Back to Journals » Cancer Management and Research » Volume 12
The Efficacy and Safety of Apatinib in Refractory/Relapse Advanced Pediatric Solid Tumor: A Retrospective Study
Authors Sun F , Lu S, Zhen Z , Zhu J , Wang J, Huang J , Zhang Y, Li H, Cai R, Liu M, Wu L, Sun X, Zhang Y
Received 21 April 2020
Accepted for publication 2 July 2020
Published 22 July 2020 Volume 2020:12 Pages 6177—6185
DOI https://doi.org/10.2147/CMAR.S258689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Feifei Sun,1,2,* Suying Lu,1,2,* Zijun Zhen,1,2 Jia Zhu,1,2 Juan Wang,1,2 Junting Huang,1,2 Yu Zhang,1,3 Hui Li,1,4 Ruiqing Cai,1,2 Meiling Liu,1,2 Liuhong Wu,1,2 Xiaofei Sun,1,2 Yizhuo Zhang1,2
1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 2Department of Pediatric Oncology, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 3Department of Pathology, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China; 4Department of Imaging, Sun Yat-Sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofei Sun; Yizhuo Zhang Email [email protected]; [email protected]
Background: The prognosis of recurrent or refractory advanced childhood solid tumor patients is very poor and new therapeutic strategies are in urgent need. This study aimed to determine the efficacy and safety of apatinib in pediatric refractory/relapse advanced solid tumor patients.
Patients and Methods: The study retrospectively reviewed recurrent or refractory advanced pediatric solid tumor patients who were treated with apatinib, an oral small-molecule tyrosine kinase inhibitor (TKI) that targets vascular endothelial growth factor receptor-2 (VEGFR2), at the Sun Yat-sen University Cancer Center (China) from January 2016 to March 2019.
Results: Fifty-six patients were included in the safety evaluation and 49 patients were included in the efficacy evaluation. The objective responses rate (ORR) was 26.5% (95% CI 15– 41): 0 CR (complete response) and 13 PR (partial response). Disease control rate (DCR) (CR+PR+SD) was 79.6% (95% CI 65– 90). The median progression-free survival (PFS) was 4.0 months (95% CI 2.6– 5.4). There was no significant difference for ORR or PFS between the A (apatinib monotherapy), A+MT (apatinib combined with oral metronomic therapy) and A+SC (apatinib combined with salvage combination chemotherapy) group (p> 0.05). The most common grade 3 or 4 adverse events were neutropenia (9[16.1%]), thrombocytopenia (8[14.3%]), hand-foot syndrome (3[5.4%]), hypertension (3[5.4%]), anaemia (3[5.4%]) and mucositis (2[3.6%]). Hypertension was the most serious adverse event and one death that occurred was considered as drug-related.
Conclusion: Apatinib showed promising clinical activity in heavily treated recurrent or refractory advanced childhood solid tumor patients. However, it is necessary to pay special attention to monitoring blood pressure when using apatinib in children. Prospective randomized controlled clinical trial is warranted.
Keywords: apatinib, angiogenesis, cancer, pediatric, vascular endothelial growth factor receptor
Introduction
Over the past few decades, survival rates for pediatric cancer have markedly improved.1 However, certain high-risk or relapsed/refractory pediatric cancers still show a very poor prognosis. There is a particularly urgent need for new therapeutic strategies for these patients.
