Back to Journals » Infection and Drug Resistance » Volume 12
The efficacy and safety of ceftaroline in the treatment of acute bacterial infection in pediatric patients – a systemic review and meta-analysis of randomized controlled trials
Authors Chen CW, Chang SP, Huang HT, Tang HJ, Lai CC
Received 31 December 2018
Accepted for publication 10 April 2019
Published 15 May 2019 Volume 2019:12 Pages 1303—1310
DOI https://doi.org/10.2147/IDR.S199978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Chih-Wei Chen,1,2 Shen-Peng Chang,3 Hui-Ting Huang,4 Hung-Jen Tang,5 Chih-Cheng Lai6
1Division of Neurosurgery, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan; 2Department of Occupational Safety and Health/Institute of Industrial Safety and Disaster Prevention, College of Sustainable Environment, Chia Nan University of Pharmacy and Science, Tainan, Taiwan; 3Department of Pharmacy, Chi Mei Medical Center, Liouying, Taiwan; 4School of Pharmacy, China Medical University, Taichung, Taiwan; 5Department of Medicine, Chi Mei Medical Center, Tainan, Taiwan; 6Department of Intensive Care Medicine, Chi Mei Medical Center, Liouying, Taiwan
Objectives: This meta-analysis aims to assess the clinical efficacy and safety of ceftaroline in treating acute bacterial infections – community-acquired pneumonia (CAP) and skin and skin structure infection (SSSI) in pediatric patients.
Methods: The Pubmed, Embase, ClinicalTrials.gov. and the Cochrane databases were searched up to December 31, 2018. Only randomized controlled trials (RCTs) evaluating ceftaroline and other comparators in the treatment of acute bacterial infection in pediatric patients were included. The primary outcome was the clinical cure rate and the secondary outcome was the risk of adverse event.
Results: Three RCTs were included. Overall, ceftaroline had a clinical cure rate at end of therapy (EOT) and test of cure (TOC) similar to comparators in the treatment of acute bacterial infection (at EOT, OR, 1.93; 95% CI, 0.88–4.25, I2=0%, and at TOC, OR, 1.36; 95% CI, 0.64–2.91, I2=14%). In addition, ceftaroline had a clinical failure rate at EOT and TOC similar to comparators in the treatment of acute bacterial infection (at EOT, OR, 0.62; 95% CI, 0.22–1.76, I2=0%, and at TOC, OR, 0.68; 95% CI, 0.24–1.91, I2=0%). No significant differences were found for the risk of treatment-emergent adverse events (TEAE) in all and different degrees between ceftaroline and comparators (OR, 0.81; 95% CI, 0.37–1.78, I2=56%). The risks of TEAE and severe adverse events related to study drug were similar between ceftaroline and comparators (TEAE related to study drug, OR, 0.98; 95% CI, 0.52–1.82, I2=0%, severe adverse event related to study drug, OR, 1.09; 95% CI, 0.22–5.44, I2=22%).
Conclusions: The clinical efficacy of ceftaroline is as good as comparator therapy in the treatment of acute bacterial infections – CAP and SSSI, and this antibiotic is well tolerated as the comparators.
