidence.17 So, it is essential to conduct well-designed clinical trials for providing high-quality EBM evidence.
The effect of TCM in the treatment of chronic metabolic diseases including GLMD has been gradually accredited with its multiple advantages, such as overall regulation, co-regulation of glucose and lipid metabolism, improving symptoms and signs, and enhancing patients’ quality of life as well as long-term prognosis. Recently, there are some randomized controlled trials (RCTs) conducted to evaluate the efficacy of TCM for CHD and diabetes.20–24,37–39 In diabetic macroangiopathy, there is an ongoing RCT about the efficacy and safety of Shen-Qi Hua-Yu formula in the treatment of diabetic lower extremity artery disease.17 Besides, recent clinical study has shown that Jiangtang Tongmai capsule could reduce the carotid intima-media thickness and the area of arterial plaque in the treatment of diabetic macroangiopathy.40 However, there is no clinical study on the effect of TCM in treating DCHD.
In clinical, FTZ is used as a hospital preparation for treatment of hyperlipidemia, T2DM, and atherosclerosis for more than a decade, which proves its effectiveness and safety. In addition, previous studies have demonstrated that FTZ could adjust the lipid metabolism by regulating the expression of PPARα, liver X receptor α, and other nuclear receptors, improve insulin resistance through the regulation of IRS1-PI3K-protein kinase B (PKB) signaling pathway and achieve its anti-atherosclerosis effect by decreasing scavenger receptor CD36 and SRA.26–28,41 These results indicate that FTZ may be an effective drug for the prevention and treatment of DCHD.
Based on the principle of “Tiao Gan Qi Shu Hua Zhuo”, FTZ was formed and contains eight single herbs. Due to its multi-components and multi-targets mechanisms, FTZ has many functions, such as modifying the lipid and the blood glucose and improving endothelial function, etc. Therefore, we have made a comprehensive consideration of assessment indicators to reflect the curative effect of FTZ from multiple dimensions, which is a beneficial exploration for the clinical efficacy evaluation of CHM.
Based on the overall understanding of the pathological mechanism of DCHD, we conducted a multicenter, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of Chinese Medicine FTZ in treating DCHD. The value of this trial is to provide scientific and rigorous EBM evidence for the efficacy and safety of FTZ in the treatment of DCHD. On the other hand, this study is the first trial of FTZ to observe cardiovascular outcomes through long-term follow-up after treating DCHD, which is of great value. Furthermore, the results of this trial are important to further explore the effectiveness of the comprehensive strategy “Tiao Gan Qi Shu Hua Zhuo” in the prevention and treatment of GLMD.
However, this study also has some limitations. One of which is the lack of evaluation on the long-term effect of the FTZ. In this study, compared with the course of chronic diseases, the time of one-year treatment was relatively short. Due to the short follow-up time and limited budget, the potential role of the FTZ in reducing cardiovascular events may remain unclear. After opening the blind, this trial will continue to conduct a cohort study to observe the effect of FTZ in cardiovascular events, all-cause mortality, and other endpoints, for fully revealing the effects and long-term benefits of FTZ. Besides, this study, a small sample size pilot clinical study, is conducted mainly in Guangdong province of South China. So, it is necessary to conduct a multicenter clinical trial about the effect of FTZ in the prevention and treatment of DCHD nationwide in further study.
Abbreviations
AEs, adverse events; ALT, alanine aminotransferase; CHD, coronary heart disease; CHM, Chinese herbal medicine; CK, creatine kinase; CRF, case report form; DCAD, diabetic coronary artery disease; DCHD, diabetic coronary heart disease; EBM, evidence-based medicine; ECG, electrocardiogram; ERC, ethical review committee; FMD, flow-mediated dilation; FTZ, Fufang Zhenzhu Tiaozhi capsule; GLMD, glucolipid metabolic disorders; RCT, randomized controlled trial; TCM, traditional Chinese medicine; T2DM, type 2 diabetes mellitus.
Data Sharing Statement
The clinical trial data used to support the findings of this study have not been made available because the status of this trial is recruiting.
Ethical Approval
The Ethics Committee at the First Affiliated Hospital of Guangdong Pharmaceutical University approved this trial (Approval no. 2016-39) and this trial was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors appreciate the help and efforts of all research staff for participating in this trial and recruiting and treating the patients.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC1704200), the Major basic and applied basic research projects of Guangdong Province of China (2019B030302005), and the Key Project of National Natural Science Foundation of China (81530102).
Disclosure
The authors declare that they have no conflicts of interests.