Angiogenesis plays a key role in cancer growth and development. A correlation between high VEGF/VEGFR levels and poor outcomes in pediatric solid tumors such as neuroblastoma, Wilms tumor, Ewing sarcoma, osteosarcoma and rhabdomyosarcoma suggested that angiogenesis inhibitors might offer a therapeutic approach.2–14
Apatinib, an oral VEGFR-2 TKI, was approved by both the FDA (Food and Drug Administration) and CFDA (China Food and Drug Administration) for the treatment of patients with metastatic gastric cancer. It was also demonstrated to improve progression-free survival (PFS) and overall survival (OS) in patients with breast cancer,15 non-small cell lung cancer,16 ovarian cancer,17,18 hepatocellular carcinoma,19 bone and soft tissue sarcomas,20 etc. So far, the safety and efficacy data of apatinib in the treatment of pediatric cancers are still insufficient. As a result, apatinib has not been approved for the treatment of pediatric cancers. However, the status of lacking new drugs and clinical trials in pediatric cancer patients in china makes off-label therapy with apatinib is a very common phenomenon in the real world. In order to clarify the safety and effectiveness of apatinib in pediatric patients, we retrospectively reviewed the data of heavily treated refractory/relapse advanced pediatric solid tumor patients treated with apatinib between January 2016 and March 2019 at Sun Yat-sen University Cancer Center.
Patients and Methods
Eligibility Criteria
Children and young adults (<18 years old when primary diagnose) with refractory/relapsed advanced solid tumors (excluding central nervous system tumors) treated with apatinib in their first or further relapse or progression between January 2016 and March 2019 at Sun Yat-sen University Cancer Center were retrospectively evaluated. All patients 1) had tumors that were not amenable to curative treatment (with unresectable lesions or distant metastasis). 2) had at least one measurable lesion according to Response Evaluation Criteria for Solid Tumors (RECIST). 3) had failed after at least two lines of chemotherapy regimens.
Treatment Schedule
Written informed consent was obtained from all patients when they began treatment for apatinib. The subjects were generally classified into three subgroups according to the therapeutic regimen: apatinib monotherapy (A), apatinib combined with oral metronomic therapy (A+MT) and apatinib combined with salvage combination chemotherapy (A+SC). Oral apatinib was given at a dose of 250 mg (<25 kg), 500 mg (25 kg≤weight<50 kg) and 750 mg (weight≥50 kg) once daily continuously. Dose reduction (in patients≥25 kg) or interruptions for drug-related toxicity were allowed. One treatment cycle was 28 days long. Apatinib treatment continued until disease progression, unacceptable toxicity or patient withdrawal. Dose-limiting toxicity was defined as possibly/probably/definitely drug-related grade 3–4 toxic responses. For group A+MT, metronomic therapy included etoposide (oral: 25mg/m2 once daily for 21 days every month) or cyclophosphamide (oral: 50mg/m2 once daily) plus vinorelbine (oral: 40mg/m2 once weekly for 3 weeks every 4 weeks). For group A+SC, the majority (16/22, 72.7%) of patients received regimen VIT (intravenous vincristine 1.5mg/m2 on day 1, plus orally temozolomide 100mg/m2 on day 1–5 and intravenous irinotecan 50mg/m2 on day 1–5); the other regimens included intravenous topotecan+vindesine, Etoposide+cisplatin, and cyclophosphamide+pirarubicin. Detailed information on treatment option is shown in Table 1.
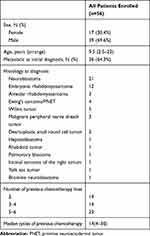 |
Table 1 Baseline Patients Characteristics |
Outcomes Evaluation
All patients underwent physical examination, laboratory tests (including haematology and serum chemistry) and 12-lead electrocardiogram every 3 weeks. Computed tomography (CT) or magnetic resonance imaging (MRI) was performed before treatment and after every 2 months during apatinib monotherapy or when new symptoms developed. Tumor response was evaluated according to RECIST 1.1. Progression-free survival, proportion of disease control, duration of response and safety were recorded. Progression-free survival was defined as the time interval from the beginning of first apatinib treatment to disease progression or death for any cause or last survival assessment without progression for patients alive. Duration of response was assessed in patients who achieved a response and defined as the time from the date of the first documented response until the date of documented progression or death from any cause. Proportion of disease control was defined as the proportion of patients who achieved a complete response, a partial response, or stable disease.
Toxicities Assessment
Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0 (NCI CTC v4.0) and recorded.
Statistical Analysis
Kaplan–Meier survival curves were used for PFS estimation. Data analyses were performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA).