Keywords: ceftaroline, acute bacterial infection, pediatric, community-acquired pneumonia, skin and skin structure infection
Introduction
Acute bacterial infections including community-acquired pneumonia (CAP) and skin and skin structure infections (SSSIs) are common causes of hospitalization among pediatric patients.1,2 Moreover, these bacterial infections may be associated with significant morbidity and mortality if no appropriate antibiotic is prescribed in time.1,3 However, the emergence of antibiotic-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) has limited the choices of antibiotics, and further made the early use of appropriate antibiotic more complicated in this critical condition.4,5 Currently, vancomycin, clindamycin and linezolid are the commonly used antibiotics for MRSA infections in pediatrics.5,6
Ceftaroline, an active metabolite of the prodrug – ceftaroline fosamil is a new broad-spectrum cephalosporin. In the in vitro studies, ceftaroline has shown good activity against most Gram-positive bacteria, such as Streptococcus spp., and Staphylococcus spp. (including MRSA) and many Gram-negative bacteria, such as Moraxella catarrhalis, Haemophilus influenza and non-ESBL Escherichia coli, and Klebsiella spp.7–12 For the clinical isolates obtained from pediatric patients, the in vitro activity of ceftaroline remains great.11,12 Clinically, several randomized trials13–15 have investigated the clinical efficacy and safety of ceftaroline in the treatment of acute bacterial infections, including CAP and SSSI in pediatric patients. But, there has been no meta-analysis comparing the efficacy and safety of ceftaroline and other comparators in treating acute bacterial infection in pediatric patients. Therefore, we performed a comprehensive meta-analysis to provide better evidence on the efficacy and safety of ceftaroline in pediatric patients with acute bacterial infections – CAP and SSSI.
Methods
Study search and selection
All clinical studies were identified by a systematic review of the literature in the PubMed, Embase, ClinicalTrials.gov. and Cochrane databases until December 31, 2018 using the following search terms – “ceftaroline”, “infant”, “youth”, “teenager”, “child”, “children”, “adolescent”, “teenagers and teens”. Only clinical studies that compared the clinical efficacy and adverse effects of ceftaroline and other comparators in pediatric patients were included. Two reviewers (Chang & Huang) searched and examined publications independently to avoid bias. When they disagreed, the third author (Chen) resolved the issue. The following data were extracted from every included study: year of publication, study design, countries and duration, type of infection, antibiotic regimens of ceftaroline and comparators, outcomes and adverse events. The assessment of clinical outcome used the modified ITT (MITT) population which was consisted of all patients in the intention to treat (ITT) population who had a confirmed diagnosis in accordance with the study protocol criteria. The evaluation of safety used the ITT population that included all patients who received any amount of intravenous (IV) study drug.
Definitions and outcomes
The primary outcome was overall clinical cure with resolution of clinical signs and symptoms of pneumonia or SSSI, or improvement to the extent that no further antimicrobial therapy was necessary at the end of therapy (EOT) and test of cure (TOC). The EOT visit took place within 48 hrs after the last dose of oral study drug or within 24 hrs if the patient had continued to receive IV study drug. The TOC visit was at 8–15 days after the last dose of IV or oral study drug (whichever was given last). Secondary outcomes included the clinical failure rate at EOT and TOC, the risk of adverse events (AE) including mild, moderate, severe and serious degree, and discontinuation because of AEs. Clinical failure at the EOT was defined as the discontinuation of study drug because of insufficient therapeutic effect or because of an AE and the patient requiring further alternative antimicrobial therapy. Clinical failure at TOC was defined as incomplete resolution or worsening of signs and symptoms requiring additional non-study antibacterial treatment.
Data analysis
This study used the Cochrane Risk of Bias Assessment tool to assess the quality of enrolled RCTs and the risk of bias.16 The software Review Manager, version 5.3 was used to conduct the statistical analyses. The degree of heterogeneity was evaluated with the Q statistic generated from the χ2 test. The proportion of statistical heterogeneity was assessed by the I2 measure. Heterogeneity was considered significant when the p-value was less than 0.10 or the I2 was more than 50%. The random-effects model was used when they are significant heterogeneous, and the fixed-effect model was used when the data were homogenous. Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated for outcome analyses.