References
1. Naito R, Miyauchi K. Coronary Artery Disease and Type 2 Diabetes Mellitus. Int Heart J. 2017;58(4):475–480. doi:10.1536/ihj.17-191
2. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi:10.1056/NEJMoa1008862
3. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi:10.1056/NEJMoa1608664
4. Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377(13):1228–1239. doi:10.1056/NEJMoa1612917
5. Liu L, Chen X, Liu Y, et al. The association between fasting plasma glucose and all-cause and cause-specific mortality by gender: the rural Chinese cohort study. Diabetes Metab Res Rev. 2019;35(4):e3129. doi:10.1002/dmrr.3129
6. Hinnen D, Kruger DF. Cardiovascular risks in type 2 diabetes and the interpretation of cardiovascular outcome trials. Diabetes Metab Syndr Obes. 2019;12:447–455. doi:10.2147/DMSO.S188705
7. Turnbull FM, Abraira C, et al.; Control Group. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–2289. doi:10.1007/s00125-009-1470-0
8. Ginsberg HN, Elam MB, et al.; ACCORD Study Group. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362(17):1563–1574. doi:10.1056/NEJMoa1001282
9. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119(2):351–357. doi:10.1161/CIRCULATIONAHA.108.191305
10. Lehrke M, Leiter LA, Hehnke U, et al. Safety and efficacy of linagliptin in patients with type 2 diabetes mellitus and coronary artery disease: analysis of pooled events from 19 clinical trials. J Diabetes Complications. 2016;30(7):1378–1384. doi:10.1016/j.jdiacomp.2016.06.015
11. Rossaneis MA, Andrade SM, Gvozd R, et al. Factors associated with glycemic control in people with diabetes mellitus. Cien Saude Colet. 2019;24(3):997–1005. doi:10.1590/1413-81232018243.02022017
12. Kozakova M, Morizzo C, Goncalves I, et al. Cardiovascular organ damage in type 2 diabetes mellitus: the role of lipids and inflammation. Cardiovasc Diabetol. 2019;18(1):61. doi:10.1186/s12933-019-0865-6
13. Looker HC, Colombo M, Agakov F, et al. Protein biomarkers for the prediction of cardiovascular disease in type 2 diabetes. Diabetologia. 2015;58(6):1363–1371. doi:10.1007/s00125-015-3535-6
14. Nonogaki K. Dysglycemia and cardiovascular risk. J Am Coll Cardiol. 2012;60(12):1121. doi:10.1016/j.jacc.2012.03.078
15. Guo J. Research progress on prevention and treatment of glucolipid metabolic disease with integrated traditional Chinese and Western medicine. Chin J Integr Med. 2017;23(6):403–409. doi:10.1007/s11655-017-2811-3
16. Fox CS, Golden SH, Anderson C, et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: a Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38(9):1777–1803. doi:10.2337/dci15-0012
17. Leng Y, Gao H, Fu X, et al. The efficacy and safety of Chinese herbal medicine Shen-Qi Hua-Yu formula in patients with diabetic lower extremity artery disease: study protocol of a multi-center, randomized, double-blind, placebo-controlled trial. Medicine. 2020;99(3):e18713. doi:10.1097/MD.0000000000018713
18. Xu H, Shang Q, Chen H, et al. ITIH4: a New Potential Biomarker of “Toxin Syndrome” in Coronary Heart Disease Patient Identified with Proteomic Method. Evid Based Complement Alternat Med. 2013;2013:360149. doi:10.1155/2013/360149
19. Feng Y, Xu H, Qu D, et al. Study on the tongue manifestations for the blood-stasis and toxin syndrome in the stable patients of coronary heart disease. Chin J Integr Med. 2011;17(5):333–338. doi:10.1007/s11655-011-0615-4
20. Liu C, Hou Y, Wang X, et al. Clinical assessment of Shenfu injection loading in the treatment of patients with exacerbation of chronic heart failure due to coronary heart disease: study protocol for a randomized controlled trial. Trials. 2015;16(1):222. doi:10.1186/s13063-015-0729-7
21. Li S, Guo M, Mao H, et al. Qing-Xin-Jie-Yu Granules in addition to conventional treatment for patients with stable coronary artery disease (QUEST Trial): study protocol for a randomized controlled trial. Trials. 2016;17(1):451. doi:10.1186/s13063-016-1569-9
22. Qiao Y, Zhang J, Liu Y, et al. Efficacy and Safety of Zhenyuan Capsule for Coronary Heart Disease with Abnormal Glucose and Lipid Metabolism: study Protocol for a Randomized, Double-Blind, Parallel-Controlled, Multicenter Clinical Trial. Evid Based Complement Alternat Med. 2018;2018:1716430. doi:10.1155/2018/1716430