Results
Patients’ Characteristics
From October 1, 2015 to February 28, 2019, 101 patients who had taken apatinib were screened, of which 45 patients were excluded because no post-baseline efficacy evaluation and safety data were collected. At last, 56 patients were enrolled in this study. Clinical characteristics of patients are shown in Table 1. The median age at the beginning of taking apatinib was 9.5 (2.5–23) years old. All diagnoses were pathologically confirmed at Sun Yat-sen University Cancer Center, which included neuroblastoma (NBL)(n=21), Embryonic rhabdomyosarcoma (ERMS)(n=12), Alveolar rhabdomyosarcoma (ARMS)(n=3), Wilms’ tumor (WT)(n=5), Ewing sarcoma (ES)/PNET (n=4), Malignant peripheral nerve sheath tumor (n=3), Desmoplastic small round cell tumor (n=2), Hepatoblastoma (n=1), Rhabdoid tumor (n=1), Pulmonary blastoma (n=1), Intimal sarcoma of the right atrium (n=1), Yolk sac tumor (n=1) and Bromine neuroblastoma (n=1).
Thirty-six patients (64.3%) were in stage IV at initial diagnosis. Before treatment with apatinib, all patients were heavily pretreated including multiple cycles of chemotherapy and/or surgery and/or irradiation for local control. The primary and salvage chemotherapy regimens were used according to tumor type (not listed). Drugs used previously before apatinib include intravenous cyclophosphamide, pirarubicin, vincristine, ifosfamide, cisplatin, carboplatin, etoposide, methotrexate, actinomycin, irinotecan, nedaplatin, doxorubicin hydrochloride liposome, vinorelbine, temozolomide, docetaxel, topotecan, gemcitabine, fluorouracil and/or oral cyclophosphamide, vinorelbine, etoposide, celebrex or 13 cis-RA as maintenance/metronomic therapy. Two of the patients had been treated with other VEGFR inhibitors anlotinib and pazopanib, respectively.
Median cycles of previous intravenous chemotherapy were 14 cycles (range 4–30). Forty-nine (87.5%) patients had accepted at least one surgery, and 46 (82.1%) patients had received prior radiotherapy. Forty-seven (83.9%) patients presented with disease progression and 9 (16.1%) with recurrence. Apatinib alone or combined with chemotherapy was treated as 3rd line in 19 patients, 4th/5th line in 14 patients and 6th/7th line in 23 patients.
The median follow-up time was 3 months (range, 2.0–17.0 months). At the data cutoff point, only two patients (3.6%) remained on apatinib treatment. Fifty-four patients (96.4%) discontinued apatinib as a result of disease progression (29/54,53.7%), toxicity intolerance (12/54,22.2%), individual willingness withdrawal (8/54,14.8%), loss to follow-up (4/54,7.4%) and receiving other anticancer therapy (1/54,1.9%). Six patients who received apatinib less than one cycle were not involved in evaluating efficacy.
Efficacy Evaluation and Outcomes
A total of 49 patients were involved in the efficacy evaluation. No patients achieved CR, 13 (26.5%) patients achieved PR, 26 (53.1%) achieved SD, and 10 (20.4%) patients developed progressive disease (PD) (Table 2). Objective responses rate was 26.5% (95% CI 15–41). Disease control rate was 79.6% (95% CI 65–90) (Table 2). The median PFS was 4.0 months (95% CI 2.6–5.4) (Figure 1). Of the 13 patients who achieved an objective response, 8 (61.5%) had disease progression at data cutoff, only 1 (7.7%) patient remained on apatinib treatment. The median duration of response was 3.0 months (IQR 2.0–4.0). Three (23.1%) patients had remission lasting longer than 6 months. The best percentage change of the target lesion size from the baseline was a 70% reduction.