Results
Study selection and characteristics
The search program yielded 198 references, including 56 from Pubmed, 116 from Embase, 15 from the Cochrane database and 11 from ClinicalTrials.gov. After excluding 76 duplications, the remaining 122 abstracts were screened. Among them, w retrieved six articles for full-text review. Finally, three studies13–15 fulfilling the inclusion criteria were included in this meta-analysis (Figure 1). All studies13–15 were randomized, multicenter studies that were designed to compare the clinical efficacy and safety of ceftaroline with other comparators for pediatric patients with acute bacterial infections (Table 1). Two studies13,14 evaluated the efficacy of ceftaroline in the treatment of CAP and one study focused the pediatric with SSSI.15 In Korczowski et al study,15 they enrolled 163 patients and compared ceftaroline with vancomycin or cefazolin. In Cannavino et al’s study,13 they enrolled 161 patients and compared ceftaroline with ceftriaxone in a ratio of 3:1. In Blumer et al’s study,14 they enrolled 40 patients and compared ceftaroline with ceftriaxone and vancomycin in a ratio of 3:1. Most of the domains were classified as having a low risk of bias, except for blinding of participants and personnel (Figure 2).
 | Table 1 Characteristics of included studies |
 | Figure 1 Flow diagram of the study selection process. |
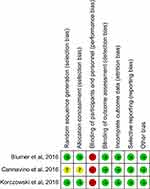 | Figure 2 Risk of bias per study and domain.Note: +, low risk; -, high risk; ?, unknown. |
Clinical efficacy
Overall, ceftaroline had a clinical cure rate at EOT and TOC similar to comparators in the treatment of acute bacterial infection (at EOT, OR, 1.93; 95% CI, 0.88–4.25, I2=0%, and at TOC, OR, 1.36; 95% CI, 0.64–2.91, I2=14%, Figure 3). In addition, ceftaroline had a clinical failure rate at EOT and TOC similar to comparators in the treatment of acute bacterial infection (at EOT, OR, 0.62; 95% CI, 0.22–1.76, I2=0%, and at TOC, OR, 0.68; 95% CI, 0.24–1.91, I2=0%, Figure 4). In the subgroup analysis of patients with CAP, the clinical cure and failure rates at EOT and TOC were similar between ceftaroline and comparators (clinical cure rate at EOT, OR, 2.48; 95% CI, 0.87–7.05, I2=0%, and at TOC, OR, 1.89; 95% CI, 0.68–5.22, I2=24%; clinical failure rate at EOT, OR, 0.77; 95% CI, 0.24–2.43, I2=0%, and at TOC, OR, 0.85; 95% CI, 0.25–2.65, I2=0%).
 | Figure 3 The overall clinical cure rates of ceftaroline and comparators in the treatment of acute bacterial infections. |
 | Figure 4 The overall clinical failure rates of ceftaroline and comparators in the treatment of acute bacterial infections. |
Adverse events
No significant differences were found for the risk of treatment-emergent adverse events (TEAE) in all and different degrees between ceftaroline and comparators (≥1 TEAE, OR, 0.81; 95% CI, 0.37–1.78, I2=56%, mild TEAE OR, 0.90; 95% CI, 0.54–1.51, I2=6%, moderate TEAE, OR, 1.02; 95% CI, 0.42–2.48, I2=24% severe TEAE OR, 0.66; 95% CI, 0.19–2.27, I2=0%, Figure 5). The risks of TEAE and severe adverse events related to study drug were similar between ceftaroline and comparators (TEAE related to study drug, OR, 0.98; 95% CI, 0.52–1.82, I2=0%, severe adverse event related to study drug, OR, 1.09; 95% CI, 0.22–5.44, I2=22%, Figure 5). Finally, the risk of discontinuation of study drug due to an adverse event was also equivalent between ceftaroline and comparators (OR, 1.44; 95% CI, 0.38–5.51, I2=0%, Figure 5).