23. Huang M, Chen G, Guan Q, et al. Effectiveness and safety of Chinese herbal medicine xuanbi antong granules for the treatment of borderline coronary lesions: study protocol for a randomised, double-blinded, placebo-controlled, multicentre clinical trial. BMJ Open. 2019;9(8):e024968. doi:10.1136/bmjopen-2018-024968
24. Jin X, Pan B, Wu H, et al. The efficacy and safety of Shenzhu Guanxin Recipe Granules for the treatment of patients with coronary artery disease: protocol for a double-blind, randomized controlled trial. Trials. 2019;20(1):520. doi:10.1186/s13063-019-3629-4
25. Guo J, Piao SH, Shi ZF, Huang P. Literature study on the distribution of TCM syndromes in Hyperlipidemia. J Guangzhou Uni Tradit Chin Med. 2013;30(5):
26. Guo J, Bei W, Hu Y, et al. A new TCM formula FTZ lowers serum cholesterol by regulating HMG-CoA reductase and CYP7A1 in hyperlipidemic rats. J Ethnopharmacol. 2011;135(2):299–307. doi:10.1016/j.jep.2011.03.012
27. Tang FT, Guo J, He W, et al. Effects of Fufang Zhenzhu Tiaozhi Prescription (), A Chinese herbal preparation, on atherosclerosis in ApoE-/- mice and related mechanisms. Chin J Integr Med. 2011. doi:10.1007/s11655-011-0794-z
28. Hu X, Wang M, Bei W, Han Z, Guo J. The Chinese herbal medicine FTZ attenuates insulin resistance via IRS1 and PI3K in vitro and in rats with metabolic syndrome. J Transl Med. 2014;12:47. doi:10.1186/1479-5876-12-47
29. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
30. Chinese Diabetes Society. China guideline for type 2 diabetes. Chin J Diabetes Mellitus. 2018;10(01):4–67. doi:10.19538/j.nk2018040108
31. Chinese Society of Cardiology of Chinese Medical. Association Editorial Board of Chinese Journal of Cardiology. Guideline for diagnosis and treatment of patients with chronic stable angina. Chin J Cardiol. 2007;35(03):195–206. doi:10.3760/j.issn.0253-3758.2007.03.002
32. Wu YL, Gao HL, Jia ZH, et al. Standard of TCM diagnosis and treatment for diabetes mellitus complicated with heart disease. World J Integr Tradit West Med. 2011;6(5):455–460. doi:10.13935/j.cnki.sjzx.2011.05.020
33. Shuo L, Jiao G. Preliminary Development of Patient Reported Outcomes Instrument for Glucolipid Metabolic Disorders. World Chin Med. 2019;14(01):12–17. doi:10.3969/j.issn.1673-7202.2019.01.003
34. Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J. 2018;206:11–23. doi:10.1016/j.ahj.2018.08.016
35. Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377(13):1228–1239. doi:10.1056/NEJMoa1612917
36. Steg PG, Bhatt DL, Simon T, et al. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N Engl J Med. 2019;381(14):1309–1320. doi:10.1056/NEJMoa1908077
37. Huang YH, Chen ST, Liu FH, et al. The efficacy and safety of concentrated herbal extract granules, YH1, as an add-on medication in poorly controlled type 2 diabetes: a randomized, double-blind, placebo-controlled pilot trial. PLoS One. 2019;14(8):e0221199. doi:10.1371/journal.pone.0221199
38. Huo J, Liu LS, Jian WY, et al. Stationary Treatment Compared with Individualized Chinese Medicine for Type 2 Diabetes Patients with Microvascular Complications: study Protocol for a Randomized Controlled Trial. Chin J Integr Med. 2018;24(10):728–733. doi:10.1007/s11655-018-2987-1
39. Yan M, Wen Y, Yang L, et al. Chinese herbal medicine Tangshen Formula treatment of patients with type 2 diabetic kidney disease with macroalbuminuria: study protocol for a randomized controlled trial. Trials. 2016;17(1):259. doi:10.1186/s13063-016-1385-2
40. Pu WR, Liu XC, Du ZQ, et al. Effect of jiangtang tongmai capsules on macroangiopathy in treating 73 patients with type 2 diabetes mellitus. Chin J Exp Tradit Med Formulae. 2015;21(13):182–185. doi:10.13422/j.cnki.syfjx.2015130182
41. Yao HX, Guo J, Tang CP, et al. Liver hypolipidemic protection of Compound Zhenzhu Tiaozhi Capsule on nonalcoholic fatty liver disease in rats and its mechanism. Chin Tradit Herb Drugs. 2011;42(10):2074–2077.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