 |
Table 2 Treatment Responses |
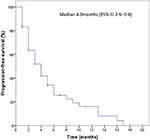 |
Figure 1 Kaplan-Meier curve of progression-free survival (PFS) for patients treated with apatinib with at least one post-baseline efficacy assessment (n=49). |
Among the 49 patients who could be evaluated the efficacy, 23 were soft tissue sarcomas (15 rhabdomyosarcomas) and 17 were neuroblastomas. In the group of patients with soft tissue sarcoma, the objective response rate was 30.4% (95% CI 13.0–53.0) and the disease control rate was 82.6% (95% CI 5.0–39.0). In the rhabdomyosarcoma subgroup, the objective response rate was 26.7% (95% CI 8.0–55.0) and the disease control rate was 80.0% (95% CI 4.0–48.0). In the group of patients with neuroblastoma, the objective response rate was 29.4% (95% CI 10.0–56.0) and the disease control rate was 76.5% (95% CI 7.0–50.0). There is no statistical difference in sensitivity to apatinib between rhabdomyosarcomas and neuroblastomas (p>0.05).
According to the different combinations of chemotherapy, 49 patients were divided into three groups. Twelve patients (24.5%) were in group A, 16 patients (32.7%) were in group A+MT and 21 patients (42.9%) were in group A+SC. Objective responses rate (ORR) of group A, A+MT and A+SC was 41.7% (95% CI 15–72), 18.8% (95% CI 4–46) and 23.8% (95% CI 8–47), respectively. Disease control rate (DCR) of group A, A+MT and A+SC was 75.0% (95% CI 43–95), 87.6% (95% CI 62–98) and 80.9% (95% CI 58–95), respectively. Median PFS of patients in the A group, A+MT group, A+SC group was 3.0 months, 3.0 months, 5.0 months, respectively. There is no statistical difference between any two groups (p>0.05) (Table 2).
Patients with hand-foot syndrome had a median PFS of 8.0 (95% CI 1.7–14.3) months, compared to 3.0 (95% CI 1.6–4.4) months in those without, but the significant difference was not achieved (p=0.163) (Figure 2). The sample size was too small to draw a definite conclusion.
 |
Figure 2 Progression-free survival (PFS) for patients who had no hand-foot syndrome and had hand-foot syndrome during taking apatinib. |
Safety and Toxicity
Fifty-six patients participated in safety assessment. Toxicities observed in the study are listed in Table 3. Regardless of causality, the incidence of any-grade adverse events was 89.0%. Adverse reactions (regardless of grade) observed included neutropenia (20 [35.7%]), thrombocytopenia (15[26.8%]), liver enzymes elevation (11[19.6%]), hand-foot syndrome (10[17.9%]), bleeding (9[17.9%]), anaemia (8[14.3%]), loss of hair pigmentation (5[8.9%]), hypertension (4[7.1%]), mucositis (4[7.1%]), diarrhea (2[3.6%]), nausea/vomiting (2[3.6%]), intestinal perforation (1[1.8%]), cough (1[1.8%]), menopause (1[1.8%]), pneumothorax (1[1.8%]), and pain (1[1.8%]). Bleeding is one of the common side effects, 9(16.1%) patients experiencing 1–2 degrees of bleeding, including epistaxis (5[8.9%]), gastrointestinal hemorrhage (2[3.6%]) and bloody sputum (2[3.6%]). Grade 3–4 toxicity was observed in 39.3% (22/56) patients, including neutropenia (9[16.1%]), thrombocytopenia (8[14.3%]), hand-foot syndrome (3[5.4%]), hypertension (3[5.4%]), anaemia (3[5.4%]), and mucositis (2[3.6%]). Severe adverse toxicities were mainly hypertension. Grade 5 hypertension and treatment-related death occurred in a relapsed and refractory neuroblastoma patient. After oral administration of apatinib 250 mg once daily continuously for a month, the 4-year-old boy developed headache symptoms and was found to have blood pressure up to 160/120mmHg at home. His parents gave him a tablet of anti-hypertensive medicine immediately and discontinued apatinib. After that, his blood pressure declined to normal. Recognizing the incurable nature of relapsed and refractory neuroblastoma, his parents did not take their son to any hospital. The child lapsed into a seizure, coma and died that night.