 | Figure 5 The risk of adverse events ceftaroline and comparators in the treatment of acute bacterial infections. |
Discussion
Several findings from this meta-analysis based on three RCTs showed that ceftaroline has a clinical efficacy similar to other comparators in the treatment of pediatric patients with acute bacterial infection. These findings are supported by the following the data. First, the pooled clinical cure rate of ceftaroline in treating acute bacterial infections was 92.6% at EOT and 90.9% at TOC, respectively, and it was as good as other comparators (87.6% at EOT, and 88.6% at TOC). Second, pooled clinical failure rate of ceftaroline in this meta-analysis was less than 5%, which was similar to comparators. Third, subgroup analysis of CAP in two studies,13,14 showed no significant differences in the clinical cure and failure rate between ceftaroline and comparators. Similar findings were showed in the previous studies20,21 of adult patients. For example, the pooled analysis involving a total of 2364 records in 14 manuscripts showed the efficacy/effectiveness of ceftaroline was 81.2% in all types of pneumonia including CAP, hospital-acquired pneumonia, health care-associated pneumonia and ventilator-associated pneumonia.20 Therefore, based on the findings of these analyses, it indicated that ceftaroline can play an important role in the treatment of pediatric patients with acute bacterial infections including CAP and SSSI. Although this meta-analysis demonstrated the efficacy of ceftaroline, when the clinical feasible, the narrowest spectrum such as ampicillin for CAP or clindamycin for SSSI should be considered for antibiotic de-escalation.
In addition to the clinical efficacy of ceftaroline, the risk of adverse events is another important concern in the treatment of acute bacterial infections in pediatric patients. Most of treatment emergent adverse events among ceftaroline users were mild, and diarrhea and vomiting were the most common adverse events. In this analysis, the pooled risks of treatment-emergent adverse effects were similar between ceftaroline and comparators. Even for the risk of treatment-emergent adverse events and serious adverse events due to study drugs, and discontinuation of study drug due to an adverse event, there was no significant difference in the safety issue between ceftaroline and comparators. All of these findings suggest that ceftaroline is as safe as other comparators in the treatment of acute bacterial infections in the pediatric population.
This meta-analysis has several limitations. First, although ceftaroline as a new cephalosporin with broad-spectrum activity, we cannot evaluate the association between in vitro activity and the in vivo response of different antibiotic-resistant organisms, especially for MRSA and PRSP. Among three enrolled studies,13–15 only Korczowski et al’s study15 demonstrated that the rate of clinical cure at TOC and microbiological eradication in patients with MRSA was higher in ceftaroline group than comparator group. In Blumer et al’s study,14 only one case of CAP due to MRSA was enrolled and the clinical cure was achieved at the TOC visit. In Cannavion et al’s trial,13 they excluded MRSA associated with CAP due to they used ceftriaxone as comparator. Therefore, we still need further studies to prove the clinical efficacy of ceftaroline against MRSA infections. Second, only three RCTs were enrolled in this meta-analysis, and only the clinical setting of CAP and SSSI was assessed as acute bacterial infections. Fortunately, three trials17–19 aim to investigate the efficacy of ceftaroline in the clinical setting of late-onset sepsis, meningitis and osteomyelitis in children are ongoing. We can obtain more data to analysis after these trials are completed in the near future. Finally, the age of the study population in this meta-analysis varied, and some specific groups such as neonates or immunocompromised patients were not included. In addition, the history of previous exposure to health care facility is lack among these enrolled studies. All these issues may limit the generalizability of the findings in this meta-analysis.
In conclusion, based on the findings of this meta-analysis of three RCTs, the clinical efficacy of ceftaroline is as good as comparator therapy in the treatment of acute bacterial infections – CAP and SSSI, and this antibiotic is well tolerated as the comparators. Thus, ceftaroline can be recommended as an appropriate antibiotic therapy for pediatric patients with acute bacterial infections.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126:204–213. doi:10.1542/peds.2009-3109
2. Lautz TB, Raval MV, Barsness KA. Increasing national burden of hospitalizations for skin and soft tissue infections in children. J Pediatr Surg. 2011;46:1935–1941. doi:10.1016/j.jpedsurg.2011.05.008
3. Hedrick J. Acute bacterial skin infections in pediatric medicine: current issues in presentation and treatment. Paediatr Drugs. 2003;5 Suppl 1:35–46.
4. Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 Suppl 5:S368–S377. doi:10.1086/533593
5. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. doi:10.1093/cid/cir531
6. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi:10.1128/CMR.00081-09
7. Shirley DA, Heil EL, Johnson JK. Ceftaroline fosamil: a brief clinical review. Infect Dis Ther. 2013;2:95–110. doi:10.1007/s40121-013-0010-x
8. Pfaller MA, Mendes RE, Castanheira M, Flamm RK, Jones RN, Sader HS. Ceftaroline activity tested against bacterial isolates causing community-acquired respiratory tract infections and skin and skin structure infections in pediatric patients from United States Hospitals: 2012-2014. Pediatr Infect Dis J. 2017;36:486–491. doi:10.1097/INF.0000000000001477
9. Jones RN, Farrell DJ, Mendes RE, Sader HS. Comparative ceftaroline activity tested against pathogens associated with community-acquired pneumonia: results from an international surveillance study. J Antimicrob Chemother. 2011;66 Suppl 3:iii69–iii80. doi:10.1093/jac/dkr101
10. Farrell DJ, Castanheira M, Mendes RE, Sader HS, Jones RN. In vitro activity of ceftaroline against multidrug-resistant staphylococcus aureus and streptococcus pneumoniae: a review of published studies and the AWARE surveillance program (2008-2010). Clin Infect Dis. 2012;55 Suppl 3:S206–S214. doi:10.1093/cid/cis563
11. Sader HS, Mendes RE, Farrell DJ, Flamm RK, Jones RN. Ceftaroline activity tested against bacterial isolates from pediatric patients: results from the assessing worldwide antimicrobial resistance and evaluation program for the United States (2011-2012). Pediatr Infect Dis J. 2014;33:837–842. doi:10.1097/INF.0000000000000307
12. Sader HS, Flamm RK, Farrell DJ, Jones RN. Activity analyses of staphylococcal isolates from pediatric, adult, and elderly patients: AWARE ceftaroline surveillance program. Clin Infect Dis. 2012;55 Suppl 3:S181–S186. doi:10.1093/cid/cis560
13. Cannavino CR, Nemeth A, Korczowski B, et al. A randomized, prospective study of pediatric patients with community-acquired pneumonia treated with ceftaroline versus ceftriaxone. Pediatr Infect Dis J. 2016;35:752–759. doi:10.1097/INF.0000000000001159
14. Blumer JL, Ghonghadze T, Cannavino C, et al. A multicenter, randomized, observer-blinded, active-controlled study evaluating the safety and effectiveness of ceftaroline compared with ceftriaxone plus vancomycin in pediatric patients with complicated community-acquired bacterial pneumonia. Pediatr Infect Dis J. 2016;35:760–766. doi:10.1097/INF.0000000000001160
15. Korczowski B, Antadze T, Giorgobiani M, et al. A multicenter, randomized, observer-blinded, active-controlled study to evaluate the safety and efficacy of ceftaroline versus comparator in pediatric patients with acute bacterial skin and skin structure infection. Pediatr Infect Dis J. 2016;35:e239–e247. doi:10.1097/INF.0000000000001191
16. Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration‘s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi:10.1136/bmj.d5928
17.
18.
19.
20. Sotgiu G, Aliberti S, Gramegna A, et al. Efficacy and effectiveness of Ceftaroline Fosamil in patients with pneumonia: a systematic review and meta-analysis. Respir Res. 2018;19:205. doi:10.1186/s12931-018-0905-x
21. Karve S, Hackett J, Levinson J, Gibson E, Battersby A. Ceftaroline fosamil treatment outcomes compared with standard of care among hospitalized patients with complicated skin and soft tissue infections. J Comp Eff Res. 2016;5:393–405. doi:10.2217/cer-2015-0024
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