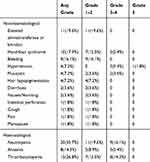 |
Table 3 Possible Treatment-Related Adverse Events |
At the data cutoff point, 56 patients received apatinib with a median of 96 days (range 4–402). Twelve (21.4%) patients discontinued the treatment as a result of adverse events, including hypertension (n=4), hand-foot syndrome (n=3), hemorrhage (n=2), neutropenia (n=1), oral mucositis (n=1) and diarrhea (n=1).
Discussion
Antiangiogenesis is a potential therapeutic target for childhood solid tumors. Several Phase II studies analyzed the effectiveness of bevacizumab, a monoclonal antibody for VEGF, combining with chemotherapy in advanced/metastatic pediatric solid carcinoma, such as neuroblastoma,21 glioma,22,23 soft tissue sarcoma20,24 and osteosarcoma.25 But these studies have not yielded evidence of survival benefits of the addition of bevacizumab to chemotherapy. Recently, several VEGFR-TKIs, such as lenvatinib and regorafenib, have been shown promising results in pediatric osteosarcoma in phase II trials. A phase II clinical study has shown apatinib’s encouraging efficacy for the treatment of metastatic sarcomas in adolescents and adults.20 Pazopanib was shown to be effective in some case reports of pediatric osteosarcoma,26,27 desmoid tumors28 and synovial sarcoma.29 However, clinical data on efficacy/safety of VEGFR-TKIs are still limited in the literature on pediatric patients. We searched ClinicalTrials.gov website on June 1, 2020 with the keyword “child”, “apatinib” and “interactive studies” and subsequently excluded withdraw/retrospective studies. A total of 14 clinical studies including patients under 18 years old diagnosed with soft tissue sarcoma, osteosarcoma, glioma and lymphoma. Eleven of them are studies for bone and/or soft tissue sarcomas. Eight of these studies were not really for children because they only included adolescents over 14 and adults. Only one rhabdomyosarcoma study included children younger than 8 years old. Clinical studies of apatinib in early childhood tumors such as neuroblastoma and Wilms tumor are absent.
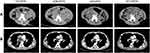
This retrospective study investigated the efficacy and safety of an oral VEGFR inhibitor apatinib alone or combined with chemotherapy in 56 pediatric patients with refractory/relapsed advanced solid tumors. We observed that 26.5% of patients achieved an objective response. The median progression-free survival was 4.0 months (95% CI 2.6–5.4). One of the best responses occurred in a patient diagnosed as the right atrium intimal sarcoma with left vertical spine muscle metastasis, who has sustained remission for more than 6 months after oral administration of apatinib alone (Figure 3). It demonstrated the effectiveness of the drug in a subset of patients. Neuroblastoma and rhabdomyosarcoma are the most common types of recurrent/refractory extracranial solid tumors and also the main tumor species causing the death in children. The results of our study show that there is no statistical difference in sensitivity to apatinib between rhabdomyosarcomas and neuroblastomas.
VEGF-TKIs are considered to have synergistic effects with chemotherapy. In this study, patients were divided into three groups: group A (apatinib monotherapy), group A+MT (apatinib combined with metronomic chemotherapy) and group A+SC (apatinib combined with salvage combination chemotherapy). In our study, the main metronomic therapy (MT) was oral cyclophosphamide plus vinorelbine, which was commonly used in pediatric cancers.30 The metronomic therapy concepts continuously use of low-dose chemotherapeutic, anti-angiogenetic and immunomodulating drugs, which is demonstrated to obtain a sustained antitumor effect through modulating the tumor microenvironment.31 However, a recent randomized clinical trial showed that the metronomic chemotherapy only reached 3.5% ORR in progressive pediatric solid malignant tumors and did not prolong progression-free survival compared with placebo.32 In a phase II study, apatinib combined with MC reached 54% ORR and 8-month PFS in patients with platinum-resistant or platinum-refractory ovarian cancer.17 The salvage chemotherapy regimen in this study was mainly VIT (irinotecan, temozolomide and vincristine). VIT was reported to be promising and frequently used in combination with antiangiogenic agents in childhood tumors.33–35 The result of the study showed that median PFS of patients in the A group, A+MC group, A+SC group was 3.0 months, 3.0 months, 5.0 months, respectively. There was no significant difference between groups and failed to prove the synergistic effect of apatinib and metronomic therapy or salvage chemotherapy. However, it is worth noting that the number of cases in each subgroup is limited.
We observed grade 3–5 toxicity were mainly neutropenia, thrombocytopenia, hypertension and hand-foot syndrome. The most serious adverse was hypertension. In 56 patients, 3 had grade 3–4 hypertension and 1 had treatment-related death. All the four patients discontinuing taking drugs immediately after the onset of hypertension. This indicated that when using VEGFR-TKI in children, more attention should be paid to monitoring blood pressure. If blood pressure is more than 120/80mmHg, anti-hypertensive drugs should be taken simultaneously with apatinib. For uncontrolled hypertension (grade 3–4), it is suggested to discontinue apatinib. The hand-foot syndrome was proposed to be a predictive biomarker for assessing the efficacy of anti-angiogenic drugs.36,37 In our study, patients who had hand-foot syndrome had a median PFS of 8.0 (95% CI 1.7–14.3) months, compared to 3.0 (95% CI 1.6–4.4) months in those who did not, but the significant difference was not achieved (p=0.163).
This study had several limitations. The main limitation is that the study was a retrospective study with no placebo control for comparison, and selection bias could not be ruled out. Second, the number of patients in each subgroup was too limited to verify whether there was a synergistic effect with apatinib and chemotherapeutic drugs. The patient population was very heterogenous, and the multiple subgroups further limit conclusions in an already relatively small number of children. Third, the urine protein concentration was not regularly monitored during follow-up in this study, resulting in a significantly lower incidence of albuminuria than in previous reports.38
Conclusion
In conclusion, this study is a retrospective review of oral VEGFR2 inhibitor apatinib in pediatric cancer patients. We have seen that apatinib is effective in part of refractory/relapsed pediatric cancer patients, but the side effects of hypertension should be alert. The result may provide a valuable reference for supporting further clinical studies. Prospective randomized controlled clinical trial is warranted.
Abbreviations
TKI, small-molecule tyrosine kinase inhibitor; VEGFR2, vascular endothelial growth factor receptor-2; ORR, objective responses rate; DCR, disease control rate; PFS, progression-free survival.
Data Sharing Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn).
Ethical Statement
The data of all patients in this study were anonymized. The ethical board of Sun Yat-sen University Cancer Center approved the retrospective study and also determined that informed consent was not required (B2019-179-01).
Acknowledgments
The authors would like to thank the patients and families who participated in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi:10.3322/caac.20073
2. Fukuzawa M, Sugiura H, Koshinaga T, Ikeda T, Hagiwara N, Sawada T. Expression of vascular endothelial growth factor and its receptor Flk-1 in human neuroblastoma using in situ hybridization. J Pediatr Surg. 2002;37(12):1747–1750. doi:10.1053/jpsu.2002.36712
3. Komuro H, Kaneko S, Kaneko M, Nakanishi Y. Expression of angiogenic factors and tumor progression in human neuroblastoma. J Cancer Res Clin Oncol. 2001;127(12):739–743. doi:10.1007/s004320100293
4. Ren Y, Chan HM, Fan J, et al. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene. 2006;25(25):3501–3508. doi:10.1038/sj.onc.1209395
5. Puppo M, Battaglia F, Ottaviano C, et al. Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1alpha and −2alpha. Mol Cancer Ther. 2008;7(7):1974–1984. doi:10.1158/1535-7163.MCT-07-2059
6. Mangieri D, Nico B, Coluccia AM, Vacca A, Ponzoni M, Ribatti D. An alternative in vivo system for testing angiogenic potential of human neuroblastoma cells. Cancer Lett. 2009;277(2):199–204. doi:10.1016/j.canlet.2008.12.014
7. Skoldenberg EG, Larsson A, Jakobson A, et al. The angiogenic growth factors HGF and VEGF in serum and plasma from neuroblastoma patients. Anticancer Res. 2009;29(8):3311–3319.
8. Wu H, Zhang J, Dai R, Xu J, Feng H. Transferrin receptor-1 and VEGF are prognostic factors for osteosarcoma. J Orthop Surg Res. 2019;14(1):296. doi:10.1186/s13018-019-1301-z
9. Wang L, Zhang D, Chen XR, Fan YX, Wang JX. Expression of vascular endothelial growth factor (VEGF) and VEGF-C in serum and tissue of Wilms tumor. Chin Med J (Engl). 2011;124(22):3716–3720.
10. Wagner KD, El Mai M, Ladomery M, et al. Altered VEGF splicing isoform balance in tumor endothelium involves activation of splicing factors Srpk1 and Srsf1 by the Wilms’ tumor suppressor Wt1. Cells. 2019;8(1):41. doi:10.3390/cells8010041
11. Capasso L, Florio M, Lillo M, et al. Vascular endothelial growth factor expression as a biomarker of prognosis in patients with chondrosarcoma, Ewing’s sarcoma and osteosarcoma. Current concepts. J Biol Regul Homeost Agents. 2019;33(2 Suppl. 1):39–43.
12. Kumar R, Sankineani S, Rastogi S, et al. Expression of vascular endothelial growth factor in Ewing’s sarcoma. Int Orthop. 2012;36(8):1669–1672. doi:10.1007/s00264-012-1564-z
13. Miyoshi K, Kohashi K, Fushimi F, et al. Close correlation between CXCR4 and VEGF expression and frequent CXCR7 expression in rhabdomyosarcoma. Hum Pathol. 2014;45(9):1900–1909. doi:10.1016/j.humpath.2014.05.012
14. Krawczyk MA, Styczewska M, Sokolewicz EM, et al. Tumour expressions of hypoxic markers predict the response to neo-adjuvant chemotherapy in children with inoperable rhabdomyosarcoma. Biomarkers. 2019;24(6):538–548. doi:10.1080/1354750X.2019.1606275
15. Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14(1):820. doi:10.1186/1471-2407-14-820
16. Wu F, Zhang S, Xiong A, et al. A phase II clinical trial of apatinib in pretreated advanced non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e831–e842. doi:10.1016/j.cllc.2018.06.002
17. Lan CY, Wang Y, Xiong Y, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a Phase 2, single-arm, prospective study. Lancet Oncol. 2018;19(9):1239–1246. doi:10.1016/S1470-2045(18)30349-8
18. Miao M, Deng G, Luo S, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 2018;148(2):286–290. doi:10.1016/j.ygyno.2017.12.013
19. Lu W, Jin XL, Yang C, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther. 2017;18(6):433–438. doi:10.1080/15384047.2017.1323589
20. Liao Z, Li F, Zhang C, et al. Phase II trial of VEGFR2 inhibitor apatinib for metastatic sarcoma: focus on efficacy and safety. Exp Mol Med. 2019;51(3):1–11. doi:10.1038/s12276-019-0221-7
21. Modak S, Kushner BH, Basu E, Roberts SS, Cheung NK. Combination of bevacizumab, irinotecan, and temozolomide for refractory or relapsed neuroblastoma: results of a phase II study. Pediatr Blood Cancer. 2017;64(8):e26448. doi:10.1002/pbc.26448
22. Peters KB, Lipp ES, Miller E, et al. Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol. 2018;137(2):349–356. doi:10.1007/s11060-017-2724-1
23. Grill J, Massimino M, Bouffet E, et al. Phase II, open-label, randomized, multicenter trial (HERBY) of bevacizumab in pediatric patients with newly diagnosed high-grade glioma. J Clin Oncol. 2018;36(10):951–958. doi:10.1200/JCO.2017.76.0611
24. Chisholm JC, Merks JHM, Casanova M, et al. Open-label, multicentre, randomised, phase II study of the EpSSG and the ITCC evaluating the addition of bevacizumab to chemotherapy in childhood and adolescent patients with metastatic soft tissue sarcoma (the BERNIE study). Eur J Cancer. 2017;83:177–184. doi:10.1016/j.ejca.2017.06.015
25. Navid F, Santana VM, Neel M, et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer. 2017;141(7):1469–1477. doi:10.1002/ijc.30841
26. Umeda K, Kato I, Saida S, Okamoto T, Adachi S. Pazopanib for second recurrence of osteosarcoma in pediatric patients. Pediatr Int. 2017;59(8):937–938. doi:10.1111/ped.13307
27. Longhi A, Paioli A, Palmerini E, et al. Pazopanib in relapsed osteosarcoma patients: report on 15 cases. Acta Oncol. 2019;58(1):124–128. doi:10.1080/0284186X.2018.1503714
28. Agresta L, Kim H, Turpin BK, et al. Pazopanib therapy for desmoid tumors in adolescent and young adult patients. Pediatr Blood Cancer. 2018;65(6):e26968. doi:10.1002/pbc.26968
29. Casanova M, Basso E, Magni C, et al. Response to pazopanib in two pediatric patients with pretreated relapsing synovial sarcoma. Tumori. 2017;103(1):e1–e3. doi:10.5301/tj.5000548
30. Minard-Colin V, Ichante JL, Nguyen L, et al. Phase II study of vinorelbine and continuous low doses cyclophosphamide in children and young adults with a relapsed or refractory malignant solid tumour: good tolerance profile and efficacy in rhabdomyosarcoma–a report from the societe francaise des cancers et leucemies de l’Enfant et de l’adolescent (SFCE). Eur J Cancer. 2012;48(15):2409–2416. doi:10.1016/j.ejca.2012.04.012
31. Heng-Maillard MA, Verschuur A, Aschero A, et al. SFCE METRO-01 four-drug metronomic regimen phase II trial for pediatric extracranial tumor. Pediatr Blood Cancer. 2019;66(7):e27693. doi:10.1002/pbc.27693
32. Pramanik R, Agarwala S, Gupta YK, et al. Metronomic chemotherapy vs best supportive care in progressive pediatric solid malignant tumors: a randomized clinical trial. JAMA Oncol. 2017;3(9):1222–1227. doi:10.1001/jamaoncol.2017.0324
33. Setty BA, Stanek JR, Mascarenhas L, et al. VIncristine, irinotecan, and temozolomide in children and adolescents with relapsed rhabdomyosarcoma. Pediatr Blood Cancer. 2018;65(1):e26728. doi:10.1002/pbc.26728
34. Buyukkapu Bay S, Kebudi R, Gorgun O, Zulfikar B, Darendeliler E, Cakir FB. Vincristine, irinotecan, and temozolomide treatment for refractory/relapsed pediatric solid tumors: a single center experience. J Oncol Pharm Pract. 2019;25(6):1343–1348. doi:10.1177/1078155218790798
35. Venkatramani R, Malogolowkin M, Davidson TB, May W, Sposto R, Mascarenhas L. A Phase I study of vincristine, irinotecan, temozolomide and bevacizumab (vitb) in pediatric patients with relapsed solid tumors. PLoS One. 2013;8(7):e68416. doi:10.1371/journal.pone.0068416
36. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi:10.1056/NEJMoa1303989
37. Kobayashi K, Kawakami K, Yokokawa T, et al. Association of hand-foot skin reaction with regorafenib efficacy in the treatment of metastatic colorectal cancer. Oncology. 2019;96(4):200–206. doi:10.1159/000495989
38. Glade Bender JL, Lee A, Reid JM, et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children’s oncology group phase I consortium report. J Clin Oncol. 2013;31(24):3034–3043. doi:10.1200/JCO.2012.47.0914
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.